Abstract
Rationale
In preclinical and clinical studies, medications enhancing the GABA neurotransmission attenuate nicotine reward. Pregabalin, a GABA analogue, presumably interacts with brain glutamate and GABA neurotransmission. The goal of this study was to determine pregabalin's effects on smoking behavior, nicotine withdrawal, craving for cigarettes, and cognitive performance.
Methods
Twenty-four smokers participated in an outpatient double-blind, placebo-controlled, crossover study. Subjects had a 4-day treatment period with either pregabalin (300 mg/day) or placebo and following a washout period were then crossed over for 4 days to the other treatment. In each treatment period, starting at midnight of Day 1, participants were asked to stop smoking until the experimental session on Day 4. During the experimental session measures of ad lib smoking behavior, tobacco withdrawal, craving for cigarettes, and cognitive performance were obtained.
Results
Pregabalin treatment, compared to placebo, did not reduce the smoking behavior during the first 3 days of treatment or during ad lib smoking period. Pregabalin treatment attenuated some tobacco withdrawal symptoms including ratings of anxious, irritable, and frustrated in abstinent smokers. Pregabalin treatment also attenuated the subjective ratings of “liking” in response to smoking. Under pregabalin treatment, smokers made more errors in a sustained attention task.
Conclusions
These findings provide limited support for pregabalin as a treatment for nicotine addiction.
Keywords: GABA, pregabalin, nicotine, cigarette smoking, attentional bias
Introduction
Cigarette smoking continues to be the leading cause of preventable death in the United States. In spite of significant reductions during the past 30 years, smoking rates continue to be widespread, with an estimated 46.6 million of U.S. adults (20.6 %) smoking cigarettes. Despite the availability of effective pharmacotherapies including nicotine replacement therapy (NRT), bupropion, and varenicline (Fiore et al. 2008; Herman and Sofuoglu 2010), 70 to 90% of smokers resume smoking within a year of treatment. Thus, there is a great need to develop more effective pharmacotherapies for tobacco addiction. Given the grave consequences of the tobacco epidemic both to the individuals and the society, innovative approaches to medications development are warranted.
A number of behavioral and pharmacological studies have demonstrated that the rewarding effects of nicotine are mediated by the mesolimbic dopamine system. Especially critical for nicotine reward, similar to other drugs of abuse, is its capacity to induce dopamine release in the nucleus accumbens (Olmstead et al. 2007). The nucleus accumbens also contains GABAergic synapses that dampen dopamine release induced by nicotine (Mansvelder et al. 2002). Consistent with these observations, treatment with gamma-vinyl-GABA (Vigabatrin), which increases GABA levels by inhibiting the breakdown of GABA, attenuated nicotine-induced place preference, nicotine self-administration, and nicotine-induced dopamine release in the nucleus accumbens of rats (Dewey et al. 1999; Paterson and Markou 2002). Similarly, baclofen, a GABAB receptor agonist, attenuated nicotine self-administration in rats (Fattore et al. 2002; Paterson et al. 2004). Altogether, these studies suggest that medications enhancing the GABA system may attenuate the rewarding effects of nicotine.
Few clinical studies have examined the effect of GABA medications on tobacco dependence. In a study with 16 smokers, a single 20 mg dose of the GABAB agonist baclofen or placebo was administered following overnight abstinence from smoking (Cousins et al. 2001). While baclofen did not change ad lib smoking behavior, it attenuated the ratings of “like cigarette's effects,” suggesting that baclofen may attenuate some of the subjective rewarding effects of nicotine. In a recent pilot clinical trial, baclofen (up to 20 mg four times a day), compared to placebo, reduced cigarette consumption in smokers who were not ready to quit smoking (Franklin et al. 2009). Another study examined the dose-dependent effects of tiagabine, a GABA enhancer, on the acute physiological and subjective effects of intravenous (IV) nicotine in 12 smokers (Sofuoglu et al. 2005). Tiagabine treatment at 8 mg, compared to placebo or 4 mg tiagabine, attenuated the rewarding effects of intravenous nicotine, including “good effects” and “drug liking” in overnight abstinent smokers. Tiagabine treatment at 8 mg, compared to placebo or 4 mg tiagabine, also decreased craving for cigarettes, and improved performance on a reaction time task, the classic Stroop test (Stroop 1935). These findings suggested that medications enhancing synaptic GABA levels may have utility for the treatment of tobacco addiction. Unfortunately, the association of tiagabine with adverse events, especially seizures, limited the investigation of this medication for novel indications, including for tobacco addiction (Wade et al. 2010).
In this study, we examined the potential utility of pregabalin, as a treatment for tobacco addiction. Pregabalin is a structural analog of GABA and has a similar pharmacological profile as gabapentin (Ben-Menachem 2004). It is used for the treatment of seizure disorder and pain syndromes. Both drugs may enhance GABA neurotransmission or reduce glutamate release through multiple mechanisms (Errante et al. 2002; Urban et al. 2005). In this randomized, double-blind, crossover study, we examined pregabalin’s effects on smoking behavior, tobacco withdrawal, and craving for cigarettes as well as cognitive performance on a sustained attention task. We chose a sustained attention task given that pregabalin treatment has been shown to impair attentional function in healthy volunteers (Hindmarch et al. 2005). We hypothesized that pregabalin treatment will reduce smoking behavior and attenuate the severity of tobacco withdrawal symptoms and mildly impair attentional function.
Materials and Methods
Participants
Seven female and 17 male non-treatment seeking smokers were recruited from the New Haven, Connecticut area. Seven additional participants were enrolled but dropped out of the study and were not included in the analyses. The reason for drop outs included drug use (n=1), personal reasons (n=2), falling on ice (n=1), dizziness (n=1), vertigo (n=1), and excessive sleepiness (n=1). Among 24 completers, 16 participants were African-American, 7 were Caucasian, and 1 was Hispanic. The average age (SD) of the participants was 33.3 (9.3). On average, participants smoked 17.8 (6.1) cigarettes/day, and had a Fagerstrom Test for Nicotine Dependence (Heatherton et al. 1991) score of 5.9 (1.8; ranging from 3 to 9), indicating moderate dependence. Participants were smokers who smoked at least 10 cigarettes per day for the past year. They had normal physical, laboratory and psychiatric examinations and were not dependent on alcohol or drugs other than nicotine. Participants provided written, signed consent before participating in the study. This study was approved by the VA Connecticut Healthcare System Human Subjects Subcommittee. Experimental sessions were conducted in the Biostudies Unit located at the VA Connecticut Healthcare System and participants were paid for participation.
Procedures
In this outpatient randomized, double-blind, crossover study, smokers had two 4-day treatment periods, in which they were assigned to a random sequence of pregabalin (300 mg/day) or placebo treatment. These treatment periods were separated by a minimum of 4 days to provide enough time for medication washout. During the first 3-days of each treatment period, participants had twice daily clinic visits to receive the study medications and to complete outcome measures. Starting at midnight of Day 1, participants were asked to stop smoking until the morning of Day 4, about 58 hours of abstinence. To assure compliance with non-smoking instructions, participants were paid $40 extra for not smoking in each treatment phase. Abstinence from smoking was verified with expired carbon monoxide (CO; < 10 parts-per-million).
On Day 4 (Lab Session), a urine sample was obtained for urine drug screening. One hour following study medication (pregabalin or placebo) administration, sustained attention was assessed with the Sustained Attention to Response Test (SART) described below. Next, participants started a 30-minute cue reactivity assessment period in which urges to smoke were assessed for neutral and smoking cues. Subjects then started a 2.5 hour smoking period, during which measures of smoking behavior were collected.
SART
The Sustained Attention to Response Test (SART), is a Go No-Go task (Robertson et al. 1997). It assesses the ability to withhold responses to an infrequently occurring target (No-Go trials). Reaction times (RTs) and errors on Go trials are also assessed. Two hundred and twenty five single digits (25 × 9 digits) are presented on a computer monitor for 250 ms each, immediately followed by a mask for 900 ms. Subjects must press a spacebar in response to every digit except the “3.” They are instructed to give equal importance to speed and accuracy (see (Sofuoglu et al. 2008) for details). For the No-Go trials, “3”s, fewer errors indicated better response inhibition. In contrast, the number of errors on Go trials, non “3”s, the reaction time reflected the response activation function, with fewer errors and faster reaction time indicating greater response activation. The SART took about 10 minutes to administer.
Cue reactivity assessment
This procedure is adapted from Sayette and Hufford (Sayette and Hufford 1994). First, participants were exposed to neutral cues, for which they opened a small box and take out a roll of tape. They were then instructed to hold the tape for two minutes and then put it back into the box, and close the lid. For the smoking cue exposure, another small box was placed on the desk which contained a recently opened box of their preferred brand of cigarettes. Participants were instructed to open the box, to take out one of the cigarettes, and to look at it for a few seconds. They were then instructed to light the cigarette (without taking a puff), and hold the cigarette in their smoking hand in the manner they would if they were between puffs when smoking. They were then instructed to continue to look at the cigarette. Finally, they were asked to stub the cigarette out in an ashtray, put the extinguished cigarette back into the box, and close the lid. For both neutral and smoking cue exposure, physiological measures (heart rate and blood pressure) and urges to smoke cigarettes were assessed just before and after exposure.
Ad lib Smoking Procedure
Two hours following the medication treatment, subjects smoked a cigarette of their own brand, followed by a 2-h ad lib smoking period where measures of smoking topography were obtained using the CReSSmicro System (Borgwadlt KC, Inc). The ad lib smoking period was conducted in a ventilated room where subjects were monitored through a two-way mirror. For this period, smokers were instructed to smoke as they would normally do and were allowed to read magazines or listen to music but were not allowed to sleep.
Drugs
Pregabalin (Lyrica®) dose was 150 mg/day on day 1, 200 mg/day on day 2, and 300 mg/day afterwards. The medication was administered in the clinic by the study nurse during the twice daily clinic visits. This dose is within the range of usual daily dose of pregabalin used for the treatment of seizure disorder or pain, which ranges from 150 to 600 mg/day. The dose was limited to 300mg/day to minimize cognitive impairment associated with pregabalin treatment. Following oral administration, peak plasma levels of pregabalin are reached within 1 to 2 hours. The elimination half-life of pregabalin ranges from 6 to 8 hours, requiring multiple daily dosing.
Measures
The main outcome measures were behavioral, biochemical, subjective, and cognitive measures. Smoking behavior during the ad lib smoking was assessed with smoking topography measures including number of cigarettes, average number of puffs for each cigarette, puff duration, and inter puff interval. In addition, CO measurement before and after the session were obtained for the CO boost as a biochemical measure of smoking using Vitalograph CO monitor (Lenexa, KS). Compliance with the smoking abstinence was assessed by daily CO measurements.
The subjective measures included the Drug Effects Questionnaire (DEQ), Brief Questionnaire on Smoking Urges (BQSU), Minnesota Nicotine Withdrawal Symptom Checklist (MNWSC), and Profile of Mood States (POMS). The DEQ was used to measure the subjective effects of smoking under each of the treatment conditions. The DEQ consisted of 5 items: drug strength, good effects, bad effects, head rush and like the drug (Soria et al. 1996). Participants rated these effects on a 100 mm scale, from “not at all” to “extremely.” The DEQ was given just after the sample smoking on day 4. Participants were instructed to use this scale to rate the effects of the cigarette that they had just smoked. The BQSU, a 10-item scale, was originally developed by Tiffany and Drobes (Cox et al. 2001; Tiffany and Drobes 1991). Smokers are asked how strongly they agree or disagree with items on a 7-point Likert scale. This scale has two factors: Factor 1 reflects an urge to smoke for stimulation and Factor 2 reflects an urge to smoke to relieve negative mood and withdrawal (Cox et al. 2001). This scale has been found to be highly reliable and reflects levels of nicotine deprivation (Bell et al. 1999; Morgan et al. 1999).
The POMS includes 65 items (rated on a scale from 0, "not at all," to 4, "very much so" for the past 24 hours) that make up six subscales: tension-anxiety, depression-dejection, anger-hostility, vigor-activity, fatigue-inertia, and confusion-bewilderment (McNair et al. 1971). As a global measure of affective state, a total mood disturbance score was calculated by summing the scores on the six subscales, with vigor-activity negatively weighted. The MNWSC measures withdrawal symptoms from tobacco and includes items of cigarette craving, irritability/anger, anxiety, difficulty concentrating, restlessness, increased appetite, depressed or sad mood, and insomnia (Hughes and Hatsukami 1986; Hughes and Hatsukami 1997). We used a modified version of the MNWSC in which participants were asked to rate these symptoms on a 100 mm scale, from “not at all” to “extremely” (Buchhalter et al. 2005). The BQSU and POMS were given daily during the study. The BQSU was also given just before and after each craving induction and at the end of the session on day 4. The MNWSC was administered at the beginning of experimental session on Day 4.
Cognitive performance was measured with the Sustained Attention to Response Test (SART), as described above.
Statistical Analysis
Study outcomes were analyzed using a mixed-effect repeated-measures crossover model using the Statistical Analysis System, Version 9.1.3. (SAS Institute Inc. 2007). Each model included fixed main effect terms for treatment (placebo or pregabalin), and time of measurement (day in the study or time since medication administration), as well as the interaction of these two effects. We also included a random effect for participant and a blocking factor for treatment sequence. Values of p< 0.05 were considered statistically significant, based on two-tailed tests, unless otherwise specified. Significant treatment or treatment by time interactions (p<0.05) were followed by post hoc comparisons, with Tukey adjustments to prevent the Type I error rate.
Results
Smoking Behavior
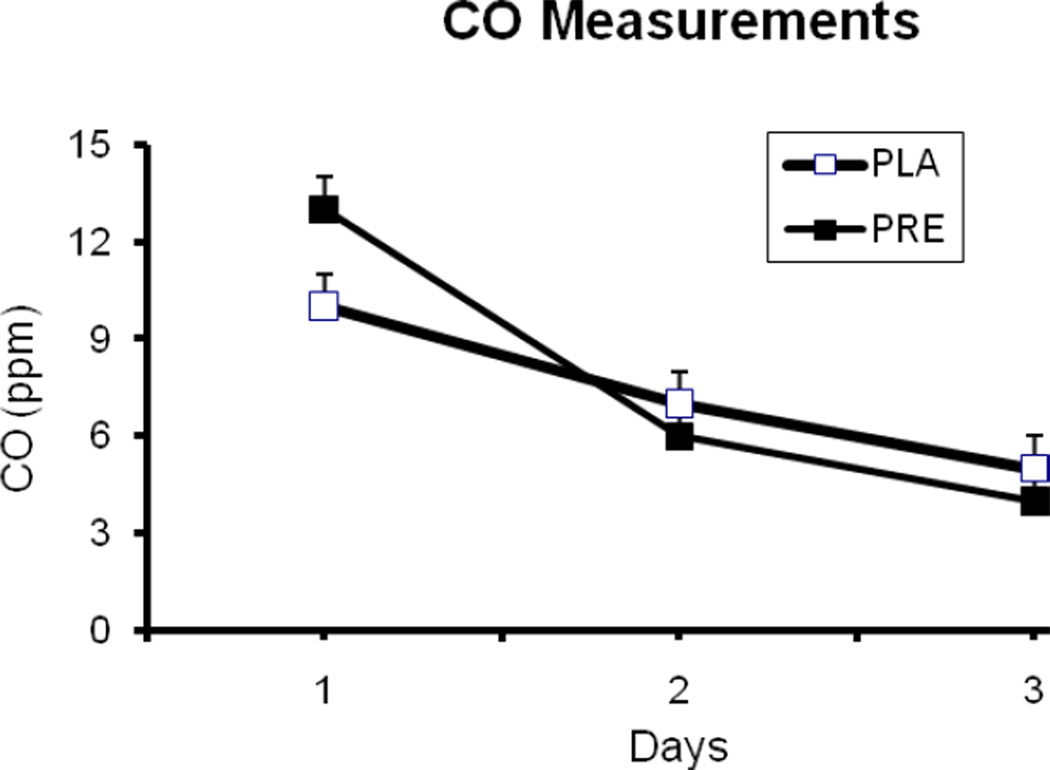
For daily breath CO measurements, there were significant group differences at baseline, before treatment was initiated (Figure 1). Analysis of the CO measurements, including baseline values as covariates, did not show significant main effects for treatment or treatment-by-time interaction (p>0.05). For the ad lib smoking session, there was no medication effect on the CO boost, change from the beginning to the end of the session (p>0.05). Under placebo treatment, the average (SD) values CO levels before and after the session were 4.9 (5.8) and 14.3 (7.8) ppm. The corresponding values under pregabalin treatment were 4.7 (5.3) and 14.2 (6.1), respectively. For smoking topography measures, there were no significant treatment effects (p>0.05). The average (SD) values for number of cigarettes smoked under placebo and pregabalin treatment were 4.5 (2.1) and 4.3 (1.6), respectively. Similarly, the average values for puff volume [54.6 (18.9) vs. 57.3 (18.8 ml)] and the number of puffs [10. 6 (3.6) vs. 10.8 (3.5)] were similar under placebo and pregabalin treatments, respectively.
Figure 1.
The average (with SEM) daily expired carbon monoxide (CO levels). Abstinence from smoking started on the evening of Day 1.
Cognitive performance
As shown in Table 1, pregabalin treatment increased the number of errors to no-go targets (3s) (p<0.05), but had no effect on go-targets or reaction time.
Table 1.
Summary statistics (Mean, SD) on the SART (n = 24).
| Placebo | Active | |
|---|---|---|
| Errors on 3s (no-go) (/25) | 10.5 (6.5) | 12.4 (6.9)* |
| Errors on non-3s (go) (/200) | 16.6 (19.7) | 15.0 (16.1) |
| Mean RT for correct presses (ms) | 423.4 (110.7) | 437.1 (107.3) |
p < .05 by t-test
Subjective Responses
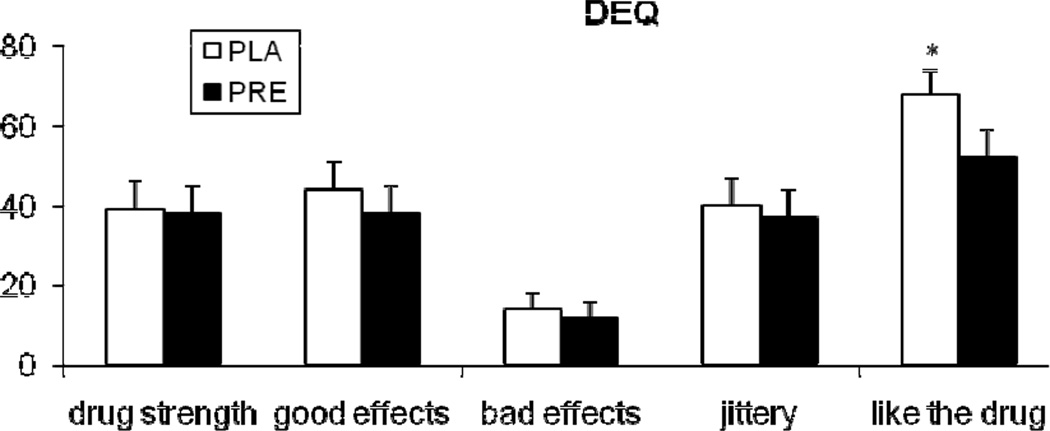
Pregabalin treatment attenuated the ratings for “drug liking,” referring to the cigarette that they had just smoked (treatment main effect; F (1, 20.8) = 5.3; p<0.05), which was assessed by the DEQ in the ad lib smoking period (Figure 2). Other DEQ items did not show significant medication effects.
Figure 2.
The average (with SEM) ratings of subjective responses to sample smoking measured with the Drug Effects Questionnaire (DEQ). The measurements were obtained after 2 puffs of a cigarette. *indicates a significant treatment effect.
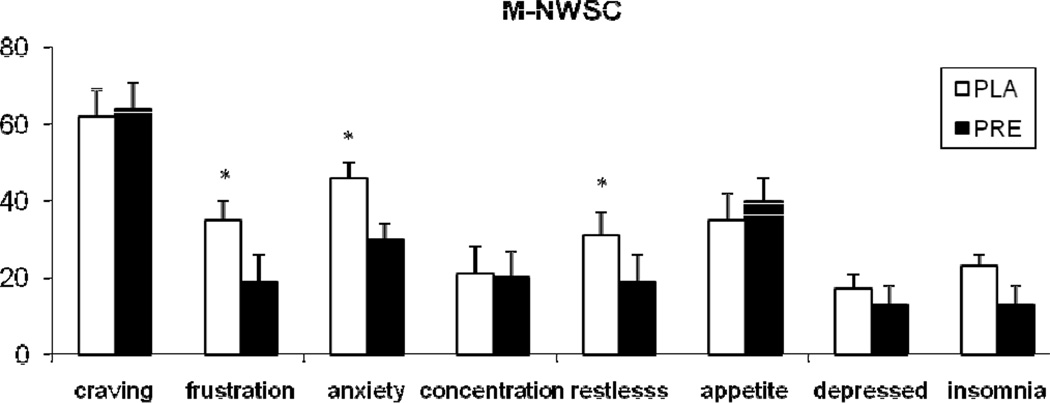
For the total score of MNWSC, treatment effect was not significant. Among individual items (Figure 3), pregabalin reduced the ratings of “frustration” (treatment main effect; F (1, 22) = 5.5; p<0.05), “restlessness” (treatment main effect; F (1, 22) = 4.2; p=0.05), and “anxiety” (treatment main effect; F (1, 22) = 4.6; p<0.05). For the POMS, pregabalin treatment was associated with higher ratings of “confusion-bewilderment” score (treatment main effect F (1, 161) = 3.8; p=0.05).
Figure 3.
The average (with SEM) ratings of Minnesota Nicotine Withdrawal Symptom Checklist (MNWSC). The measurements were obtained following 2.5 days of smoking abstinence. *indicates a significant treatment effect.
During the experimental sessions, exposure to smoking cues, increased heart rate (time main effect; F (9, 434) = 3.3; p<0.001) and BQSU factor 1 (time main effect; F (7, 346) = 55; p<0.0001) and factor 2 (time main effect; F (7, 346) = 23.7; p<0.0001) scores. The average (SD) changes in heart rate in response to smoking cues were 2.0 (1.5) and 1.2 (1.5), under placebo and pregabalin treatment, respectively. For the BQSU factor 1 score, the average changes in responses to smoking cues were 0.5 (0.7) and 1.2 (0.8), under pregabalin and placebo treatment, respectively. For the BQSU factor 2, the corresponding values were 1.1 (0.8) and 0.8 (0.6), respectively. No treatment or treatment-by-time interaction was observed for these outcomes.
Safety measures
No treatment effect was observed for the heart rate and blood pressure measurements obtained during the outpatient phase and experimental sessions. A total of seven smokers (4 in pregabalin and three in placebo groups) dropped out the study. There were 3 adverse events likely related to pregabalin treatment including dizziness (n=1), vertigo (n=1), and excessive sleepiness (n=1), which led to discontinuation of the study medication.
Discussion
Pregabalin treatment, compared to placebo, did not reduce ad lib smoking behavior in abstinent smokers. Pregabalin treatment attenuated some nicotine withdrawal symptoms including ratings of anxious, irritable, and frustrated in abstinent smokers. In addition, pregabalin attenuated the subjective ratings of “drug liking” in response to smoking following abstinence. These findings partially support our study hypotheses regarding pregabalin’s effects in smokers. The main findings of the study are discussed further below.
Pregabalin did not reduce smoking behavior during the 2.5 days of smoking abstinence or during the ad lib smoking session. No alternatives to smoking were provided during the ad lib smoking session, which might have enhanced the sensitivity of our model to detect medication effects on smoking behavior (Bisaga et al. 2007; Rohsenow et al. 2008). However, smokers were paid a bonus for not smoking during the abstinence period, which also did not show any treatment effect.
Pregabalin treatment attenuated the ratings of anxious, irritable, and frustrated following 2.5 days of tobacco abstinence. These selective effects of pregabalin are consistent with its anti-anxiety properties which have been demonstrated in randomized clinical trials (Feltner et al. 2008; Montgomery et al. 2008; Montgomery et al. 2006). Consistent with its effects on tobacco withdrawal, pregabalin attenuated alcohol withdrawal symptoms in clinical studies (Di Nicola et al. 2010; Martinotti et al. 2010). In our study, pregabalin, however, did not change the reactivity to smoking cues. Pregabalin treatment attenuated the rating of “drug liking” in response to sample smoking in abstinent smokers. No treatment effects were observed for the rating of other items. The first few cigarette puffs following abstinence is regarded to be highly rewarding and linked to relapse in smokers trying to quit (Brandon et al. 1990; Kenford et al. 1994; Shiffman and Kirchner 2009; Strong et al. 2011). Thus, medications attenuating the rewarding effects from smoking may be useful for smoking cessation.
Pregabalin treatment, compared to placebo, increased the number of errors in the SART, a sustained attention task. Consistent with the SART results, pregabalin treatment increased the POMS subscale on confusion-bewilderment, suggesting that sedation is associated with pregabalin treatment. Further, in a previous study in healthy volunteers, 3 days of pregabalin treatment (150 mg three times daily), compared to placebo, impaired eye tracking, and attentional and working memory functions (Hindmarch et al. 2005). Other studies suggested that these cognitive effects are generally mild and transient in a dose range from 150 to 600 mg/day (French et al. 2003). It is important to note that smokers who are trying to quit smoking display cognitive impairments and commonly complain of difficulty concentrating. Thus, medications further impairing cognitive performance may not be useful for smoking cessation. Our findings provide limited support for the potential use of pregabalin for smoking cessation. In a recent pilot clinical trial (Sood et al. 2010), gabapentin, a medication with a similar pharmacological profile, did not show efficacy for smoking cessation. Although similar in their mechanism of action, pregabalin and gabapentin may differ in their pharmacokinetics and clinical efficacy (Bockbrader et al. 2010). Some studies suggest that pregabalin may be more effective than gabapentin for the treatment of seizure disorder or fibromyalgia (Delahoy et al. 2010; Tzellos et al. 2010). Given that pregabalin has shown efficacy for the treatment of alcohol withdrawal, one possible use for pregabalin is for treatment of alcoholic smokers. Important to note that pregabalin has some abuse potential (Schwan et al. 2010) and should be used carefully especially in addicted individuals.
This study also had several limitations. First, participants were non-treatment seeking smokers who were not motivated to quit. Therefore, generalizability of our findings to treatment-seeking smokers may be limited. On the other hand, a number of studies have also shown that smoking behavior in non-treatment-seeking smokers is sensitive to both pharmacological and behavioral interventions in human laboratory settings (Benowitz et al. 1998; Cousins et al. 2001; Madden and Bickel 1999). Second, we used one dose of pregabalin, within the range of clinically used doses. As a result the study did not examine dose-dependent effects of pregabalin. However, pregabalin doses over 300 mg were more likely to cause cognitive impairment, limiting its usefulness for smoking cessation. Third, the use of smoking topography device and the artificial nature of smoking through a mouthpiece might have a potential disruptive effect on smoking behavior. Lastly, the study had a 4-day treatment duration, long-enough for pregabalin to reach steady-state levels in plasma. It is possible that longer treatment duration might be associated with different treatment effects. However, long-term studies with a similar medication, gabapentin, also did not show efficacy for smoking cessation (Sood et al. 2010).
Acknowledgments
This research was supported by the Veterans Administration Mental Illness Research, Education and Clinical Center (MIRECC) and National Institute on Drug Abuse (NIDA) grants R01 DA020752, K02-DA021304 (MS), and K12-DA-019446 (AIH). Dr. Sofuoglu serves as an expert witness on behalf of Pfizer in lawsuits related to varenicline.
Contributor Information
Aryeh I. Herman, Yale University, School of Medicine, Department of Psychiatry and VA Connecticut Healthcare System, West Haven, CT
Andrew J. Waters, Uniformed Services University of the Health Sciences, Bethesda, Maryland
Sherry A. McKee, Yale University, School of Medicine, Department of Psychiatry and VA Connecticut Healthcare System, West Haven, CT
Mehmet Sofuoglu, Yale University, School of Medicine, Department of Psychiatry and VA Connecticut Healthcare System, West Haven, CT.
References
- Bell SL, Taylor RC, Singleton EG, Henningfield JE, Heishman SJ. Smoking after nicotine deprivation enhances cognitive performance and decreases tobacco craving in drug abusers [In Process Citation] Nicotine Tob Res. 1999;1:45–52. doi: 10.1080/14622299050011141. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E. Pregabalin pharmacology and its relevance to clinical practice. Epilepsia. 2004;45(Suppl 6):13–18. doi: 10.1111/j.0013-9580.2004.455003.x. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Zevin S, Jacob P., 3rd Suppression of nicotine intake during ad libitum cigarette smoking by high-dose transdermal nicotine. J Pharmacol Exp Ther. 1998;287:958–962. [PubMed] [Google Scholar]
- Bisaga A, Padilla M, Garawi F, Sullivan MA, Haney M. Effects of alternative reinforcer and craving on the choice to smoke cigarettes in the laboratory. Hum Psychopharmacol. 2007;22:41–47. doi: 10.1002/hup.816. [DOI] [PubMed] [Google Scholar]
- Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clinical pharmacokinetics. 2010;49:661–669. doi: 10.2165/11536200-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: the process of relapse. Addict Behav. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- Buchhalter AR, Acosta MC, Evans SE, Breland AB, Eissenberg T. Tobacco abstinence symptom suppression: the role played by the smoking-related stimuli that are delivered by denicotinized cigarettes. Addiction. 2005;100:550–559. doi: 10.1111/j.1360-0443.2005.01030.x. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Stamat HM, de Wit H. Effects of a single dose of baclofen on self-reported subjective effects and tobacco smoking. Nicotine Tob Res. 2001;3:123–129. doi: 10.1080/14622200110042624. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Delahoy P, Thompson S, Marschner IC. Pregabalin versus gabapentin in partial epilepsy: a meta-analysis of dose-response relationships. BMC neurology. 2010;10:104. doi: 10.1186/1471-2377-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey SL, Brodie JD, Gerasimov M, Horan B, Gardner EL, Ashby CR., Jr A pharmacologic strategy for the treatment of nicotine addiction. Synapse. 1999;31:76–86. doi: 10.1002/(SICI)1098-2396(199901)31:1<76::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Di Nicola M, Martinotti G, Tedeschi D, Frustaci A, Mazza M, Sarchiapone M, Pozzi G, Bria P, Janiri L. Pregabalin in outpatient detoxification of subjects with mild-to-moderate alcohol withdrawal syndrome. Human psychopharmacology. 2010;25:268–275. doi: 10.1002/hup.1098. [DOI] [PubMed] [Google Scholar]
- Errante LD, Williamson A, Spencer DD, Petroff OA. Gabapentin and vigabatrin increase GABA in the human neocortical slice. Epilepsy research. 2002;49:203–210. doi: 10.1016/s0920-1211(02)00034-7. [DOI] [PubMed] [Google Scholar]
- Fattore L, Cossu G, Martellotta MC, Fratta W. Baclofen antagonizes intravenous self-administration of nicotine in mice and rats. Alcohol Alcohol. 2002;37:495–498. doi: 10.1093/alcalc/37.5.495. [DOI] [PubMed] [Google Scholar]
- Feltner D, Wittchen HU, Kavoussi R, Brock J, Baldinetti F, Pande AC. Long-term efficacy of pregabalin in generalized anxiety disorder. International clinical psychopharmacology. 2008;23:18–28. doi: 10.1097/YIC.0b013e3282f0f0d7. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services; 2008. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]
- Franklin TR, Harper D, Kampman K, Kildea-McCrea S, Jens W, Lynch KG, O'Brien CP, Childress AR. The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study. Drug Alcohol Depend. 2009;103:30–36. doi: 10.1016/j.drugalcdep.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Kugler AR, Robbins JL, Knapp LE, Garofalo EA. Dose-response trial of pregabalin adjunctive therapy in patients with partial seizures. Neurology. 2003;60:1631–1637. doi: 10.1212/01.wnl.0000068024.20285.65. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addictions. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Herman AI, Sofuoglu M. Comparison of available treatments for tobacco addiction. Curr Psychiatry Rep. 2010;12:433–440. doi: 10.1007/s11920-010-0134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindmarch I, Trick L, Ridout F. A double-blind, placebo- and positive-internal-controlled (alprazolam) investigation of the cognitive and psychomotor profile of pregabalin in healthy volunteers. Psychopharmacology (Berl) 2005;183:133–143. doi: 10.1007/s00213-005-0172-7. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. Effects of three doses of transdermal nicotine on post-cessation eating, hunger and weight. J Subst Abuse. 1997;9:151–159. doi: 10.1016/s0899-3289(97)90013-4. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. Jama. 1994;271:589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Bickel WK. Abstinence and price effects on demand for cigarettes: a behavioral- economic analysis. Addiction. 1999;94:577–588. doi: 10.1046/j.1360-0443.1999.94457712.x. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Martinotti G, di Nicola M, Frustaci A, Romanelli R, Tedeschi D, Guglielmo R, Guerriero L, Bruschi A, De Filippis R, Pozzi G, Di Giannantonio M, Bria P, Janiri L. Pregabalin, tiapride and lorazepam in alcohol withdrawal syndrome: a multi-centre, randomized, single-blind comparison trial. Addiction (Abingdon, England) 2010;105:288–299. doi: 10.1111/j.1360-0443.2009.02792.x. CINEBMJ, Pmid. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Dropperman L. Manual for profile of mood states. San Diego, CA: Educational and industrial testing services; 1971. [Google Scholar]
- Montgomery S, Chatamra K, Pauer L, Whalen E, Baldinetti F. Efficacy and safety of pregabalin in elderly people with generalised anxiety disorder. The British journal of psychiatry : the journal of mental science. 2008;193:389–394. doi: 10.1192/bjp.bp.107.037788. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Tobias K, Zornberg GL, Kasper S, Pande AC. Efficacy and safety of pregabalin in the treatment of generalized anxiety disorder: a 6-week, multicenter, randomized, double-blind, placebo-controlled comparison of pregabalin and venlafaxine. The Journal of clinical psychiatry. 2006;67:771–782. doi: 10.4088/jcp.v67n0511. CINEBMHF, Pmid. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Davies GM, Willner P. The Questionnaire of Smoking Urges is sensitive to abstinence and exposure to smoking-related cues. Behav Pharmacol. 1999;10:619–626. doi: 10.1097/00008877-199911000-00008. [DOI] [PubMed] [Google Scholar]
- Olmstead TA, Sindelar JL, Easton CJ, Carroll KM. The cost-effectiveness of four treatments for marijuana dependence. Addiction. 2007;102:1443–1453. doi: 10.1111/j.1360-0443.2007.01909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Froestl W, Markou A. The GABAB receptor agonists baclofen and CGP44532 decreased nicotine self-administration in the rat. Psychopharmacology. 2004;172:179–186. doi: 10.1007/s00213-003-1637-1. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased GABA neurotransmission via administration of gamma-vinyl GABA decreased nicotine self-administration in the rat. Synapse. 2002;44:252–253. doi: 10.1002/syn.10073. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Manly T, Andrade J, Baddeley BT, Yiend J. 'Oops!': performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia. 1997;35:747–758. doi: 10.1016/s0028-3932(97)00015-8. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Tidey JW, Miranda R, McGeary JE, Swift RM, Hutchison KE, Sirota AD, Monti PM. Olanzapine reduces urge to smoke and nicotine withdrawal symptoms in community smokers. Exp Clin Psychopharmacol. 2008;16:215–222. doi: 10.1037/1064-1297.16.3.215. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. The SAS System for Windows. Cary, NC: SAS Institute Inc; 2007. [Google Scholar]
- Sayette MA, Hufford MR. Effects of cue exposure and deprivation on cognitive resources in smokers. J Abnorm Psychol. 1994;103:812–818. doi: 10.1037//0021-843x.103.4.812. [DOI] [PubMed] [Google Scholar]
- Schwan S, Sundstrom A, Stjernberg E, Hallberg E, Hallberg P. A signal for an abuse liability for pregabalin--results from the Swedish spontaneous adverse drug reaction reporting system. European journal of clinical pharmacology. 2010;66:947–953. doi: 10.1007/s00228-010-0853-y. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Kirchner TR. Cigarette-by-cigarette satisfaction during ad libitum smoking. Journal of abnormal psychology. 2009;118:348–359. doi: 10.1037/a0015620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Mouratidis M, Yoo S, Culligan K, Kosten T. Effects of tiagabine in combination with intravenous nicotine in overnight abstinent smokers. Psychopharmacology (Berl) 2005:1–7. doi: 10.1007/s00213-005-0010-y. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Waters AJ, Mooney M, Kosten T. Riluzole and D-amphetamine interactions in humans. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:16–22. doi: 10.1016/j.pnpbp.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood A, Ebbert JO, Wyatt KD, Croghan IT, Schroeder DR, Sood R, Hays JT. Gabapentin for smoking cessation. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2010;12:300–304. doi: 10.1093/ntr/ntp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria R, Stapleton JM, Gilson SF, Sampson-Cone A, Henningfield JE, London ED. Subjective and cardiovascular effects of intravenous nicotine in smokers and non-smokers. Psychopharmacology. 1996;128:221–226. doi: 10.1007/s002130050129. [DOI] [PubMed] [Google Scholar]
- Strong DR, Leventhal AM, Evatt DP, Haber S, Greenberg BD, Abrams D, Niaura R. Positive reactions to tobacco predict relapse after cessation. Journal of abnormal psychology. 2011 doi: 10.1037/a0023666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Tzellos TG, Toulis KA, Goulis DG, Papazisis G, Zampeli VA, Vakfari A, Kouvelas D. Gabapentin and pregabalin in the treatment of fibromyalgia: a systematic review and a meta-analysis. Journal of clinical pharmacy and therapeutics. 2010;35:639–656. doi: 10.1111/j.1365-2710.2009.01144.x. [DOI] [PubMed] [Google Scholar]
- Urban MO, Ren K, Park KT, Campbell B, Anker N, Stearns B, Aiyar J, Belley M, Cohen C, Bristow L. Comparison of the antinociceptive profiles of gabapentin and 3-methylgabapentin in rat models of acute and persistent pain: implications for mechanism of action. The Journal of pharmacology and experimental therapeutics. 2005;313:1209–1216. doi: 10.1124/jpet.104.081778. [DOI] [PubMed] [Google Scholar]
- Wade JF, Dang CV, Nelson L, Wasserberger J. Emergent complications of the newer anticonvulsants. The Journal of emergency medicine. 2010;38:231–237. doi: 10.1016/j.jemermed.2008.03.032. [DOI] [PubMed] [Google Scholar]