Abstract
While there is general agreement that cardiovascular disease (CVD) development is influenced by a combination of genetic, environmental, and behavioral contributors, the actual mechanistic basis of how these factors initiate or promote CVD development in some individuals while others with identical risk profiles do not, is not clearly understood. This review considers the potential role for mitochondrial genetics and function in determining CVD susceptibility from the standpoint that the original features that molded cellular function were based upon mitochondrial-nuclear relationships established millions of years ago and were likely refined during prehistoric environmental selection events that today, are largely absent. Consequently, contemporary risk factors that influence our susceptibility to a variety of age-related diseases, including CVD were probably not part of the dynamics that defined the processes of mitochondrial – nuclear interaction, and thus, cell function. In this regard, the selective conditions that contributed to cellular functionality and evolution should be given more consideration when interpreting and designing experimental data and strategies. Finally, future studies that probe beyond epidemiologic associations are required. These studies will serve as the initial steps for addressing the provocative concept that contemporary human disease susceptibility is the result of selection events for mitochondrial function that increased chances for prehistoric human survival and reproductive success.
With the exception of the worldwide Spanish influenza epidemic of 1918, cardiovascular disease (CVD) has been the leading cause of mortality and morbidity in the United States every year since 19001. Consequently, many studies have investigated the potential causes of cardiovascular disease, and it is generally accepted that oxidative stress mediated changes within the cardiovascular milieu are among the most popular postulated mechanisms of CVD development2–7. Oxidative stress is caused by a collective grouping of reactive oxygen and nitrogen species (ROS and RNS, respectively) that are capable of disrupting cell function and exerting cytotoxic effects when generated in amounts beyond the antioxidant capacity of the cell. The concept that oxidative stress is important in the pathogenesis of CVD was conceived from studies that noted the cytotoxic and atherogenic properties of oxidized LDL (oxLDL) cholesterol8–12. Subsequently, it became apparent that vascular dysfunction can be linked to increased oxidant stress; oxidant stress can have several biological effects, including the peroxidation of polyunsaturated fatty acids in membrane or plasma lipoproteins, direct inhibition of mitochondrial respiratory chain enzymes, inactivation of membrane sodium channels, and DNA damage2, 3, 5–7, 13–22. These findings are consistent with the notion that CVD risk factors increase oxidative stress and contribute to a pro-inflammatory environment5, 11, 12, 23–35. Whereas the majority of these studies regard atherosclerotic disease, oxidative stress also has been implicated as an important factor in many other forms of cardiovascular related maladies, including hypertension and cardiometabolic disease/syndrome.36–39. Although atherosclerosis and hypertension are often a pathologies ultimately associated with cardiometabolic syndrome, individuals with hypertension or atherosclerosis do not always have cardiometabolic disease. The classic traits of visceral obesity and insulin resistance are associated with cardiometabolic syndrome, although other traits typically linked with metabolic syndrome are common as well. Multiple early definitions of metabolic syndrome have been related from different organizations including: the International Diabetes Federation (IDF)40, the revised National Cholesterol Education Program (NCEP; ATP III criteria)41, the World Health Organization (WHO)42, and the European Group for the Study of Insulin Resistance (EGIR)43. Based on a joint interim statement in 2009 from the American Heart Association (AHA), National Heart Lung Blood Institute (NHLBI), World Health Federation, International Atherosclerosis Society, International Association for the Study of Obesity, and International Diabetes Federation (IDF) consensus statement44, the criteria for clinical diagnosis of the metabolic syndrome include having three of five of the following (or drug treatment for them): some form of insulin resistance (impaired glucose tolerance or impaired fasting glucose [≥ 100 mg/dL]), hypertension [systolic ≥ 130 and/or diastolic ≥ 85 mm Hg], dyslipidemia (higher triglycerides [≥ 150mg/dL, 1.7 mmol/L], and lower HDL [males < 40mg/dL, 1.0 mmol/L; females < 50 mg/dL, 1.3 mmol/L]) and country-specific elevated waist circumference and abdominal obesity (USA, AHA/NHLBI ATP III thresholds: males ≥ 94–102 cm; females ≥ 80–88 cm) and all of these risk factors have been linked to oxidative stress45, 46,47, 48. Among the potential cellular origins of oxidative stress, studies have shown multiple sources to be important, including NAD(P)H oxidase, xanthine oxidase, and myeloperoxidase13, 49–60. More recently, the mitochondrion, both a source and target of oxidants related to cardiovascular disease development, has garnered attention61–64,65–73.
THE MECHANISMS OF INDIVIDUAL CVD SUSCEPTIBILITY ARE NOT CLEARY UNDERSTOOD
While significant progress in understanding the pathology, progression and development of CVD has been made, the determinants of why some individuals with identical CVD risk factor profiles develop disease while others will not, are not clearly understood. Currently, less than 5% of CVD appears to result from single mutations, such as those regulating lipoprotein synthesis74, 75. It has been estimated that 70%–80% of CVD is attributable to modifiable, non-genetic factors, which is consistent with the notion that environmental factors heavily influence the risk of disease development74. In addition to endogenous and environmental risk factors (i.e., hypercholesterolemia and tobacco smoke exposure, respectively), CVD susceptibility is also increased by age, family history76–81, and ethnicity (reviewed in82. Some studies have shown that differences in cardiovascular function exist between racial groups, however the basis of these differences is currently unclear83–88. Consequently, it is thought that CVD is a multi-factorial disorder that involves both environmental and genetic factors89, 90. A corollary of this idea however, is that individual response to environmental factors can be genetically influenced.
The Mendelian concept, or the “common disease, common variants” hypothesis suggests that common forms of disease such as CVD have a multi-factorial and polygenic basis: genetic variants present in many normal individuals, each with a relatively small effect, alone or in combination with modifier genes and environmental factors contribute to overall CVD risk89, 90. Hence, it has been hypothesized that multiple genes involved in vascular regulation, lipoprotein metabolism, inflammation, metabolic control and redox tone (the balance between oxidant generation and neutralization by antioxidants) and their interaction with risk factors influence CVD susceptibility77, 78. In this regard, studies have looked for connections between polymorphic gene mutations and CVD development. However, many original associations were lost in larger scale studies, or were not as predictive for risk as plasma markers such as cholesterol levels75, 91–97. Consequently, while important in advancing the understanding of gene “groups” that may be involved in influencing predilection to disease development, the underlying genetic and physiologic basis of why these differences exist is not well understood.
Because CVD usually develops over decades, its etiology should entail subtle changes in the vascular/endothelial environment over time, collectively resulting in the initiation and progression of disease. Therefore features of CVD development should involve genetic and cellular mechanisms that: i) play important roles in multiple cell functions involving the regulation and expression of multiple genes (e.g., growth, death, signaling, and bioenergetics); ii) are capable of gradual decline or dysfunction over time (an “aging” mechanism); iii) are susceptible to oxidative damage (risk factors), and; iv) explain risk associated with ethnicity.
The mitochondrion and its genome may account for these features in CVD development. The mitochondrion: i) is a multifunctional organelle which is a central focal point for proper cell function due to its role in energy production, cell growth, apoptosis, thermogenesis and redox signaling98–102; ii) has an “aging” mechanism – there are thousands of copies of mitochondrial DNA (mtDNA) per cell, allowing for the accumulation of mtDNA mutations and damage over time that cause an age-related decline in mitochondrial function103, 104; iii) is vulnerable contemporary CVD risk factors and oxidative stress which increase mitochondrial damage and alter function in cardiovascular tissues61–64, and; iv) harbors the mtDNA which displays marked regional variation and has proven useful in population and molecular anthropological studies105. By contrast, most ancient nDNA polymorphisms are common to all global populations106. Similarly, maternal family history of cardiovascular disease has also been reported to convey greater risk than paternal history77–81. Although this association is controversial and has been suggested to be due to offspring – maternal nutritional effects that were experienced in utero107, 108, studies of in utero risk factor exposure have shown mtDNA damage64. Consequently, these observations are consistent with the notion that mitochondria play significant roles in the etiology of CVD.
MITOCHONDRIA ARE MULTIFUNCTIONAL ORGANELLES
Mitochondria are ancient bacterial endosymbionts with their own DNA, RNA, and protein synthesis systems109. Mitochondria are multifunctional organelles, and serve as the sites for electron transport, oxidative phosphorylation (OXPHOS), the citric acid cycle, β-oxidation, steroidogenesis, and many other important cellular functions including growth, oxidant generation and programmed cell death102. In fact, the primary function of the mitochondrion is dependent upon the current requirements and environment of the cell. For instance, the primary function of a mitochondrion within an endothelial cell may be the regulated generation of oxidants for cell signaling, whereas within a cardiac myocyte, it may be the generation of ATP, or, a combination of functions therein (e.g. ATP and oxidant generation). This unique feature of mitochondrial functional biology makes it the central focal point in terms of the mechanistic basis of many forms of age-related diseases, including CVD.
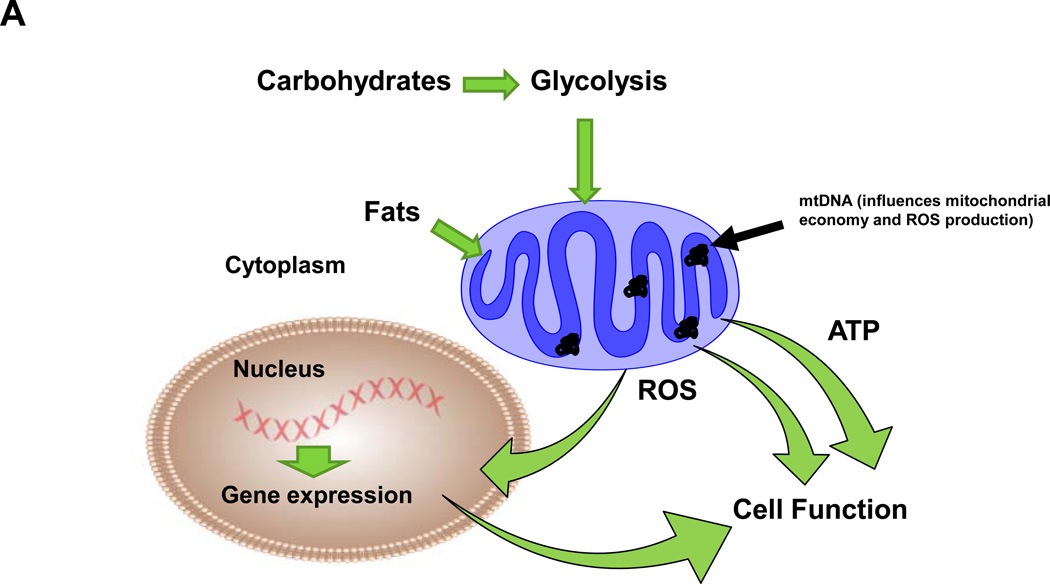
Mitochondria fundamentally execute the conversion of caloric energy into molecular energy, thermal energy, and oxidants (Figure 1). They achieve these tasks by coupling electron transport with proton translocation and OXPHOS. The energy released during the movement of electrons along the electron transport chain is used to pump protons across the inner membrane at complexes I, III, and IV, which creates a transmembrane electrochemical gradient. This potential energy is utilized by ATP synthase (complex V) to condense ADP and Pi to form ATP. The energy not utilized for proton pumping is lost in the form of heat (thermogenesis). Electrons are also donated directly to oxygen (O2) during electron transport to form superoxide (O2˙−) which can be converted to hydrogen peroxide (H2O2) and contribute to cell redox signaling processes, or in the presence of nitric oxide (˙NO), form peroxynitrite (ONOO−), an oxidant which can react with carbon dioxide (CO2) to form nitrosperoxycarbonate (ONOOCO2−), a nitrating agent24, 110.
Figure 1.
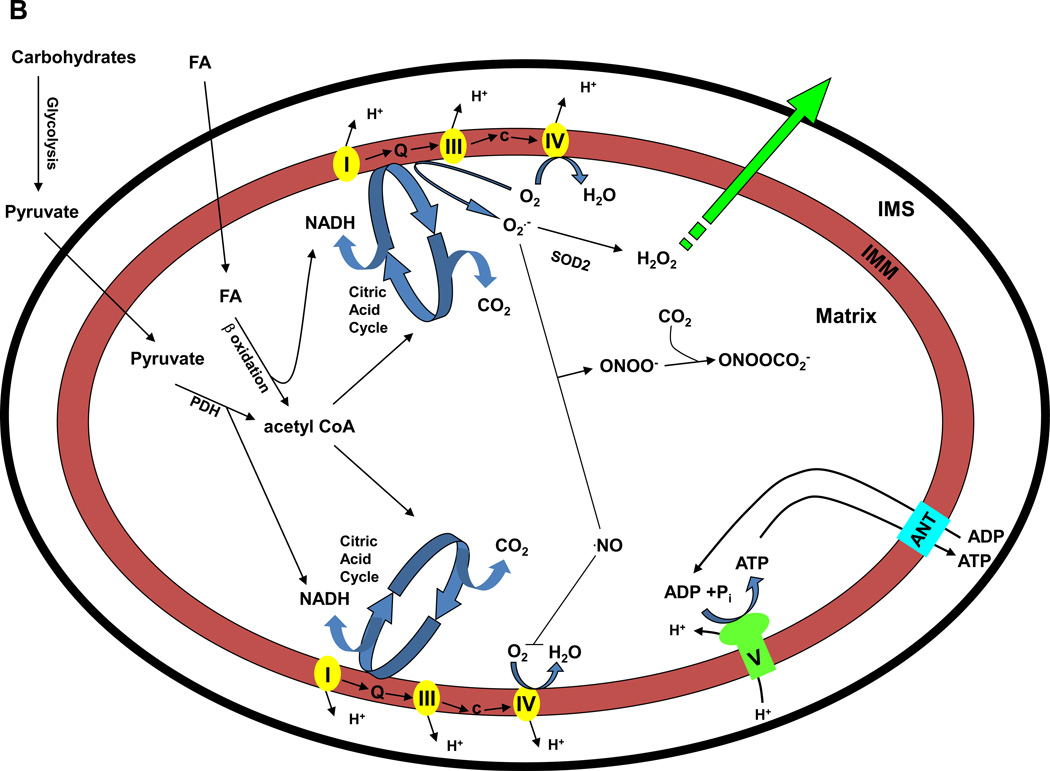
A) Fundamental aspects of mitochondrial function. Caloric energy (carbohydrates and fats) are converted into molecular (ATP) and thermal (heat, energy lost during electron transport) energy and oxidants (reactive oxygen species-ROS). While ATP is utilized for energy requiring cell functions, mitochondrial generated ROS influence redox cell signaling processes, including induction of nuclear gene expression (via redox sensitive transcription factors) which contribute to cell function. Differences in mtDNA sequences are proposed to influence mitochondrial oxygen utilization (economy) and ROS production that impact cell function. The conversion of caloric energy into these respective components is dependent on overall organelle economy (influenced by the mtDNA encoded subunits), degree of positive or negative energy balance and uncoupling proteins. ATP and ROS are utilized for cellular functions (energy requiring processes and redox signaling); mitochondrial ROS also serve as a means for communication to the nuclear compartment and regulation of certain nuclear genes. B) Carbohydrates are metabolized to glucose that is further converted to pyruvate (glycolysis) in the cytoplasm and transported into the mitochondrion. Acetyl CoA is formed from pyruvate via oxidative decarboxylation (pyruvate dehydrogenase), where it enters the citric acid cycle that yields reducing equivalents (NADH and FADH2) for electron transport located within the mitochondrial inner membrane. NADH is oxidized at complex I (NADH:Coenzyme Q oxidoreductase or NADH dehydrogenase) of the transport chain while FADH is oxidized at complex II (Succinate:Coenzyme Q oxidoreductase or Succinate dehydrogenase, part of the citric acid cycle). Electrons are next passed to Coenzyme Q (Q). Complex III (Coenzyme Q:Cytochrome c oxidoreductase or Cytochrome bc1 complex) passes electrons from reduced coenzyme Q (Q) to cytochrome c (c), a peripheral membrane protein that alternately binds cytochrome c1 (of complex III) and to complex IV (Cytochrome c oxidase). Complex IV catalyzes the one electron oxidations of four consecutive reduced cytochrome c molecules and the concomitant four electron reduction of one O2 molecule to yield H2O. During electron transport, protons are pumped across the inner membrane from the matrix into the intermembrane space, creating an electrochemical gradient. The free energy resulting from this gradient is utilized to condense a molecule of inorganic phosphate (Pi) with ADP at complex V (ATP synthase or F1F0 – ATPase) to yield ATP. ATP is subsequently transported out of the matrix by the inner membrane bound adenine nucleotide translocase (ANT) with the exchange of ADP. Fats bypass glycolytic metabolism in the cytoplasm and undergo β-oxidation in the mitochondrion to yield acetyl CoA (plus NADH and FADH2 per cycle of oxidation), which enters the citric acid cycle to generate substrates for electron transport. During electron transport, superoxide (O2˙−) is generated when electrons are added to O2; O2˙− is converted to hydrogen peroxide (H2O2) in the mitochondrion by manganese superoxide dismutase (MnSOD or SOD2). H2O2 (which is freely diffusible) can participate in cell signaling processes (H2O2 levels are regulated by a number of antioxidants within the mitochondrion and the cell, not illustrated). Alternatively, O2˙− reacts with nitric oxide (˙NO) to form peroxynitrite (ONOO−), an oxidant, which in the presence of carbon dioxide (CO2) forms nitrosoperoxycarbonate (ONOOCO2−), a nitrating agent.
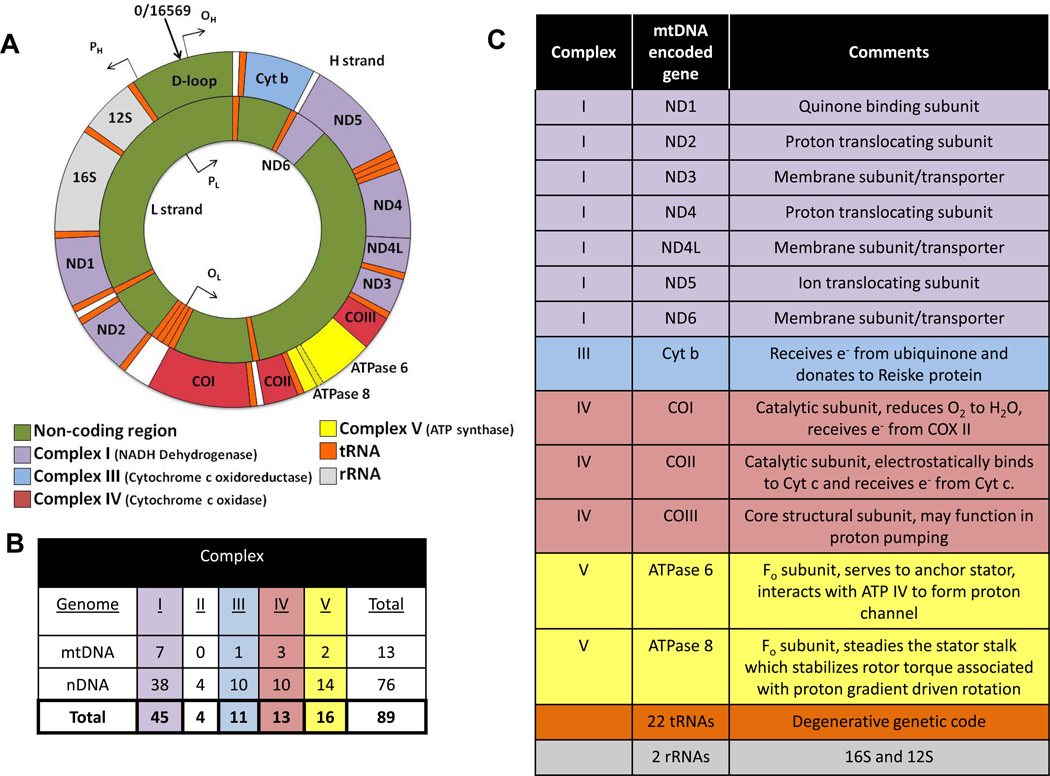
Each cell contains hundreds to thousands of mitochondria and each mitochondrion contains 5 – 10 copies of maternally inherited mtDNA. The mammalian mtDNA encodes 13 polypeptides, two rRNAs (12S and 16S) and 22 tRNAs that are essential for OXPHOS and proper cell function (Figure 2). The nucleus encodes all the remaining mitochondrial proteins for the organelle. Interestingly, the mtDNA retained key structural subunits required for the catalytic activity for four of the five OXPHOS enzyme complexes (I, III, IV and V). Consequently, mutations in these mtDNA encoded subunits could alter features in mitochondrial metabolism or economy (bioenergetic function).
Figure 2.
A) Sequence organization of the mammalian mtDNA. Colors indicate mtDNA encoded subunits for respective electron transport complexes, ATP synthase, tRNAs and rRNAs. ATPase 6 and 8 subunits overlap in sequence. The origins of heavy strand (guanosine rich) and light strand DNA synthesis are indicated by OH and OL, respectively. Transcriptional promoters for the heavy and light strands are represented by PH and PL, respectively. The D-loop (displacement loop) is a ~1 kb non-coding region within the mtDNA. The mtDNA genetic code is highly degenerate, so that only 22 are required for protein translation. When uridine is in the wobble position, all four members of a codon family can be read by one mitochondrial tRNA, whereas pairs of codons can be read when guanine or uridine is in the wobble position. Hence, 8 mitochondrial tRNAs recognize four member codon families, while 14 tRNAs recognize codon pairs. B) Table presenting the number of mtDNA and nDNA encoded subunits for each electron transport complex (I–IV) and ATP synthase (V) in the mammalian mtDNA. C) Table summarizing the known (or putative) function of each of the mtDNA encoded genes.
MITOCHONDRIAL ECONOMY
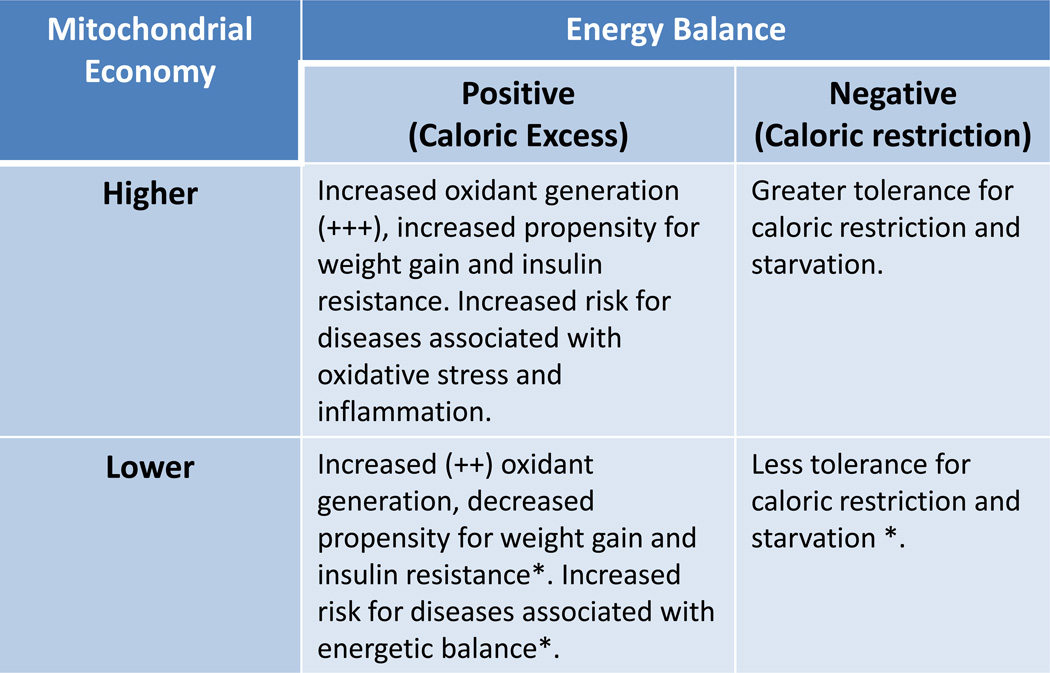
Mitochondrial economy describes how the organelle utilizes the oxygen it consumes in terms of molecular (ATP) and thermal energy production, plus oxidant generation. This economy is therefore dependent upon a myriad of factors including local concentrations of both reactive nitrogen and oxygen species, mitochondrial antioxidants, cytokines, electron transport efficiency, metabolic reducing equivalent availability (NADH, FADH2, and those from β-oxidation of fatty acids), cellular energetic demand, uncoupling protein (UCP) activities, and overall organelle integrity (damage to membranes, DNA, and proteins). Among these factors, electron transport efficiency as it relates to overall “mitochondrial economy” has received limited attention and has not been considered seriously in relation to CVD development. Mitochondria that utilize less oxygen to generate the same amount of ATP compared to mitochondria that use more oxygen for ATP production are, relatively speaking, more economical. Hence, economical mitochondria will have a higher ATP/O2 compared to those that are relatively less economical. Under conditions of excess substrate and low energetic demand (positive energy balance), mitochondria with high ATP/O2 will have a greater proportion of electron carriers in a reduced state (occupied by electrons) compared to those with lower ATP/O2 and thus, will be more prone to donate electrons to oxygen to form oxidants and are suggested to include African haplotypes (Figure 3, higher mitochondrial economy). However, under chronic conditions of substrate excess and low energetic demand even mitochondria that are less economical will generate increased oxidants. Mitochondria with low ATP/O2 utilize more electrons and O2 to generate ATP, resulting in greater energy loss in the form of heat compared to those with higher ATP/O2 and are suggested to include Eurasian and northern migratory haplotypes (Figure 3, lower mitochondrial economy).
Figure 3.
Table summarizing the anticipated characteristics of cells/tissues/individuals harboring mitochondria having higher or lower economy in regard to cellular-tissue oxidant generation and individual propensity for weight gain under positive or negative energy balance. An asterisk (*) indicates relative to higher economy, however under conditions of persistent positive energy balance, even cells/tissues/individuals with decreased (lower) mitochondrial economy will exhibit the same features as those with higher economy due to chronic stress. It is predicted that these features will be related to specific mtDNA sequences, or shared mutations between mtDNA haplotypes (representing mtDNA haplogroups).
PREHISTORIC SELECTION, MITOCHONDRIAL ECONOMY AND EVOLUTION
As humans migrated from Africa, they encountered changes in climate and diet as they moved northward111, 112. To successfully survive and reach reproductive age, it was necessary to develop a biological system to deal with these challenges. Consequently, selective pressures on prehistoric humans were exclusively related to reproductive success and survival of their offspring to reproductive age. Post-reproductive challenges beyond the successful rearing of offspring to reproductive age, were not major selective pressures in a genetically adaptive sense. As our ancestors moved northward, they accumulated a greater frequency of mtDNA missense mutations relative to silent substitutions100, 113. It has been proposed that these mutations altered aspects of mitochondrial economy that enabled these northern migrants to generate more heat/calorie consumed99, 100, 113. These changes were tolerated because the diet of these migrants changed from a low protein, low fat vegetarian diet to a high protein, high fat diet consisting of animal fats111, 112; hence, the decreased ATP generation/calorie (due to increased heat production) associated with these mtDNAs may have been offset by higher caloric intake. Consequently, by changing aspects of mitochondrial function in settings of warm climates and vegetarian diets to a system designed to be more thermogenic in a setting of cold climates with increased caloric intake, these changes in mitochondrial function may have contributed to increased probability for survival of the young to reproductive age.
Changes in mitochondrial function and/or protein levels associated with specific animal adaptations in vertebrates have been previously noted. For example, hibernating 13-lined ground squirrels (Spermophilus lateralis) demonstrate upregulated nad2 (mitochondrial-encoded ETC complex I subunit) mRNA in heart, liver, and skeletal muscle during hibernation114 coincidental with 95% decreased metabolic rate compared to resting levels and decreased core body temperature maintained below 10°C115. Smaller mtDNA-encoded cytochrome b and c spectra in S. lateralis liver mitochondria have also been reported during hibernation, which may decrease the capacity of complex III116 affecting ROS formation and CVD117, mtDNA damage, and retrograde signaling (discussed below in “THE IMPLICATIONS OF THE MITOCHONDRIAL PARADIGM…AND DISEASE DEVELOPMENT”). In an avian model, the bar-headed goose (Anser indicus) that migrates over the Himalayas (altitudes up to 9000 meters) must sustain high metabolic rates in the context of severe hypoxia. A. indicus has evolved more subsarcolemmal mitochondria bordering capillaries with increased densities within increased numbers of oxidative fibers, enabling them to sustain high metabolic rates for flight under hypoxic conditions compared to low altitude birds118. This evolutionary adaptation to hypoxia has more recently been shown to involve decreased maximal cytochrome c oxidase (COX) activity, a higher affinity of COX for reduced cytochrome c, and proportional decreases in COX3/COX1 and COX4/COX3 protein expression. The decreased COX3 subunit (mtDNA encoded) in the bar-headed goose also has a nonsynonymous substitution at a conserved site in vertebrates, which based on structural modeling suggests it would alter the interactions of COX3 and COX1 accounting for the increased economy and evolutionary mechanisms of high-altitude adaptation119. Finally, although not extensively studied, aspects of adaptive evolution of the mtDNA encoded subunits across placental mammals has been examined, potentially providing a framework for future characterization of mtDNA mutations in regard to their impact on cellular function and physiology120.
MITOCHONDRIAL OXIDANT PRODUCTION
The perception that mitochondrial oxidant production is analogous to “pollution” or an unnecessary by-product of electron transport is inaccurate. While many reports have implicated mitochondrial oxidant generation as an important form of cellular stress that contributes to disease development, which is certainly a correct interpretation65, 66, 121–137,65, 66, 68, 70, 72, 138–145, it also reflects a contemporary viewpoint. Mitochondrial produced oxidants most likely originally served as a signaling system for the benefit of the host (the eukaryotic cell), a concept that has not been widely contemplated. From an evolutionary perspective, the cellular functions of the mitochondrion developed over millions of years of endosymbiosis with its nucleated host. Because it is possible that our proto-eukaryotic ancestors spent a significant amount of time during their evolutionary existence under conditions of limited caloric availability, they likely evolved systems of mitochondrial – nuclear interactions designed for increased survival and reproductive success under conditions of punctuated caloric restriction. Consequently, selection for a system that had a rapid feedback/signaling mechanism (production of oxidants) linked to energy production that would induce caloric storage when energetic demands were met would be strong. This notion would suggest that mitochondrial oxidants may have originally served as stimuli for i) insulin production and ii) signaling molecules for insulin signaling pathways in non-insulin producing tissues. Hence, the mitochondrial oxidants may have initially served as a means for regulating caloric utilization and storage. Under conditions of excess substrate and low cellular energetic requirements (positive energy balance), mitochondria would increase oxidant production, triggering signaling pathways that would have led to storage of calories146–150. As energy demand increased or food availability became low, mitochondrial oxidant production would decrease, as would caloric storage pathways. In this regard, studies have shown that mitochondrial oxidant generation or the alteration of mitochondrial UCP levels can impact insulin secretion and also affect aspects of insulin sensitivity68, 147, 151, 152. While many studies have also shown that mitochondrial oxidant production inhibits insulin production and sensitivity, these studies are often performed under chronic conditions of hyperglycemia and therefore represent contemporary stress factors rather than prehistoric. Regardless, studies have shown that a connection exists between mitochondrial oxidant production and insulin secretion153, 154, and more recent work suggests that oxidants impact insulin signaling pathways in non-insulin producing tissues146, 155, 156. A final consideration is that these systems were designed to increase survival for reproductive purposes and hence, may function more robustly in the young (by virtue of their importance for survival and from a gene pool perspective). This concept is supported by the observation that insulin sensitivity is higher in the young compared to old157.
THE IMPLICATIONS OF THE MITOCHONDRIAL PARADIGM FOR CONTEMPORARY SOCIETY AND CONCEPTS OF DISEASE DEVELOPMENT
As previously discussed, it has been hypothesized that mtDNA mutations fixed into prehistoric populations altered aspects of mitochondrial economy that enabled our ancestors to survive and reproduce at different geographic latitudes and diets100. Today, these variants in mitochondrial function may influence individual disease susceptibility due to differences in mitochondrial oxidant production related to mtDNA haplotype100, 158. With the development of greater physical inactivity, increased lifespan and excessive caloric intake seen in Western societies, these variants in mitochondrial function and genetics may influence predisposition towards disease development. Individuals with greater mitochondrial economy will have increased basal levels of endogenous mitochondrial oxidant stress under conditions of excessive caloric intake, physical inactivity (positive energy balance, Figure 3) and exposure to CVD risk factors compared to those with less economy and thus, will appear more susceptible to diseases related to oxidative stress such as CVD. Furthermore, those individuals with less mitochondrial economy will appear less susceptible to diseases related to oxidative stress, yet will not be completely immune to such disease under conditions of high caloric intake and/or physical inactivity (Figure 3). Chronic, excessive caloric intake and low energetic demands will still result in sustained mitochondrial oxidant generation over time that will induce cellular damage; hence even those individuals with lower mitochondrial economy will potentially develop CVD or cardiometabolic diseases with persistent exposure to these stressors. Conversely, individuals harboring mitochondria with greater economy will be more tolerant towards caloric restriction compared to those having less economy (Figure 3, negative energy balance). This of course, sets up the dilemma that individuals more prone for weight gain under conditions of positive energy balance will be also more resistant to weight loss under conditions of negative energy balance although it has been shown that regular steady exercise may prevent or diminish the influence of mtDNA haplotype on some physiologic measures including aerobic capacity159 and ROS-induced damage to skeletal muscle160.
Evidence supporting these concepts is becoming recognized; several studies have shown that specific mtDNA mutations and haplotypes are associated with differences in oxygen consumption, increased risk for diseases thought or known to have an environmental component (e.g., deafness, blindness, Alzheimer’s disease, diabetes and cancer)104, 160–181. Similarly, studies have shown that the mtDNA haplotype can influence tumor growth and age-related deafness in mice182, 183. It has also been suggested that human longevity significantly co-segregates with mtDNA haplotypes that have temperate and arctic origins174, 175, yet they may have increased predilection for clinical illnesses associated with energetic insufficiencies such as blindness and CNS defects100. Alternatively, mitochondrial haplotypes thought to be associated with increased mitochondrial economy may be more prone to certain types of cancer and age-related diseases associated with oxidative stress and/or somatic mutation100, 182. More recent studies suggest a link between mtDNA haplotypes and CVD in certain populations184. While studies in cybrid culture have provided conflicting results regarding the concept that the mtDNA influences cellular bioenergetics158, 185–187, studies in conplastic strains of mice suggest that mtDNA background influences aspects of cognition, behavior, reproductive behavior, and susceptibility to autoimmune disease188–191. An extension of the concept that the mtDNA alters organelle economy (bioenergetics) which influences disease susceptibility is that it may also play a role in modulating nuclear gene expression since the majority of proteins functioning within the mitochondrion are encoded by the nucleus. If this is the case, it would represent another historical clue regarding the evolution of the eukaryotic cell and endosymbiosis, and thus, provide the basis for an additional paradigm in that the mtDNA influences the selection of certain nuclear – mitochondrial gene combinations and mitochondrial retrograde signaling192–194. If true, this would have serious implications regarding the use of transgenics derived from different strains of mice (e.g., backcrossing one strain on the background of another) and there are likely to be tissue-specific effects on mitochondrial-nuclear signaling195–197 influenced by energy balance, ROS, exercise, and diet.
THE ROLE OF DIET AND MITOCHONDRIAL FUNCTION
The composition and caloric content of the diet likely influence mitochondrial and cellular interactions. Excessive caloric intake without increased energy expenditure (a net positive energy balance), will result in increased weight gain, oxidant stress and disease risk. Whilst the effects of positive energy balance on mitochondrial function are a matter of debate concerning the question of whether mitochondrial dysfunction or positive energy balance drives the development of insulin resistance, diabetes, and cardiometabolic diseases198–202, it is evident that caloric restriction decreases mitochondrial oxidant production and cardiovascular risk203–205. Interestingly, it has also been shown that methionine restriction without caloric restriction can decrease mitochondrial oxidant production and mtDNA damage in rodents206–208, while the same percent of carbohydrate does not209, 210. In contrast, methionine supplementation in rats has been shown to increase ROS production and mtDNA damage in rat liver but not the heart211. Diets with higher unsaturated/polyunsaturated fat content (ie. fats from natural vegetable oils, nuts, and fish) compared to those with higher saturated fat (pork, beef, chicken, dairy, eggs, coconut oil, and some seafood) have been shown to decrease CVD212–215, and decrease mitochondrial ROS production216. Polyphenols such as resveratrol contained in red grapes, red wine, and peanuts have been shown to induce mitochondrial biogenesis through activation of sirtuin 1 (NAD-dependent deacetylase sirtuin-1 or SIRT1) and peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α)217, improving health, survival and decreasing diabetic complications218, 219. Coincidental with dietary and mitochondrial interactions are conflicting reports regarding low-carbohydrate versus low-fat diets and their effects on disease progression. Ketogenic diets, high-fat with adequate protein and low-carbohydrates, mimic caloric restriction by forcing the body to burn fats rather than carbohydrates and have been shown to decrease mitochondrial ROS production through increased NADH oxidation220. Ketogenic diets are clinically used to treat many acute and chronic neurological diseases such as stroke221, epilepsy222, 223, mitochondrial myopathy224 and cardiovascular disease225.
The interaction of diet on mitochondrial function and bioenergetics in general involves the capability of the mitochondria to effectively and rapidly signal to the cell that excess reducing equivalents exist (high cytosolic NADH/NAD+ and ATP/AMP). This has been shown to occur in both rodents and humans through low level mitochondrial ROS generation146, 226. Hoehn et. al. also demonstrated increased MnSOD expression improved glucose and insulin tolerance in mice fed high fat diets compared to controls146. Further, evidence from that study suggests that mitochondrial ROS production (which would be higher under conditions of excess reducing equivalent availability and low ATP demand) may serve as a cell signal that decreases GLUT4 translocation to the cellular plasma membrane and induces temporary insulin resistance (by limiting cellular glucose uptake) in adipocytes and myotubes146. Decreased influx of glucose would decrease NADH/NAD+, increase AMP/ATP, stimulate increased flux of electrons through the electron transport chain (ETC), decrease membrane potential and decrease ROS formation. However, under conditions of persistent positive energy balance, individuals with increased adiposity would also supply reducing equivalents via β-oxidation, and chronic ROS formation would ensue, contributing to post-translational oxidation of lipids, proteins and mtDNA, down regulation of metabolism, and vicious cycle of ROS-mediated mitochondrial dysfunction.
Under positive energy balance, excess reduced carbohydrates, fats and other foodstuffs lead to a chronic cellular redox shift toward an overload of reduced cytosolic NADH creating a ‘reductive stress’227. Elevated levels of these high energy electron carriers, NADH and the reduced form of flavin adenine dinucleotide, FADH2, would come from glycolysis and the Krebs cycle. Under these circumstances, most dehydrogenases and all NAD+-dependent enzymes would function abnormally because of the relative deficiency of NAD+ and inhibitory feedback mechanisms well described in most biochemistry text books. The NADH/NAD varies in response to changes in metabolism228–230 and is often used as a measure of the intracellular redox or metabolic state of the organism231. Since NADH cannot penetrate the inner mitochondrial membrane directly, various shuttling mechanisms exist to transport the NADH reducing equivalents into the mitochondria for oxidation. The malate-aspartate shuttle is required in yeast for increased life span mediated through calorie restriction232. Mitochondrial glycerol-3-phosphate shuttle also helps to funnel cytosolic reducing equivalents to the mitochondria for respiration233 and when knocked out in plants has been shown to increase the NADH/NAD ratio234.
Carbohydrate metabolism generates a ratio of 5 NADH/FADH2 (per pyruvate), while fat metabolism generates a ratio of 2 NADH/FADH2 (per acetyl CoA) which feed into the ETC at complex I for NADH and succinate dehydrogenase (complex II) for FADH2. These reducing equivalents converge on coenzyme Q and complex III. Mitochondrial oxidant production has been shown to originate from complexes I and III of the electron transport chain (ETC)235–239 through both forward and reverse electron flux240, 241. Hence, under conditions of excess reducing equivalents and low energy demand (positive energy balance), a “bottleneck” can occur at coenzyme Q and complex III that increases cytosolic NADH/NAD+, mitochondrial membrane potential, and ROS formation242. Moreover, it is possible that these effects are compounded in overweight individuals by virtue of their increased adiposity which further contributes to reducing equivalent availability through β-oxidation. Consistent with the notion that coenzyme Q may play an important role in modulating the effects of excess reducing equivalent availability are reports that mitochondrially-targeted coenzyme Q supplementation protects against endogenous oxidative stress243 and that supplementation of Co-Q has helped alleviate myopathic symptoms244. Interestingly, coenzyme Q deficiency may exacerbate cardiometabolic245, neurological246, and other diseases including diabetes and cancer247. It has also been reported that coenzyme Q deficiency induces mitochondrial degradation by mitophagy248.
SUMMARY
While there has been significant progress in understanding the pathological processes involved in CVD progression and development, the continuing status of CVD as the leading cause of death and morbidity in the Western world for the past century implies a lack of understanding regarding the basis of individual CVD susceptibility. Numerous studies have delineated important CVD risk factors, and although there is general agreement that they share a common feature of increasing vascular oxidant stress, the actual mechanistic basis of how they initiate or promote CVD development in some individuals and not in others with identical risk profiles, is not clearly understood. It is widely thought that CVD development is influenced by a combination of genetic, environmental, and behavioral factors that influence an individual’s biological response to known disease risk factors. A consideration currently lacking from these analyses is the potential role for mitochondrial genetics and function in determining CVD susceptibility. The mitochondrion is directly involved in the inter-relative aspects of caloric conversion to energy, thermogenic output, and oxidant production, and has been previously shown in numerous studies to be associated with cardiovascular dysfunction. Another aspect not commonly considered is that mitochondrial-nuclear relationships were established millions of years ago and were likely refined during prehistoric environmental selection events that today, are largely absent. By contrast, contemporary risk factors that influence our susceptibility to a variety of age-related diseases, including CVD were probably not part of the “equation” so to speak, that defined the processes of mitochondrial – nuclear interaction. Consequently, these diseases which are mostly post-reproductive are the by-product of our rapidly changing environment induced by technology; an environment for which our eukaryotic system was not designed. In this regard, the selective conditions that contributed to cellular functionality and evolution should be given more consideration when interpreting and designing experimental data and strategies. Finally, future studies that probe beyond epidemiologic/molecular epidemiologic associations are required. These studies will serve as the initial steps for addressing the provocative concept that contemporary human disease susceptibility is the result of selection events for mitochondrial function that increased chances for prehistoric human survival and reproductive success.
ACKNOWLEDGEMENT
The author’s studies were funded by NIH grants HL77419, HL94518, P60 DK79626, NIH training grants in Cardiovascular Pathophysiology (T32 HL007918) and Hypertension (T32 HL007457), an American Heart Association predoctoral fellowship (09PRE2240046), and the Howard Hughes Med-to-Grad Program fellowship (56005705).
Reference List
- 1.American Heart Association. Heart Disease and Stroke Statistics - 2006 Update. Circulation. 2006 Feb 14;105:1–67. [Google Scholar]
- 2.Carpenter KL, Taylor SE, van der Veen C, Mitchinson MJ. Evidence of lipid oxidation in pulmonary artery atherosclerosis. Atherosclerosis. 1995;118:169–172. doi: 10.1016/0021-9150(95)05642-a. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B. Free radicals, reactive oxygen species and human disease: a critical evaluation with special reference to atherosclerosis. Br J Exp Pathol. 1989;70:737–757. [PMC free article] [PubMed] [Google Scholar]
- 4.Halliwell B. Lipid peroxidation, antioxidants and cardiovascular disease: how should we move forward? Cardiovasc Res. 2000;47(3):410–418. doi: 10.1016/s0008-6363(00)00097-3. [DOI] [PubMed] [Google Scholar]
- 5.Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20:707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 6.Massaeli H, Pierce GN. Involvement of lipoproteins, free radicals, and calcium in cardiovascular disease processes. Cardiovasc Res. 1995;29:597–603. [PubMed] [Google Scholar]
- 7.Freeman BA, White CR, Gutierrez H, Paler-Martinez A, Tarpey MM, Rubbo H. Oxygen radical-nitric oxide reactions in vascular diseases. Adv Pharmacol. 1995;34:45–69. doi: 10.1016/s1054-3589(08)61080-7. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;321:1196–1197. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 9.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid V, Mitchinson MJ, Skepper J. Cytotoxicity of oxidised low density lipoprotein to mouse peritoneal macrophages: an ultrastructural study. J Pathol. 1993;171:321–328. doi: 10.1002/path.1711710413. [DOI] [PubMed] [Google Scholar]
- 11.Holland JA, Ziegler LM, Meyer JW. Atherogenic levels of low-density lipoprotein increase hydrogen peroxide generation in cultured human endothelial cells: Possible mechanism of heightened endocytosis. J Cell Physiol. 1996;166:144–151. doi: 10.1002/(SICI)1097-4652(199601)166:1<144::AID-JCP17>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 12.Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993;91:2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. American Journal of Cardiology. 2003 Feb 6;91(3):7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 14.Diaz MN, Frei B, Vita JA, Keaney JF. Mechanisms of disease - Antioxidants and atherosclerotic heart disease. N Engl J Med. 1997 Aug 7;337(6):408–416. doi: 10.1056/NEJM199708073370607. [DOI] [PubMed] [Google Scholar]
- 15.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases - The role of oxidant stress. Circ Res. 2000 Nov 10;87(10):840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 16.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. Journal of Hypertension. 2000 Jun;18(6):655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 17.Iuliano L. The oxidant stress hypothesis of atherogenesis. Lipids. 2001;36:S41–S44. doi: 10.1007/s11745-001-0680-1. [DOI] [PubMed] [Google Scholar]
- 18.Chisolm GM, Steinberg D. The oxidative modification hypothesis of atherogenesis: An overview. Free Radic Biol Med. 2000 Jun 15;28(12):1815–1826. doi: 10.1016/s0891-5849(00)00344-0. [DOI] [PubMed] [Google Scholar]
- 19.Keith M, Geranmayegan A, Sole MJ, et al. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:1352–1356. doi: 10.1016/s0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 20.Holvoet P, Perez G, Zhao Z, Brouwers E, Bernar H, Collen A. Malondialdehyde-modified low density lipoproteins in patients with atherosclerotic disease. J Clin Invest. 1995;95:2611–2619. doi: 10.1172/JCI117963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt AM, Hori O, Chen JX, et al. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96:1395–1403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hazen SL, Heinecke JW. 3-chlorotyrosine, a specific marker of myeloperosidase-catalyzed oxidation, is markedly elevated in low density lipoprotein siolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radi R, Rodriguez M, Castro L, Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys. 1994;308:89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 24.Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys. 1998;356:1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- 25.Minetti M, Scorza G, Pietraforte D. Peroxynitrite induces long lived tyrosyl radicals in oxyhemoglobin of red blood cells through a reaction involving CO2 and a ferryl species. Biochemistry. 1999;38:2078–2087. doi: 10.1021/bi982311g. [DOI] [PubMed] [Google Scholar]
- 26.van Jaarsveld H, Kuyl JM, Alberts DW. Exposure of rats to low concentrations of cigarette smoke increases myocardial sensitivity to ischaemia/reperfusion. Basic Res Cardiol. 1992;87:393–399. doi: 10.1007/BF00796524. [DOI] [PubMed] [Google Scholar]
- 27.Alexander RW. Atherosclerosis as disease of redox-sensitive genes. Trans Am Clin Climatol Assoc. 1998;109:129–145. [PMC free article] [PubMed] [Google Scholar]
- 28.Alexander RW. Hypertension and the pathogenesis of atherosclerosis: Oxidative stress and the mediation of arterial inflammatory response: A new perspective. Hypertension. 1995;25:155–161. doi: 10.1161/01.hyp.25.2.155. [DOI] [PubMed] [Google Scholar]
- 29.Parthasarathy S, Rankin SM. Role of oxidized low density lipoprotein in atherogenesis. Prog Lipid Res. 1992;31:127–143. doi: 10.1016/0163-7827(92)90006-5. [DOI] [PubMed] [Google Scholar]
- 30.Berliner JA, Navab M, Fogelman AM, et al. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995;91:2488–2496. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- 31.Taylor AE, Johnson DC, Kazemi H. Environmental tobacco smoke and cardiovascular disease. Circulation. 1992;86:1–4. doi: 10.1161/01.cir.86.2.699. [DOI] [PubMed] [Google Scholar]
- 32.Glantz S, Parmley W. Passive smoking and heart disease; epidemiology, physiology, and biochemistry. Circulation. 1991;83:1–12. doi: 10.1161/01.cir.83.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Steenland K. Passive smoking and the risk of heart disease. JAMA. 1992;267:94–99. [PubMed] [Google Scholar]
- 34.Glantz S, Parmley W. Passive smoking and heart disease. JAMA. 1995;273:1047–1053. [PubMed] [Google Scholar]
- 35.Steenland K, Thun M, Lally C, Heath C. Environmental Tobacco Smoke and Coronary Heart Disease in the American Cancer Society CPS-II Cohort. Circulation. 1996;94:622–628. doi: 10.1161/01.cir.94.4.622. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. American Journal of Physiology-Renal Physiology. 2004 Apr 1;286(4):F606–F616. doi: 10.1152/ajprenal.00269.2003. [DOI] [PubMed] [Google Scholar]
- 37.Touyz RM, Tabet F, Schiffrin EL. Redox-dependent signalling by angiotensin II and vascular remodelling in hypertension. Clinical and Experimental Pharmacology and Physiology. 2003 Nov;30(11):860–866. doi: 10.1046/j.1440-1681.2003.03930.x. [DOI] [PubMed] [Google Scholar]
- 38.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2005 Oct;289(4):R913–R935. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- 39.Cohen RA, Tong X-Y. Vascular oxidative stress: the common link in hypertensive and diabetic vascular disease. J Cardiovascular Pharm. 2010;55(4):308–316. doi: 10.1097/fjc.0b013e3181d89670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006 May;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 41.National Cholesterol Education Program N. Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 42.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998 Jul;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 43.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diabet Med. 1999 May;16(5):442–443. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 44.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Circulation. 2009 Oct 20;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 45.Codoner-Franch P, Tavarez-Alonso S, Murria-Estal R, Megias-Vericat J, Tortajada-Girbes M, onso-Iglesias E. Nitric oxide production is increased in severely obese children and related to markers of oxidative stress and inflammation. Atherosclerosis. 2011 Jan 19; doi: 10.1016/j.atherosclerosis.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 46.Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011 Feb 3; doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Briet M, Schiffrin EL. The role of aldosterone in the metabolic syndrome. Curr Hypertens Rep. 2011 Apr;13(2):163–172. doi: 10.1007/s11906-011-0182-2. [DOI] [PubMed] [Google Scholar]
- 48.Alberici LC, Vercesi AE, Oliveira HC. Mitochondrial energy metabolism and redox responses to hypertriglyceridemia. J Bioenerg Biomembr. 2011 Feb;43(1):19–23. doi: 10.1007/s10863-011-9326-y. [DOI] [PubMed] [Google Scholar]
- 49.Datla SR, Griendling KK. Reactive Oxygen Species, NADPH Oxidases, and Hypertension. Hypertension. 2010 Sep;56(3):325–330. doi: 10.1161/HYPERTENSIONAHA.109.142422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lassegue B, Griendling KK. NADPH Oxidases: Functions and Pathologies in the Vasculature. Arteriosclerosis Thrombosis and Vascular Biology. 2010 Apr;30(4):653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction - Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008 Feb 29;102(4):488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 52.Harrison DG, Cai H, Landmesser U, Griendling KK. Interactions of angiotensin II with MAD(P)H oxidase, oxidant stress and cardiovascular disease. Journal of the Renin-Angiotensin-Aldosterone System. 2003 Jun;4(2):51–61. doi: 10.3317/jraas.2003.014. [DOI] [PubMed] [Google Scholar]
- 53.Harrison DG, Gongora MC. Oxidative Stress and Hypertension. Medical Clinics of North America. 2009 May;93(3):621-+. doi: 10.1016/j.mcna.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 54.Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111(8):1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warnholtz A, Nickenig G, Schulz E, et al. Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin-angiotensin system. Circulation. 1999;99(15):2027–2033. doi: 10.1161/01.cir.99.15.2027. [DOI] [PubMed] [Google Scholar]
- 56.Baldus S, Mullerleile K, Chumley P, et al. Inhibition of xanthine oxidase improves myocardial contractility in patients with ischemic cardiomyopathy. Free Radic Biol Med. 2006 Oct 15;41(8):1282–1288. doi: 10.1016/j.freeradbiomed.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.White CR, Darley-Usmar V, Berrington WR, et al. Circulating plasma xanthine oxidase contributes to vascular dysfunction in hypercholesterolemic rabbits. Proc Natl Acad Sci USA. 1996;93:8745–8749. doi: 10.1073/pnas.93.16.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nicholls SJ, Hazen SL. Myeloperoxidase, modified lipoproteins, and atherogenesis. Journal of Lipid Research. 2009 Apr;50:S346–S351. doi: 10.1194/jlr.R800086-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Podrez EA, Abu-Soud HM, HAzen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic Biol Med. 2000;28(12):1717–1725. doi: 10.1016/s0891-5849(00)00229-x. [DOI] [PubMed] [Google Scholar]
- 60.Wang Z, Nicholls SJ, Rodriguez ER, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nature Medicine. 2007 Oct;13(10):1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 61.Ballinger SW, Patterson C, Knight-Lozano CA, et al. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106:544–549. doi: 10.1161/01.cir.0000023921.93743.89. Ref Type: Generic. [DOI] [PubMed] [Google Scholar]
- 62.Chuang GC, Yang Z, Westbrook DG, et al. Pulmonary ozone exposure induces vascular dysfunction, mitochondrial damage, and atherogenesis. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2009 Aug;297(2):L209–L216. doi: 10.1152/ajplung.00102.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knight-Lozano CA, Young CG, Burow DL, et al. Cigarette Smoke Exposure and Hypercholesterolemia increase mitochondrial damage in cardiovascular tissues. Circulation. 2002;105:849–854. doi: 10.1161/hc0702.103977. [DOI] [PubMed] [Google Scholar]
- 64.Yang Z, Knight CA, Mamerow M, et al. Prenatal Environmental Tobacco Smoke Exposure Promotes Adult Atherogenesis and Mitochondrial Damage in apoE−/− mice fed a chow diet. Circulation. 2004;110:3715–3720. doi: 10.1161/01.CIR.0000149747.82157.01. [DOI] [PubMed] [Google Scholar]
- 65.Rodriguez-Iturbe B, Sepassi L, Quiroz Y, Ni ZM, Vaziri ND. Association of mitochondrial SOD deficiency with salt-sensitive hypertension and accelerated renal senescence. Journal of Applied Physiology. 2007 Jan;102(1):255–260. doi: 10.1152/japplphysiol.00513.2006. [DOI] [PubMed] [Google Scholar]
- 66.Chen C, Korshunov VA, Massett MP, Yan C, Berk BC. Impaired vasorelaxation in inbred mice is associated with alterations in both nitric oxide and super oxide pathways. Journal of Vascular Research. 2007;44(6):504–512. doi: 10.1159/000106751. [DOI] [PubMed] [Google Scholar]
- 67.Bernal-Mizrachi C, Gates AC, Weng S, et al. Vascular respiratory uncoupling increases blood pressure and atherosclerosis. Nature. 2005 May 26;435(7041):502–506. doi: 10.1038/nature03527. [DOI] [PubMed] [Google Scholar]
- 68.Gates AC, Bernal-Mizrachi C, Chinault SL, et al. Respiratory uncoupling in skeletal muscle delays death and diminishes age-related disease. Cell Metabolism. 2007 Dec;6(6):497–505. doi: 10.1016/j.cmet.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 69.Teshima Y, Akao M, Jones SP, Marban E. Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circ Res. 2003 Aug 8;93(3):192–200. doi: 10.1161/01.RES.0000085581.60197.4D. [DOI] [PubMed] [Google Scholar]
- 70.Kim HS, Park KG, Koo TB, Huh S, Lee IK. The modulating effects of the overexpression of uncoupling protein 2 on the formation of reactive oxygen species in vascular cells. Diabetes Research and Clinical Practice. 2007 Sep;77:S46–S48. doi: 10.1016/j.diabres.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 71.Lee KU, Lee IK, Han J, et al. Effects of recombinant adenovirus-mediated uncoupling protein 2 overexpression on endothelial function and apoptosis. Circ Res. 2005 Jun 10;96(11):1200–1207. doi: 10.1161/01.RES.0000170075.73039.5b. [DOI] [PubMed] [Google Scholar]
- 72.Park JY, Park KG, Kim HJ, et al. The effects of the overexpression of recombinant uncoupling protein 2 on proliferation, migration and plasminogen activator inhibitor 1 expression in human vascular smooth muscle cells. Diabetologia. 2005 May;48(5):1022–1028. doi: 10.1007/s00125-005-1712-8. [DOI] [PubMed] [Google Scholar]
- 73.Blanc J, Alves-Guerra MC, Esposito B, et al. Protective role of uncoupling protein 2 in atherosclerosis. Circulation. 2003 Jan 28;107(3):388–390. doi: 10.1161/01.cir.0000051722.66074.60. [DOI] [PubMed] [Google Scholar]
- 74.Willett WC. Balancing life-style and genomics research for disease prevention. Science. 2002 Apr 26;296(5568):695–698. doi: 10.1126/science.1071055. [DOI] [PubMed] [Google Scholar]
- 75.Stephens JW, Humphries SE. The molecular genetics of cardiovascular disease: clinical implications. Journal of Internal Medicine. 2003 Feb;253(2):120–127. doi: 10.1046/j.1365-2796.2003.01104.x. [DOI] [PubMed] [Google Scholar]
- 76.Sundquist K, Li XJ. Differences in maternal and paternal transmission of coronary heart disease. American Journal of Preventive Medicine. 2006 Jun;30(6):480–486. doi: 10.1016/j.amepre.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 77.Nilsson PM, Nilsson JA, Berglund G. Family burden of cardiovascular mortality: risk implications for offspring in a national register linkage study based upon the Malmo Preventive Project. Journal of Internal Medicine. 2004 Feb;255(2):229–235. doi: 10.1046/j.1365-2796.2003.01287.x. [DOI] [PubMed] [Google Scholar]
- 78.Schildkraut JM, Myers RH, Cupples A, Kiely DK, Kannel WB. Coronary risk associated with age and sex of parental heart disease in the Framingham study. Am J Cardiol. 1989;10:555–559. doi: 10.1016/0002-9149(89)90477-3. [DOI] [PubMed] [Google Scholar]
- 79.Sesso HD, Lee IM, Gaziano JM, Rexrode KM, Glynn RJ, Buring JE. Maternal and paternal history of myocardial infarction and risk of cardiovascular disease in men and women. Circulation. 2001 Jul 24;104(4):393–398. doi: 10.1161/hc2901.093115. [DOI] [PubMed] [Google Scholar]
- 80.Ferrieres J, Lascaux-Lefebvre V, Arveiler D, et al. Influence of parental history of cardiovascular risk factors on multiple metabolic syndrome. European Heart Journal. 2000 Aug;21:28. [Google Scholar]
- 81.Wada K, Tamakoshi K, Yatsuya H, et al. Association between parental histories of hypertension, diabetes and dyslipidemia and the clustering of these disorders in offspring. Preventive Medicine. 2006 May;42(5):358–363. doi: 10.1016/j.ypmed.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 82.Forouhi NG, Sattar N. CVD risk factors and ethnicity - A homogeneous relationship? Atherosclerosis Supplements. 2006 Apr;7(1):11–19. doi: 10.1016/j.atherosclerosissup.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Sullivan J. Salt sensitivity: Definition, conception, methodlolgy, and long term issues. Hypertension. 1991;17:I61–I68. doi: 10.1161/01.hyp.17.1_suppl.i61. [DOI] [PubMed] [Google Scholar]
- 84.Luft F, Grim C, Fineberg N, et al. Effeccts of volume expansion and contraction in normotensive whites, blacks, and subjects of different ages. Am J Cardiol. 1979;59:643–650. doi: 10.1161/01.cir.59.4.643. [DOI] [PubMed] [Google Scholar]
- 85.Weinberger M, Miller J, Luft F, et al. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8:II127–II134. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 86.Luft F, Miller J, Grim C. Salt sensitivity and resistance of blood pressure: Age and race as factors in physiological responses. Hypertension. 1991;17:I102–I108. doi: 10.1161/01.hyp.17.1_suppl.i102. [DOI] [PubMed] [Google Scholar]
- 87.Luft F. Heterogenious response to chagnes in dietary salt intake: The salt sensitivity paradigm. Am J Clin Nutr. 1997;65:612S–617S. doi: 10.1093/ajcn/65.2.612S. [DOI] [PubMed] [Google Scholar]
- 88.Falkner B, Katz S, Canessa M, et al. The response to long term sodium loading in young blacks. Hypertension. 1986;8:I165–I168. [Google Scholar]
- 89.American Heart Association. Relevance of genetics and genomics for prevention and treatment of cardiovascular disease: A scientific statement from the AHA council on epidemiology and prevention, the stroke council, and the functional genomics and translational biology interdisciplinary working group. Circulation. 2007;115:2878–2901. doi: 10.1161/CIRCULATIONAHA.107.183679. [DOI] [PubMed] [Google Scholar]
- 90.Hirschhorn JN, Daly MJ. Genome-wide association studies for common diseases and complex traits. Nature Reviews Genetics. 2005 Feb;6(2):95–108. doi: 10.1038/nrg1521. [DOI] [PubMed] [Google Scholar]
- 91.van Schooten FJ, Hirvonen A, Maas LM, et al. Putative susceptibility markers of coronary artery disease: association between VDR genotype, smoking, and aromatic DNA adduct levels in human right atrial tissue. Faseb Journal. 1998 Oct;12(13):1409–1417. doi: 10.1096/fasebj.12.13.1409. [DOI] [PubMed] [Google Scholar]
- 92.Connelly JJ, Wang T, Cox JE, et al. GATA2 is associated with familial early-onset coronary artery disease. Plos Genetics. 2006 Aug;2(8):1265–1273. doi: 10.1371/journal.pgen.0020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Humphries SE, Talmud PJ, Hawe E, Bolla M, Day INM, Miller GJ. Apolipoprotein E4 and coronary heart disease in middle-aged men who smoke: a prospective study. Lancet. 2001 Jul 14;358(9276):115–119. doi: 10.1016/S0140-6736(01)05330-2. [DOI] [PubMed] [Google Scholar]
- 94.Talmud PJ, Humphries SE. Gene: environment interaction in lipid metabolism and effect on coronary heart disease risk. Current Opinion in Lipidology. 2002 Apr;13(2):149–154. doi: 10.1097/00041433-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 95.Keavney B, Parish S, Palmer A, et al. Large-scale evidence that the cardiotoxicity of smoking is not significantly modified by the apolipoprotein E epsilon 2/epsilon 3/epsilon 4 genotype. Lancet. 2003 Feb 1;361(9355):396–398. doi: 10.1016/S0140-6736(03)12386-0. [DOI] [PubMed] [Google Scholar]
- 96.Wheeler JG, Keavney BD, Watkins H, Collins R, Danesh J. Four paraoxonase gene polymorphisms in 11,212 cases of coronary heart disease and 12,786 controls: meta-analysis of 43 studies. Lancet. 2004 Feb 28;363(9410):689–695. doi: 10.1016/S0140-6736(04)15642-0. [DOI] [PubMed] [Google Scholar]
- 97.Wang XS, Ria M, Kelmenson PM, et al. Positional identification of TNFSF4, encoding OX40 ligand, as a gene that influences atherosclerosis susceptibility. Nature Genetics. 2005 Apr;37(4):365–372. doi: 10.1038/ng1524. [DOI] [PubMed] [Google Scholar]
- 98.Wallace DC. A mitochondrial paradigm for degenerative diseases and ageing. Novartis Found Symp. 2001;235:247–263. doi: 10.1002/0470868694.ch20. [DOI] [PubMed] [Google Scholar]
- 99.Wallace DC. The mitochondrial genome in human adaptive radiation and disease: On the road to therapeutics and performance enhancement. Gene. 2005 Jul 18;354:169–180. doi: 10.1016/j.gene.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 100.Wallace DC. A Mitochondrial Paradigm of Metabolic and Degenerative Diseases, Aging, and Cancer: A Dawn for Evolutionary Medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med. 2005 May 15;38(10):1278–1295. doi: 10.1016/j.freeradbiomed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 102.Ballinger SW. Deregulation of mitochondrial function: a potential common theme for cardiovascular disease development. In: Miwa S, Beckman KB, Muller FL, editors. Aging Medicine: Oxidative Stress in Aging: From Model Systems to Human Diseases. Totowa, NJ: Humana Press; 2008. pp. 165–189. [Google Scholar]
- 103.Trounce I, Byrne E, Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet. 1989;1(2):44–45. doi: 10.1016/s0140-6736(89)92143-0. [DOI] [PubMed] [Google Scholar]
- 104.Holt IJ, Harding AE, Morgan-Hughes JA. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988;331:717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- 105.Schurr TG. The peopling of the New World: Perspectives from molecular anthropology. Annu Rev Anthropol. 2004;33(3):551–583. [Google Scholar]
- 106.Jorde LB, Watkins WS, Bamshad MJ, et al. The distribution of human genetic diversity: A comparison of mitochondrial, autosomal, and Y-chromosome data. American Journal of Human Genetics. 2000 Mar;66(3):979–988. doi: 10.1086/302825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barker DJ. Fetal origins of coronary disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Brit Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 109.Margulis L. Origin of Mitochondria and Hydrogenosomes. History and Philosophy of the Life Sciences. 2008;30(3–4):473–477. [Google Scholar]
- 110.Gow A, Duran D, Thom S, Ischiropoulos H. Carbon dioxide enhancement of peroxynitrite mediated protein tyrosine nitration. Arch Biochem Biophys. 1996;333:42–48. doi: 10.1006/abbi.1996.0362. [DOI] [PubMed] [Google Scholar]
- 111.Milton K. Hunter-gatherer diets – a different perspective. Am J Clin Nutr. 2000;71:665–667. doi: 10.1093/ajcn/71.3.665. [DOI] [PubMed] [Google Scholar]
- 112.Cordain L, Brand-Miller J, Eaton SB, Mann N, Holt SHA, Speth JD. Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. Am J Clin Nutr. 2000;71:682–692. doi: 10.1093/ajcn/71.3.682. [DOI] [PubMed] [Google Scholar]
- 113.Ruiz-Pesini E, Mishmar D, Brandon M, Procaccio V, Wallace DC. Effects of purifying and adaptive selection on regional variation in human mtDNA. Science. 2004;303:223–226. doi: 10.1126/science.1088434. [DOI] [PubMed] [Google Scholar]
- 114.Fahlman A, Storey JM, Storey KB. Gene up-regulation in heart during mammalian hibernation. Cryobiology. 2000 Jun;40(4):332–342. doi: 10.1006/cryo.2000.2254. [DOI] [PubMed] [Google Scholar]
- 115.Muleme HM, Walpole AC, Staples JF. Mitochondrial metabolism in hibernation: metabolic suppression, temperature effects, and substrate preferences. Physiol Biochem Zool. 2006 May;79(3):474–483. doi: 10.1086/501053. [DOI] [PubMed] [Google Scholar]
- 116.Shug AL, Ferguson S, Shrago E, Burlington RF. Changes in respiratory control and cytochromes in liver mitochondria during hibernation. Biochim Biophys Acta. 1971 Mar 2;226(2):309–312. doi: 10.1016/0005-2728(71)90097-1. [DOI] [PubMed] [Google Scholar]
- 117.Ide T, Tsutsui H, Hayashidani S, et al. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res. 2001;88:529–535. doi: 10.1161/01.res.88.5.529. [DOI] [PubMed] [Google Scholar]
- 118.Scott GR, Egginton S, Richards JG, Milsom WK. Evolution of muscle phenotype for extreme high altitude flight in the bar-headed goose. Proc Biol Sci. 2009 Oct 22;276(1673):3645–3653. doi: 10.1098/rspb.2009.0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Scott GR, Schulte PM, Egginton S, Scott AL, Richards JG, Milsom WK. Molecular evolution of cytochrome C oxidase underlies high-altitude adaptation in the bar-headed goose. Mol Biol Evol. 2011 Jan;28(1):351–363. doi: 10.1093/molbev/msq205. [DOI] [PubMed] [Google Scholar]
- 120.da Fonseca RR, Johnson WE, O'Brien SJ, Ramos MJ, Antunes A. The adaptive evolution of the mammalian mitochondrial genome. BMC Genomics. 2008;9:119. doi: 10.1186/1471-2164-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen K, Thomas SR, Albano A, Murphy MP, Keaney JF. Mitochondrial function is required for hydrogen peroxide-induced growth factor receptor transactivation and downstream signaling. J Biol Chem. 2004 doi: 10.1074/jbc.M404859200. [DOI] [PubMed] [Google Scholar]
- 122.Li A, Ito H, Rovira I, et al. A role for reactive oxygen species in endothelial cell anoikis. Circ Res. 1999;85:304–310. doi: 10.1161/01.res.85.4.304. [DOI] [PubMed] [Google Scholar]
- 123.Schulze-Osthoff K, Beyaert R, Vandervoorde V, Haegeman G, Fiers W. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J. 1993;12:3095–3104. doi: 10.1002/j.1460-2075.1993.tb05978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schulze-Osthoff K, Bakker AC, Vanhaesebroeck B, Beyaert R, Jacob WA, Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992;267(8):5317–5323. [PubMed] [Google Scholar]
- 125.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochimica et Biophysica Acta-Bioenergetics. 2010 Jun;1797(6–7):897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 126.Yin JX, Yang RF, Li SM, et al. Mitochondria-produced superoxide mediates angiotensin II-induced inhibition of neuronal potassium current. American Journal of Physiology-Cell Physiology. 2010 Apr;298(4):C857–C865. doi: 10.1152/ajpcell.00313.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Go YM, Park H, Koval M, et al. A key role for mitochondria in endothelial signaling by plasma cysteine/cystine redox potential. Free Radic Biol Med. 2010 Jan 15;48(2):275–283. doi: 10.1016/j.freeradbiomed.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arciuch VGA, Alippe Y, Carreras MC, Poderoso JJ. Mitochondrial kinases in cell signaling: Facts and perspectives. Advanced Drug Delivery Reviews. 2009 Nov 30;61(14):1234–1249. doi: 10.1016/j.addr.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 129.Gao Q, Zhao XM, Ahmad M, Wolin MS. Mitochondrial-derived hydrogen peroxide inhibits relaxation of bovine coronary arterial smooth muscle to hypoxia through stimulation of ERK MAP kinase. American Journal of Physiology-Heart and Circulatory Physiology. 2009 Dec;297(6):H2262–H2269. doi: 10.1152/ajpheart.00817.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huang SM, Zhang AH, Ding GX, Chen RH. Aldosterone-induced mesangial cell proliferation is mediated by EGF receptor transactivation. American Journal of Physiology-Renal Physiology. 2009 Jun;296(6):F1323–F1333. doi: 10.1152/ajprenal.90428.2008. [DOI] [PubMed] [Google Scholar]
- 131.Han ZS, Varadharaj S, Giedt RJ, Zweier JL, Szeto HH, Alevriadou BR. Mitochondria-Derived Reactive Oxygen Species Mediate Heme Oxygenase-1 Expression in Sheared Endothelial Cells. Journal of Pharmacology and Experimental Therapeutics. 2009 Apr;329(1):94–101. doi: 10.1124/jpet.108.145557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hasebe Y, Egawa K, Shibanuma M, Nose K. Induction of matrix metalloproteinase gene expression in an endothelial cell line by direct interaction with malignant cells. Cancer Science. 2007 Jan;98(1):58–67. doi: 10.1111/j.1349-7006.2006.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jones DP. Disruption of mitochondrial redox circuitry in oxidative stress. Chemico-Biological Interactions. 2006 Oct 27;163(1–2):38–53. doi: 10.1016/j.cbi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 134.Gutierrez J, Ballinger SW, Darley-Usmar VM, Landar A. Free radicals, mitochondria, and oxidized lipids - The emerging role in signal transduction in vascular cells. Circ Res. 2006 Oct 27;99(9):924–932. doi: 10.1161/01.RES.0000248212.86638.e9. [DOI] [PubMed] [Google Scholar]
- 135.Desouki MM, Kulawiec M, Bansal S, Das G, Singh KK. Cross talk between mitochondria and superoxide generating NADPH oxidase in breast and ovarian tumors. Cancer Biology & Therapy. 2005 Dec;4(12):1367–1373. doi: 10.4161/cbt.4.12.2233. [DOI] [PubMed] [Google Scholar]
- 136.Felty Q, Roy D. Mitochondrial signals to nucleus regulate estrogen-induced cell growth. Med Hypotheses. 2005;64(1):133–141. doi: 10.1016/j.mehy.2003.12.056. [DOI] [PubMed] [Google Scholar]
- 137.Roy D, Cai QY, Felty Q, Narayan S. Estrogen-induced generation of reactive oxygen and nitrogen species, gene damage, and estrogen-dependent cancers. Journal of Toxicology and Environmental Health-Part B-Critical Reviews. 2007;10(4):235–257. doi: 10.1080/15287390600974924. [DOI] [PubMed] [Google Scholar]
- 138.Bienengraeber M, Ozcan C, Terzic A. Stable transfection of UCP1 confers resistance to hypoxia/reoxygenation in a heart-derived cell line. J Mol Cell Cardiol. 2003;35:861–865. doi: 10.1016/s0022-2828(03)00147-0. [DOI] [PubMed] [Google Scholar]
- 139.Bernal-Mizrachi C, Gates AC, Weng S, et al. Vascular respiratory uncoupling increases blood pressure and atherosclerosis. Nature. 2005 May 26;435(7041):502–506. doi: 10.1038/nature03527. [DOI] [PubMed] [Google Scholar]
- 140.Bernal-Mizrachi C, Weng S, Li B, et al. Respiratory uncoupling lowers blood pressure through a leptin-dependent mechanism in genetically obese mice. Arteriosclerosis Thrombosis and Vascular Biology. 2002 Jun;22(6):961–968. doi: 10.1161/01.atv.0000019404.65403.71. [DOI] [PubMed] [Google Scholar]
- 141.Bernal-Mizrachi C, Weng S, Bloodsworth AC, et al. Inducible expression of uncoupling protein-1 in mouse aortic smooth muscle cells promotes hypertension and atherosclerosis. Arteriosclerosis Thrombosis and Vascular Biology. 2003 May;23(5):A76. [Google Scholar]
- 142.Asimakis G, Lick S, Patterson W. Postischemic recovery of contractile function is impaired in SOD2 (+/−) but not SOD1 (+/−) mouse hearts. Circulation. 2002;105(8):981–986. doi: 10.1161/hc0802.104502. [DOI] [PubMed] [Google Scholar]
- 143.Chen Z, Siu B, Ho Y-S, et al. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J Mol Cell Cardiol. 1998;30:2281–2289. doi: 10.1006/jmcc.1998.0789. [DOI] [PubMed] [Google Scholar]
- 144.Fisher CJ, Goswami PC. Mitochondria-targeted antioxidant enzyme activity regulates radioresistance in human pancreatic cancer cells. Cancer Biology & Therapy. 2008 Aug;7(8):1271–1279. doi: 10.4161/cbt.7.8.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ohashi M, Runge MS, Faraci FM, Heistad DD. MnSOD deficiency increases endothelial dysfunction in ApoE-deficient mice. Arteriosclerosis Thrombosis and Vascular Biology. 2006 Oct;26(10):2331–2336. doi: 10.1161/01.ATV.0000238347.77590.c9. [DOI] [PubMed] [Google Scholar]
- 146.Hoehn KL, Salmon AB, Hohnen-Behrens C, et al. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci USA. 2009 Oct 20;106(42):17787–17792. doi: 10.1073/pnas.0902380106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Boudina S, Sena S, Theobald H, et al. Mitochondrial energetics in the heart in obesity-related diabetes - Direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007 Oct;56(10):2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 148.Twig G, Elorza A, Molina AJA, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. Embo Journal. 2008 Jan 23;27(2):433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Civitarese AE, MacLean PS, Carling S, et al. Regulation of Skeletal Muscle Oxidative Capacity and Insulin Signaling by the Mitochondria! Rhomboid Protease PARL. Cell Metabolism. 2010 May 5;11(5):412–426. doi: 10.1016/j.cmet.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wiederkehr A, Wollheim CB. Minireview: Implication of mitochondria in insulin secretion and action. Endocrinology. 2006 Jun;147(6):2643–2649. doi: 10.1210/en.2006-0057. [DOI] [PubMed] [Google Scholar]
- 151.Mattiasson G, Sullivan PG. Comprehensive invited review - The emerging functions of UCP2 in health, disease, and therapeutics. Antioxidants & Redox Signaling. 2006 Jan;8(1–2):1–38. doi: 10.1089/ars.2006.8.1. [DOI] [PubMed] [Google Scholar]
- 152.Fisler JS, Warden CH. Uncoupling proteins, dietary fat and the metabolic syndrome. Nutrition & Metabolism. 2006 Sep 12;3 doi: 10.1186/1743-7075-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Leloup C, Tourrel-Cuzin C, Magnan C, et al. Mitochondrial Reactive Oxygen Species Are Obligatory Signals for Glucose-Induced Insulin Secretion. Diabetes. 2009 Mar;58(3):673–681. doi: 10.2337/db07-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pi JB, Bai YS, Zhang Q, et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007 Jul;56(7):1783–1791. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- 155.Dokken BB, Saengsirisuwan V, Kim JS, Teachey MK, Henriksen EJ. Oxidative stress-induced insulin resistance in rat skeletal muscle: role of glycogen synthase kinase-3. American Journal of Physiology-Endocrinology and Metabolism. 2008 Mar;294(3):E615–E621. doi: 10.1152/ajpendo.00578.2007. [DOI] [PubMed] [Google Scholar]
- 156.Loh K, Deng HY, Fukushima A, et al. Reactive Oxygen Species Enhance Insulin Sensitivity. Cell Metabolism. 2009 Oct 7;10(4):260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chang AM, Halter JB. Aging and insulin secretion. American Journal of Physiology-Endocrinology and Metabolism. 2003 Jan;284(1):E7–E12. doi: 10.1152/ajpendo.00366.2002. [DOI] [PubMed] [Google Scholar]
- 158.Moreno-Loshuertos R, Acin-Perez R, Fernandez-Silva P, et al. Differences in reactive oxygen species production explain the phenotypes associated with common mouse mitochondrial DNA variants. Nature Genetics. 2006 Nov;38(11):1261–1268. doi: 10.1038/ng1897. [DOI] [PubMed] [Google Scholar]
- 159.Marcuello A, Martinez-Redondo D, Dahmani Y, et al. Steady exercise removes VO(2max) difference between mitochondrial genomic variants. Mitochondrion. 2009 Sep;9(5):326–330. doi: 10.1016/j.mito.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 160.Martinez-Redondo D, Marcuello A, Casajus JA, et al. Human mitochondrial haplogroup H: the highest VO2max consumer--is it a paradox? Mitochondrion. 2010 Mar;10(2):102–107. doi: 10.1016/j.mito.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 161.Corral-Debrinski M, Shoffner JM, Lott MT, Wallace DC. Association of mitochondrial DNA damage with aging and coronary atherosclerotic heart disease. Mutat Res. 1992;275:169–180. doi: 10.1016/0921-8734(92)90021-g. [DOI] [PubMed] [Google Scholar]
- 162.Ballinger SW, Shoffner JM, Hedaya EV, et al. Maternally trasnmitted diabetes and deafness associated with a 10.4 kb mitochondrial DNA deletion. Nat Genet. 1992;1:11–15. doi: 10.1038/ng0492-11. [DOI] [PubMed] [Google Scholar]
- 163.Ballinger SW, Shoffner JM, Wallace DC. Mitochondrial Myopathies - Genetic-Aspects. Current Topics in Bioenergetics, Vol 17. 1994;17:59–98. [Google Scholar]
- 164.Shoffner JM, Lott MT, Lezza AM, Seibel P, Ballinger SW, Wallace DC. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990 Jun 15;61(6):931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- 165.Shoffner JM, Fernhoff PM, Krawiecki NS, et al. Subacute necrotizing encephalopathy: oxidative phosphorylation defects and the ATPase 6 point mutation. Neurology. 1992 Nov;42(11):2168–2174. doi: 10.1212/wnl.42.11.2168. [DOI] [PubMed] [Google Scholar]
- 166.Wallace DC, Zheng XX, Lott MT, et al. Familial mitochondrial encephalomyopathy (MERRF): genetic, pathophysiological, and biochemical characterization of a mitochondrial DNA disease. Cell. 1988 Nov 18;55(4):601–610. doi: 10.1016/0092-8674(88)90218-8. [DOI] [PubMed] [Google Scholar]
- 167.Wallace DC, Shoffner JM, Watts RL, Juncos JL, Torroni A. Mitochondrial oxidative phosphorylation defects in Parkinson's disease. Ann Neurol. 1992 Jul;32(1):113–114. doi: 10.1002/ana.410320123. [DOI] [PubMed] [Google Scholar]
- 168.Wallace DC, Singh G, Lott MT, et al. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988 Dec 9;242(4884):1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- 169.Brown MD, Sun FZ, Wallace DC. Clustering of Caucasian Leber hereditary optic neuropathy patients containing the 11778 or 14484 mutations on an mtDNA lineage. American Journal of Human Genetics. 1997 Feb;60(2):381–387. [PMC free article] [PubMed] [Google Scholar]
- 170.Brown MD, Starikovskaya E, Derbeneva O, et al. The role of mtDNA background in disease expression: a new primary LHON mutation associated with Western Eurasian haplogroup J. Human Genetics. 2002 Feb;110(2):130–138. doi: 10.1007/s00439-001-0660-8. [DOI] [PubMed] [Google Scholar]
- 171.Brown MD, Zhadanov S, Allen JC, et al. Novel mtDNA mutations and oxidative phosphorylation dysfunction in Russian LHON families. Human Genetics. 2001 Jul;109(1):33–39. doi: 10.1007/s004390100538. [DOI] [PubMed] [Google Scholar]
- 172.Brown MD, Derbeneva OA, Starikovskaya Y, Allen J, Sukernik RI, Wallace DC. The influence of mtDNA background on the disease process: a new primary Leber's Hereditary Optic Neuropathy mtDNA mutation requires European haplogroup J for expression. American Journal of Human Genetics. 2001 Oct;69(4):578. [Google Scholar]
- 173.Chagnon P, Gee M, Filion M, Robitaille Y, Belouchi M, Gauvreau D. Phylogenetic analysis of the mitochondrial genome indicates significant differences between patients with Alzheimer disease and controls in a French-Canadian founder population. American Journal of Medical Genetics. 1999 Jul 2;85(1):20–30. doi: 10.1002/(sici)1096-8628(19990702)85:1<20::aid-ajmg6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 174.De Benedictis G, Rose G, Carrieri G, et al. Mitochondrial DNA inherited variants are associated with successful aging and longevity in humans. Faseb Journal. 1999 Sep;13(12):1532–1536. doi: 10.1096/fasebj.13.12.1532. [DOI] [PubMed] [Google Scholar]
- 175.Rose G, Passarino G, Carrieri G, et al. Paradoxes in longevity: sequence analysis of mtDNA haplogroup J in centenarians. European Journal of Human Genetics. 2001 Sep;9(9):701–707. doi: 10.1038/sj.ejhg.5200703. [DOI] [PubMed] [Google Scholar]
- 176.Goto Y, Nonaka I, Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990 Dec 13;348(6302):651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- 177.van den Ouweland JMW, Lemkes HHPJ, Ruitenbeek W. Mutation in mitochondrial tRNALeu(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nature Genetics. 1992;1:368–371. doi: 10.1038/ng0892-368. [DOI] [PubMed] [Google Scholar]
- 178.van der Walt JM, Nicodemus KK, Martin ER, et al. Mitochondrial polymorphisms significantly reduce the risk of Parkinson disease. American Journal of Human Genetics. 2003 Apr;72(4):804–811. doi: 10.1086/373937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.van der Walt JM, Dementieva YA, Martin ER, et al. Analysis of European mitochondrial haplogroups with Alzheimer disease risk. Neuroscience Letters. 2004 Jul 15;365(1):28–32. doi: 10.1016/j.neulet.2004.04.051. [DOI] [PubMed] [Google Scholar]
- 180.Niemi AK, Hervonen A, Hurme M, Kurhunen PJ, Jylha M, Majamaa K. Mitochondrial DNA polymorphisms associated with longevity in a Finnish population. Human Genetics. 2003 Jan;112(1):29–33. doi: 10.1007/s00439-002-0843-y. [DOI] [PubMed] [Google Scholar]
- 181.McMahon FJ, Chen YS, Patel S, et al. Mitochondrial DNA sequence diversity in bipolar affective disorder. American Journal of Psychiatry. 2000 Jul;157(7):1058–1064. doi: 10.1176/appi.ajp.157.7.1058. [DOI] [PubMed] [Google Scholar]
- 182.Petros JA, Baumann AK, Ruiz-Pesini E, et al. mtDNA mutations increase tumorigenicity in prostate cancer. Proc Natl Acad Sci USA. 2005 Jan 18;102(3):719–724. doi: 10.1073/pnas.0408894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Johnson KR, Zheng QY, Bykhovskaya Y, Spirina O, Fischel-Ghodsian N. A nuclear-mitochondrial DNA interaction affecting hearing impairment in mice. Nature Genetics. 2001 Feb;27(2):191–194. doi: 10.1038/84831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bhopal RS, Rafnsson SB. Could mitochondrial efficiency explain the susceptibility to adiposity, metabolic syndrome, diabetes and cardiovascular diseases in South Asian populations? International Journal of Epidemiology. 2009 Aug;38(4):1072–1081. doi: 10.1093/ije/dyp202. [DOI] [PubMed] [Google Scholar]
- 185.Amo T, Yadava N, Oh R, Nicholls DG, Brand MD. Experimental assessment of bioenergetic differences caused by the common European mitochondrial DNA haplogroups H and T. Gene. 2008 Mar 31;411(1–2):69–76. doi: 10.1016/j.gene.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Amo T, Brand MD. Were inefficient mitochondrial haplogroups selected during migrations of modern humans? A test using modular kinetic analysis of coupling in mitochondria from cybrid cell lines. Biochem J. 2007 Jun 1;404:345–351. doi: 10.1042/BJ20061609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gomez-Duran A, Pacheu-Grau D, Lopez-Gallardo E, et al. Unmasking the causes of multifactorial disorders: OXPHOS differences between mitochondrial haplogroups. Human Molecular Genetics. 2010 Sep 1;19(17):3343–3353. doi: 10.1093/hmg/ddq246. [DOI] [PubMed] [Google Scholar]
- 188.Roubertoux PL, Sluyter F, Carlier M, et al. Mitochondrial DNA modifies cognition in interaction with the nuclear genome and age in mice. Nature Genetics. 2003 Sep;35(1):65–69. doi: 10.1038/ng1230. [DOI] [PubMed] [Google Scholar]
- 189.Gimsa U, Kanitz E, Otten W, Ibrahim SM. Behavior and Stress Reactivity in Mouse Strains with Mitochondrial DNA Variations. Neuroimmunomodulation: from Fundamental Biology to Therapy. 2009;1153:131–138. doi: 10.1111/j.1749-6632.2008.03960.x. [DOI] [PubMed] [Google Scholar]
- 190.Yu X, Gimsa U, Wester-Rosenlof L, et al. Dissecting the effects of mtDNA variations on complex traits using mouse conplastic strains. Genome Research. 2009 Jan;19(1):159–165. doi: 10.1101/gr.078865.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yu XH, Wester-Rosenlof L, Gimsa U, et al. The mtDNA nt7778 G/T polymorphism affects autoimmune diseases and reproductive performance in the mouse. Human Molecular Genetics. 2009 Dec 15;18(24):4689–4698. doi: 10.1093/hmg/ddp432. [DOI] [PubMed] [Google Scholar]
- 192.Schieke SM, Finkel T. Mitochondrial signaling, TOR, life span. Biol Chem. 2006 Oct;387(10–11):1357–1361. doi: 10.1515/BC.2006.170. [DOI] [PubMed] [Google Scholar]
- 193.Karu TI. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem Photobiol. 2008 Sep;84(5):1091–1099. doi: 10.1111/j.1751-1097.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- 194.Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis) Exp Gerontol. 2010 Jun;45(6):410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 195.Jahnke VE, Sabido O, Freyssenet D. Control of mitochondrial biogenesis, ROS level, and cytosolic Ca2+ concentration during the cell cycle and the onset of differentiation in L6E9 myoblasts. Am J Physiol Cell Physiol. 2009 May;296(5):C1185–C1194. doi: 10.1152/ajpcell.00377.2008. [DOI] [PubMed] [Google Scholar]
- 196.Holley AK, Dhar SK, St Clair DK. Manganese superoxide dismutase vs. p53: regulation of mitochondrial ROS. Mitochondrion. 2010 Nov;10(6):649–661. doi: 10.1016/j.mito.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 197.Srinivasan S, Koenigstein A, Joseph J, et al. Role of mitochondrial reactive oxygen species in osteoclast differentiation. Ann N Y Acad Sci. 2010 Mar;1192:245–252. doi: 10.1111/j.1749-6632.2009.05377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fridlyand LE, Philipson LH. Reactive species and early manifestation of insulin resistance in type 2 diabetes. Diabetes Obesity & Metabolism. 2006 Mar;8(2):136–145. doi: 10.1111/j.1463-1326.2005.00496.x. [DOI] [PubMed] [Google Scholar]
- 199.Samocha-Bonet D, Heilbronn LK, Lichtenberg D, Campbell LV. Does skeletal muscle oxidative stress initiate insulin resistance in genetically predisposed individuals? Trends in Endocrinology and Metabolism. 2010 Feb;21(2):83–88. doi: 10.1016/j.tem.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 200.Mariappan N, Elks CM, Sriramula S, et al. NF-kappa B-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc Res. 2010 Feb 1;85(3):473–483. doi: 10.1093/cvr/cvp305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Leduc L, Levy E, Bouity-Voubou M, Delvin E. Fetal programming of atherosclerosis: Possible role of the mitochondria. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2010 Apr;149(2):127–130. doi: 10.1016/j.ejogrb.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 202.Kofler B, Mueller EE, Eder W, et al. Mitochondrial DNA haplogroup T is associated with coronary artery disease and diabetic retinopathy: a case control study. Bmc Medical Genetics. 2009 Apr 21;10 doi: 10.1186/1471-2350-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gredilla R, Sanz A, Lopez-Torres M, Barja G. Caloric restriction decreases mitochondrial free radical generation at complex I and lowers oxidative damage to mitochondrial DNA in the rat heart. Faseb Journal. 2001 May 29;15(7):1589-+. doi: 10.1096/fj.00-0764fje. [DOI] [PubMed] [Google Scholar]
- 204.Lopez-Lluch G, Hunt N, Jones B, et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci USA. 2006 Feb 7;103(6):1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gredilla R, Barja G. Minireview: The role of oxidative stress in relation to caloric restriction and longevity. Endocrinology. 2005 Sep;146(9):3713–3717. doi: 10.1210/en.2005-0378. [DOI] [PubMed] [Google Scholar]
- 206.Caro P, Gomez J, Lopez-Torres M, et al. Forty percent and eighty percent methionine restriction decrease mitochondrial ROS generation and oxidative stress in rat liver. Biogerontology. 2008 Jun;9(3):183–196. doi: 10.1007/s10522-008-9130-1. [DOI] [PubMed] [Google Scholar]
- 207.Caro P, Gomez J, Sanchez I, et al. Forty Percent Methionine Restriction Decreases Mitochondrial Oxygen Radical Production and Leak at Complex I During Forward Electron Flow and Lowers Oxidative Damage to Proteins and Mitochondrial DNA in Rat Kidney and Brain Mitochondria. Rejuvenation Research. 2009 Dec;12(6):421–434. doi: 10.1089/rej.2009.0902. [DOI] [PubMed] [Google Scholar]
- 208.Sanz A, Caro P, Ayala V, Portero-Otin M, Pamplona R, Barja G. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. Faseb Journal. 2006 Jun;20(8):1064–1073. doi: 10.1096/fj.05-5568com. [DOI] [PubMed] [Google Scholar]
- 209.Sanz A, Gomez J, Caro P, Barja G. Carbohydrate restriction does not change mitochondrial free radical generation and oxidative DNA damage. Journal of Bioenergetics and Biomembranes. 2006 Dec;38(5–6):327–333. doi: 10.1007/s10863-006-9051-0. [DOI] [PubMed] [Google Scholar]
- 210.Sanz A, Caro P, Sanchez JG, Barja G. Effect of Lipid Restriction on Mitochondrial Free Radical Production and Oxidative DNA Damage. Understanding and Modulating Aging. 2006;1067:200–209. doi: 10.1196/annals.1354.024. [DOI] [PubMed] [Google Scholar]
- 211.Gomez J, Caro P, Sanchez I, et al. Effect of methionine dietary supplementation on mitochondrial oxygen radical generation and oxidative DNA damage in rat liver and heart. Journal of Bioenergetics and Biomembranes. 2009 Jun;41(3):309–321. doi: 10.1007/s10863-009-9229-3. [DOI] [PubMed] [Google Scholar]
- 212.Iso H, Rexrode KM, Stampfer MJ, et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. Jama-Journal of the American Medical Association. 2001 Jan 17;285(3):304–312. doi: 10.1001/jama.285.3.304. [DOI] [PubMed] [Google Scholar]
- 213.Simon JA, Hodgkins ML, Browner WS, Neuhaus JM, Bernert JT, Hulley SB. Serum Fatty-Acids and the Risk of Coronary Heart-Disease. American Journal of Epidemiology. 1995 Sep 1;142(5):469–476. doi: 10.1093/oxfordjournals.aje.a117662. [DOI] [PubMed] [Google Scholar]
- 214.Hu FB, Cho EY, Rexrode KM, Albert CM, Manson JE. Fish and long-chain omega-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation. 2003 Apr 15;107(14):1852–1857. doi: 10.1161/01.CIR.0000062644.42133.5F. [DOI] [PubMed] [Google Scholar]
- 215.Hu FB. Plant-based foods and prevention of cardiovascular disease: an overview. American Journal of Clinical Nutrition. 2003 Sep;78(3):544S–551S. doi: 10.1093/ajcn/78.3.544S. [DOI] [PubMed] [Google Scholar]
- 216.Hagopian K, Weber KL, Hwee DT, et al. Complex I-Associated Hydrogen Peroxide Production Is Decreased and Electron Transport Chain Enzyme Activities Are Altered in n-3 Enriched fat-1 Mice. Plos One. 2010 Sep 13;5(9) doi: 10.1371/journal.pone.0012696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005 Apr 29;280(17):17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 218.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006 Nov 16;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sulaiman M, Matta MJ, Sunderesan NR, Gupta MP, Periasamy M, Gupta M. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. American Journal of Physiology-Heart and Circulatory Physiology. 2010 Mar;298(3):H833–H843. doi: 10.1152/ajpheart.00418.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007 Mar 2;145(1):256–264. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mattson MP, Chan SL, Duan WZ. Modification of brain aging and neurodegenerative disorders by genes, diet, and behavior. Physiol Rev. 2002 Jul;82(3):637–672. doi: 10.1152/physrev.00004.2002. [DOI] [PubMed] [Google Scholar]
- 222.Neal EG, Chaffe H, Schwartz RH, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurology. 2008 Jun;7(6):500–506. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- 223.Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behavioural Pharmacology. 2006 Sep;17(5–6):431–439. doi: 10.1097/00008877-200609000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ahola-Erkkila S, Carroll CJ, Peltola-Mjosund K, et al. Ketogenic diet slows down mitochondrial myopathy progression in mice. Human Molecular Genetics. 2010 May 15;19(10):1974–1984. doi: 10.1093/hmg/ddq076. [DOI] [PubMed] [Google Scholar]
- 225.Dashti HM, Bo-Abbas YY, Asfar SK, et al. Ketogenic diet modifies the risk factors of heart disease in obese patients. Nutrition. 2003 Oct;19(10):901–902. doi: 10.1016/s0899-9007(03)00161-8. [DOI] [PubMed] [Google Scholar]
- 226.Anderson EJ, Lustig ME, Boyle KE, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009 Mar;119(3):573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Williamson JR, Chang K, Frangos M, et al. Hyperglycemic Pseudohypoxia and Diabetic Complications. Diabetes. 1993 Jun;42(6):801–813. doi: 10.2337/diab.42.6.801. [DOI] [PubMed] [Google Scholar]
- 228.Gaikwad A, Long DJ, Stringer JL, Jaiswal AK. In vivo role of NAD(P)H : quinone oxidoreductase 1 (NQO1) in the regulation of intracellular redox state and accumulation of abdominal adipose tissues. J Biol Chem. 2001 Jun 22;276(25):22559–22564. doi: 10.1074/jbc.M101053200. [DOI] [PubMed] [Google Scholar]
- 229.Mongan PD, Capacchione J, West S, et al. Pyruvate improves redox status and decreases indicators of hepatic apoptosis during hemorrhagic shock in swine. American Journal of Physiology-Heart and Circulatory Physiology. 2002 Oct;283(4):H1634–H1644. doi: 10.1152/ajpheart.01073.2001. [DOI] [PubMed] [Google Scholar]
- 230.MacDonald MJ, Marshall LK. Mouse lacking NAD(+)-linked glycerol phosphate dehydrogenase has normal pancreatic beta cell function but abnormal metabolite pattern in skeletal muscle. Arch Biochem Biophys. 2000 Dec 1;384(1):143–153. doi: 10.1006/abbi.2000.2107. [DOI] [PubMed] [Google Scholar]
- 231.Lin SJ, Guarente L. Nicotinamide adenine dinucleotide, a metabolic regulator of transcription, longevity and disease. Current Opinion in Cell Biology. 2003 Apr;15(2):241–246. doi: 10.1016/s0955-0674(03)00006-1. [DOI] [PubMed] [Google Scholar]
- 232.Easlon E, Tsang F, Skinner C, Wang C, Lin SJ. The malate-aspartate NADH shuttle components are novel metabolic longevity regulators required for calorie restriction-mediated life span extension in yeast. Genes & Development. 2008 Apr 1;22(7):931–944. doi: 10.1101/gad.1648308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bobyleva V, Pazienza L, Muscatello U, Kneer N, Lardy H. Short-term hypothermia activates hepatic mitochondrial sn-glycerol-3-phosphate dehydrogenase and thermogenic systems. Arch Biochem Biophys. 2000 Aug 15;380(2):367–372. doi: 10.1006/abbi.2000.1942. [DOI] [PubMed] [Google Scholar]
- 234.Shen WY, Wei YD, Dauk M, et al. Involvement of a glycerol-3-phosphate dehydrogenase in modulating the NADH/NAD(+) ratio provides evidence of a mitochondrial glycerol-3-phosphate shuttle in Arabidopsis. Plant Cell. 2006 Feb;18(2):422–441. doi: 10.1105/tpc.105.039750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends in Biochemical Sciences. 2000 Oct;25(10):502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- 236.Malinska D, Kulawiak B, Kudin AP, et al. Complex III-dependent superoxide production of brain mitochondria contributes to seizure-related ROS formation. Biochimica et Biophysica Acta-Bioenergetics. 2010 Jun;1797(6–7):1163–1170. doi: 10.1016/j.bbabio.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 237.Drose S, Brandt U. The mechanism of mitochondrial superoxide production by the cytochrome bc(1) complex. J Biol Chem. 2008 Aug 1;283(31):21649–21654. doi: 10.1074/jbc.M803236200. [DOI] [PubMed] [Google Scholar]
- 238.Lambert A, Brand M. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J. 2004 doi: 10.1042/BJ20040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Cadenas E, Davies KJA. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29(3–4):222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 240.Brand MD, Affourtit C, Esteves TC, et al. Mitochondrial superoxide: Production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med. 2004 Sep 15;37(6):755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 241.Schonfeld P, Wojtczak L. Fatty acids decrease mitochondrial generation of reactive oxygen species at the reverse electron transport but increase it at the forward transport. Biochimica et Biophysica Acta-Bioenergetics. 2007 Aug;1767(8):1032–1040. doi: 10.1016/j.bbabio.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 242.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009 Jan 1;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jauslin ML, Meier T, Smith RAJ, Murphy MP. Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. Faseb Journal. 2003 Aug;17(11):1972-+. doi: 10.1096/fj.03-0240fje. [DOI] [PubMed] [Google Scholar]
- 244.Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme Q10 on myopathic symptoms in patients treated with statins. American Journal of Cardiology. 2007 May 15;99(10):1409–1412. doi: 10.1016/j.amjcard.2006.12.063. [DOI] [PubMed] [Google Scholar]
- 245.Khatta M, Alexander BS, Krichten CM, et al. The effect of coenzyme Q(10) in patients with congestive heart failure. Annals of Internal Medicine. 2000 Apr 18;132(8):636–640. doi: 10.7326/0003-4819-132-8-200004180-00006. [DOI] [PubMed] [Google Scholar]
- 246.de Bustos F, Molina JA, Jimenez-Jimenez FJ, et al. Serum levels of coenzyme Q(10) in patients with Alzheimer's disease. Journal of Neural Transmission. 2000;107(2):233–239. doi: 10.1007/s007020050019. [DOI] [PubMed] [Google Scholar]
- 247.Dhanasekaran M, Ren J. The emerging role of coenzyme Q-10 in aging, neurodegeneration, cardiovascular disease, cancer and diabetes mellitus. Current Neurovascular Research. 2005 Dec;2(5):447–459. doi: 10.2174/156720205774962656. [DOI] [PubMed] [Google Scholar]
- 248.Rodriguez-Hernandez A, Cordero MD, Salviati L, et al. Coenzyme Q deficiency triggers mitochondria degradation by mitophagy. Autophagy. 2009 Jan;5(1):19–32. doi: 10.4161/auto.5.1.7174. [DOI] [PubMed] [Google Scholar]