Abstract
The purposes of this study were (1) determine if youth peak Achilles tendon (AT) strain, peak AT stress, and AT stiffness, measured during an isometric plantar flexion, differed after six months (mos) of growth, and (2) determine if sex, physical activity level (Physical Activity Questionnaire (PAQ-C)), and/or growth rate (GR) were related to these properties. AT stress, strain, and stiffness were quantified in 20 boys (13.47±0.81 years) and 22 girls (11.18±0.82 years) at 2 times (0 and 6 mos). GR (change in height in 6 mos) was not significantly different between boys and girls (3.5±1.4 and 3.4±1.1 cm/6 mos, respectively). Peak AT strain and stiffness (mean 3.8±0.4% and 128.9±153.6 N/mm, respectively) did not differ between testing sessions or sex. Peak AT stress (22.1±2.4 and 24.0±2.1 MPa at 0 and 6 mos, respectively) did not differ between sex and increased significantly at 6 mos due to a significant decrease in AT cross-sectional area (40.6±1.3 and 38.1±1.6 mm2 at 0 and 6 mos, respectively) with no significant difference in peak AT force (882.3±93.9 and 900.3± 65.5 N at 0 and 6 mos, respectively). Peak AT stress was significantly greater in subjects with greater PAQ-C scores (9.1% increase with 1 unit increase in PAQ-C score) and smaller in subjects with faster GRs (13.8% decrease with 1 cm/6 mos increase in GR). These results indicate that of the AT mechanical properties quantified, none differed between sex, and only peak AT stress significantly differed after 6 months and was related to GR and physical activity.
Keywords: Mechanical properties, physical activity, sex
INTRODUCTION
Little is known about Achilles tendon (AT) mechanical properties in youth and how factors such as sex, growth rate, and physical activity relate to these properties. Understanding these properties and relations is fundamental to understanding AT injury mechanisms. AT-calcaneal injuries, such as Sever’s disease, represent 2-16% of all physical activity related musculoskeletal injuries in youth 10-14 years of age (Dalton, 1992; Scharfbillig et al., 2008). Assuming 35% of the 20 million 10-14 year old children in the US. experience musculoskeletal injuries each year (CDC, 2002; Cuff et al., 2010), this equates to 0.14 to 1.1 million AT-calcaneal injuries per year. The AT plays an important role in gait, is clinically relevant due to the prevalence of youth AT injuries such as Sever’s disease, and can be studied non-invasively making it a good representative tendon to study.
Stress, strain, and stiffness have been implicated in AT overuse injury (Almekinders et al., 2002; Maganaris et al., 2004) and were therefore of interest in this investigation. These quantities have been shown to differ based on both sex (Kubo et al., 2003) and physical activity (Rosager et al., 2002). Growth rate (GR) is also thought to affect youth tendon properties due to a slower muscle growth rate compared to bone growth rate but to date no evidence has been provided to support this hypothesis (Micheli and Fehlandt, 1996; Scharfbillig et al., 2008). Asynchronous growth rates of the lower leg bones relative to the AT growth rate in combination with gastrocnemius-soleus strength gains may affect youth AT stress, AT strain, and AT stiffness. The purposes of this study were to (1) determine if peak AT stress, peak AT strain, and AT stiffness, measured during a maximal isometric plantar flexion (MIPF) effort, changed after six months of growth in a youth population during their primary growth years, and (2) test our hypotheses that sex, physical activity level, and/or growth rate are related to these properties in youth. This study is the first to report within-subject differences in AT properties in youth during a period of their primary growth years.
METHODS
The study was approved by the University of California, Davis Institutional Review Board and prior to testing, written informed parental consent and subject assent (12 years of age or older) were obtained. 22 girls and 20 boys (11.18 ± 0.82 and 13.47 ± 0.81 years, respectively) were enrolled in the study. The age ranges targeted for recruitment (10 to 12 year old girls and 12-14 year old boys) represent one standard deviation around the average age during which peak height velocity (PHV) occurs for girls and boys respectively (Berkey et al., 1993; Philippaerts et al., 2006; Rauch et al., 2004). Subjects completed the same testing at the start of the study (0 months (mos)) and approximately 6 months later (24.2 ± 1.4 weeks on average; this equates to 5.6 mos and is referred to throughout as the 6 mos testing session). A full practice testing session was completed within 10 days prior to the first testing session to familiarize subjects with the testing procedures.
Clothed body mass without shoes (measured to the nearest half kilogram (kg) using a calibrated scale) and body height without shoes (measured to the nearest half centimeter (cm) using a stadeometer) were recorded. Lower leg length (referred to as leg length throughout) was measured to the nearest millimeter and was defined as the distance between the floor and the most lateral aspect of the fibular head (Kongsgaard et al., 2005). This length provided a reasonable estimate of the bone length spanned by the Gastrocnemius-AT complex. The AT moment arm was manually measured as the distance along a line formed between the mid-substance of the AT and the most medial aspect of the medial malleolus while subjects were standing with even weight distributed among both feet. Body height, leg length, and AT moment arm were measured in triplicate and the average determined for each subject. GR was defined as the difference in height at 0 mos and 6 mos.
Each subject completed the Physical Activity Questionnaire (PAQ-C), which has been validated elsewhere (Biddle et al., 2011; Crocker et al., 1997; Janz et al., 2008), to assess their physical activity level. The average overall score (ranges from 1 to 5, with 5 representing a very physically active lifestyle) of the 9 questions was calculated to determine each subject’s PAQ-C score (Janz et al., 2008).
Tendon mechanical properties
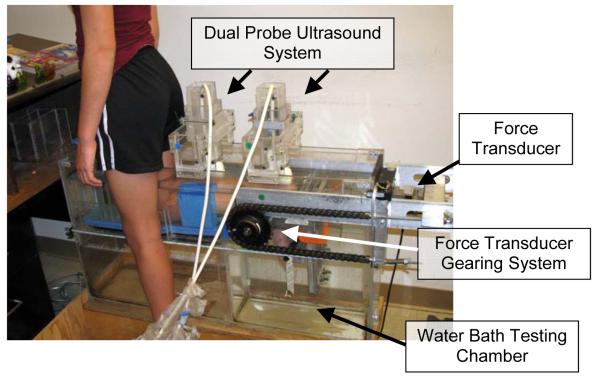
The mechanical properties of the AT were assessed using a water bath chamber (Figure 1) that included a force transducer and a dual probe ultrasound system. After completing a warm-up of six minutes of treadmill walking (2.0 miles per hour) (Hawkins et al., 2009), subjects were positioned in the testing chamber such that their knee was flexed at ninety degrees and their malleoli were aligned with the force transducer gearing system axis of rotation. The subject’s knee and shank were secured using firm foam wedges to minimize extraneous leg motion. Minimal ankle rotation was observed during MIPF efforts.
Figure 1.
The basic setup used to quantify muscle-tendon architecture and tendon strain during isometric muscle efforts. A force transducer, located at the ball of the foot, is used to quantify ankle plantar flexion torque developed by the triceps surae muscles. The ultrasound system was used to quantify tendon CSA and AT length during muscle contraction.
AT force and ultrasound images were collected simultaneously at 30 Hz using custom Labview programs (National Instruments, Austin, Texas). Subjects performed five to six 5 second ramped MIPF trials. The four trials with the greatest MIPF torque were analyzed (three for AT length and deformation, one for AT cross-sectional area (CSA)). For AT length and deformation, sagittal plane images were collected using ultrasound probes located over the calcaneus-AT and the gastrocnemius-AT junctions. The lateral gastrocnemius-AT junction was imaged for all but two subjects, who had the medial gastrocnemius imaged because of better image clarity. For AT CSA, transverse plane images were collected using one probe located at approximately 50% of the free AT length (e.g. free tendon defined as the tendon between the calcaneus-AT and the soleus-AT junctions). CSA has been previously shown to vary along the length of the AT (Magnusson and Kjaer, 2003) but has been shown to be the smallest at approximately 50% of the free AT length (position used in the current study) (Kongsgaard et al., 2011).
For all trials, force acting on the AT (FAT) was calculated by dividing plantar flexion (PF) torque by the AT moment arm. AT ultrasound images corresponding to 10 % increments of peak FAT from 0% to 100% were identified (11 images total).
The AT perimeter was digitized in triplicate (ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA) in ultrasound images corresponding to 0, 20, and 40% of peak FAT and the average CSA for these force levels calculated.
For AT length and deformation, the 11 ultrasound images were digitized in triplicate and the average location of the calcaneus-AT and gastrocnemius-AT junctions determined. The total AT length was defined as the length between the calcaneus-AT junction and the gastrocnemius-AT junction. Deformation was calculated as the change in length relative to the tendon length at 0% peak FAT (defined as the AT rest length).
AT stress, strain and stiffness were determined from the AT force-deformation, CSA and rest length data. Force and deformation data were divided by CSA and rest length, respectively to determine stress and strain, respectively. Second order polynomials were fit to the average force-deformation data and the stress-strain data between 0 and 100% of peak FAT for each subject. The stiffness and modulus were calculated as the derivative of the force-deformation and the stress-strain second order polynomial equations, respectively, at 70% of peak FAT.
Statistical Analysis
Repeated measures multiple regressions were used to determine if height, mass, and PAQ-C score differed between testing sessions or between sex (R, R Foundation for Statistical Computing, Austria). Repeated measures multiple regressions were used to assess the relationship between the explanatory factors of interest (testing session, PAQ-C score, GR, and sex) and peak PF torque, peak FAT, AT moment arm, AT rest length, AT CSA, peak AT strain, peak AT stress, and AT stiffness. Significance for all AT properties and related quantities was defined as Bonferroni adjusted p < 0.006 (p = 0.05/8 tests). Differences between the percent growth of the AT compared to the lower leg were determined using a paired t-test (p < 0.05). Differences between GR in boys and girls were determined using a t-test (p < 0.05). Mean and standard deviations are reported.
RESULTS
Height and mass differed at the start and end of the study for both boys and girls (Table 1). PAQ-C score and GR were not statistically different between sexes (p > 0.18). Peak AT stress significantly increased 6.7% at 6 mos compared with 0 mos (Table 2) and was significantly greater by 9.1% on average for a subject with a 1 unit greater PAQ-C score (Table 4) compared to another subject (assuming all other factors held constant). When GR was greater for a given subject by 1 cm/6 mos (assuming all other factors held constant), peak AT stress was significantly smaller by 13.8% on average (Table 4). Peak AT stress did not differ significantly between boys and girls (Table 4).
Table 1.
Subject demographics and descriptive measures for study population. Mean (standard 2 deviation) are reported.
| Sex | Testing Session |
Age (years) |
Height (cm) |
Mass (kg) |
Leg length (cm) |
GR (cm/6 mos) |
PAQ-C score |
|---|---|---|---|---|---|---|---|
| Boys | 0 mos | 13.47 (0.81) |
159.1 *† (11.6) |
50.0 *† (11.1) |
42.1 (0.9) |
n/a | 2.5 (0.7) |
| 6 mos | 13.94 (0.81) |
162.7 * (11.0) |
52.9 * (11.0) |
42.7 (0.8) |
3.5 (1.4) |
2.4 (0.6) |
|
| Girls | 0 mos | 11.18 (0.82) |
147.6 † (9.8) |
43.0 † (8.2) |
38.7 (0.6) |
n/a | 2.4 (0.4) |
| 6 mos | 11.65 (0.82) |
151.0 (9.8) |
45.8 (9.1) |
39.7 (0.7) |
3.4 (1.1) |
2.6 (0.3) |
Significant difference between sex (p < 0.013).
Significant difference between 0 and 6 mos (p < 0.013).
Table 2.
Stress, strain, stiffness, modulus and the associated variables at the beginning and end of the study for both boys and girls. Mean (standard deviation) are reported.
| Sex | Testing Session |
Peak AT strain (%) |
Peak AT stress †§‡ (MPa) |
AT stiffness (N/mm) |
AT modulus (MPa) |
|---|---|---|---|---|---|
| Boys | 0 mos | 3.3 (1.5) |
23.1 (12.5) |
108.7 (134.6) |
564.7 (644.5) |
| 6 mos | 3.6 (1.6) |
24.7 (9.8) |
162.6 (146.0) |
866.9 (753.5) |
|
| Girls | 0 mos | 4.0 (2.3) |
21.1 (10.1) |
117.5 (182.8) |
610.1 (882.1) |
| 6 mos | 4.2 (1.9) |
23.5 (10.1) |
128.1 (150.2) |
665.5 (820.1) |
Significant difference between testing session (p < 0.006).
Significant relationship with PAQ-C score (p < 0.006).
Significant relationship with growth rate (p < 0.006).
Table 4.
Relationship of each signficant factor on each AT property (p < 0.006). Percentages shown assume all other factors are held constant. Non-significant p values are shown in parenthenses.
| Sex |
Growth Rate
(GR) |
Testing
Session |
PAQ-C | |
|---|---|---|---|---|
| Female compared to male |
Change with 1 cm/6 mos greater GR |
6 mos compared to 0 mos |
Change with 1 unit greater PAQ-C score |
|
| Peak AT stress |
(p = 0.36) | −13.8% | 6.7% | 9.1% |
| Peak AT strain |
(p = 0.18) | (p = 0.34) | (p = 0.09) | (p = 0.10) |
| AT stiffness | (p = 0.70) | (p = 0.77) | (p = 0.03) | (p = 0.83) |
| AT Modulus | (p = 0.66) | (p = 0.61) | (p = 0.01) | (p = 0.98) |
| AT moment arm |
−12.2% | (p = 0.33) | 5.7% | (p = 0.17) |
| Peak PF torque |
(p = 0.03) | −11.0% | (p = 0.06) | (p = 0.28) |
| Peak FAT | (p = 0.16) | −11.3% | (p = 0.51) | (p = 0.04) |
| AT rest length |
(p = 0.009) | (p = 0.43) | 1.6% | −2.0% |
| AT CSA | (p = 0.38) | (p = 0.24) | −5.7% | (p = 0.10) |
Peak AT strain, AT stiffness, and AT modulus were not significantly different between 0 and 6 mos (Table 2) and had no significant relationship with sex, GR, or PAQ-C score (Table 4). AT moment arm significantly increased during the study by 5.7% on average (Tables 3) and was significantly shorter for girls compared with boys (on average 12.2% shorter for girls). PAQ-C score and GR had no significant relationship with AT moment arm (Table 3).
Table 3.
Associated variables of stress, strain, and stiffness at the beginning and end of the study for both boys and girls. Mean (standard deviation) are reported.
| Sex | Testing Session |
AT moment arm *† (cm) |
Peak PF torque ‡ (Nm) |
Peak FAT
‡ (N) |
AT Rest length †§ (mm) |
AT CSA † (mm2) |
|---|---|---|---|---|---|---|
| Boys | 0 mos | 5.3 (0.4) |
50.8 (28.8) |
947.8 (511.1) |
203.5 (23.0) |
41.3 (5.7) |
| 6 mos | 5.8 (1.2) |
51.6 (21.0) |
943.6 (357.8) |
207.1 (24.9) |
38.7 (7.4) |
|
| Girls | 0 mos | 4.8 (0.4) |
39.6 (17.8) |
822.7 (366.8) |
183.2 (24.0) |
38.8 (6.4) |
| 6 mos | 5.0 (0.2) |
42.8 (13.7) |
860.9 (255.3) |
185.6 (24.0) |
37.2 (7.7) |
Significant difference between sex (p < 0.006).
Significant difference between testing session (p < 0.006).
Significant relationship with PAQ-C score (p < 0.006).
Significant relationship with growth rate (p < 0.006).
When GR was greater for a given subject by 1 cm/6 mos compared to another subject (assuming all other factors held constant), peak PF torque and peak FAT were significantly smaller on average by 11.0% and 11.3%, respectively. Peak PF torque and peak FAT did not differ significantly at 6 mos compared to 0 mos and were not related to PAQ-C score or sex (Table 4).
AT rest length was significantly smaller by 2.0% on average for a subject with a 1 unit greater PAQ-C score compared with another subject (assuming all other factors held constant). AT rest length was not significantly different between sexes and was not significantly related to GR (Table 4). Although the AT rest length increased over the study, the percent growth of the lower leg (2.1 ± 0.6%) and AT percent growth (1.7 ± 0.9%) were not statistically different (p = 0.29).
AT CSA significantly decreased between testing sessions by 5.7% on average. Sex, GR, and PAQ-C score had no significant relationship with AT CSA (Table 4).
DISCUSSION
The purposes of this study were to (1) determine if peak AT stress, peak AT strain, and AT stiffness, measured during an MIPF, changed after six months of growth, and (2) test our hypotheses that sex, physical activity level, and/or GR are related to these properties in youth. This study is unique in that within-subject comparisons, before and after 6 mos of growth, identified changes in AT mechanical properties during growth rather than inferring this relationship from a sample of youth and a sample of older adults (Magnusson et al., 2003; Waugh et al., 2012). The current study design also allowed for the relationships between GR and AT properties as well as differences in AT properties between the biological age-matched boys and girls to be determined, which prior to this study, had not been reported.
Properties at 0 and 6 mos
Peak AT stress increased at 6 mos compared to 0 mos (+ 6.7%) due to a decrease in AT CSA (−5.7%) and no difference in peak FAT. Peak stresses reported may represent the highest MIPF stresses in the AT because the AT CSA used likely represent the narrowest part of the AT. Peak stress (average 23 MPa) was less than previously reported in children (46.7 ± 11.8 MPa (Waugh et al., 2012)) and adults (30-42 MPa (Kongsgaard et al., 2011; Magnusson et al., 2003)). The differences are likely due to both AT CSA and peak FAT. Waugh, et al. (2012) found similar AT CSA (39 mm2) in younger subjects (~9 years of age) while larger adult CSAs have been reported (47-67 mm2 (Kongsgaard et al., 2011; Magnusson et al., 2003)). Peak FAT (900 N) was less than previously reported in children (~1850 N (Waugh et al., 2012)) and adults (~1970 N (Kongsgaard et al., 2011). The lower peak FAT in the current population compared with those reported by Waugh, et al. (2012) may be due to gastrocnemius force contributions being higher in an extended knee position compared to the flexed knee position used in this study. The lower peak FAT in the current population compared with adults (Kongsgaard et al., 2011) likely results from the immature muscular strength in the younger age versus the older age.
Peak AT strain, AT stiffness, and AT modulus did not significantly differ between 0 and 6 mos. Peak strain (average 3.8%) was on the low end of previously reported peak AT strains of 4 to 8.0% (Arampatzis et al., 2007a; Kubo et al., 2007; Rosager et al., 2002; Waugh et al., 2012). Stiffness (average 128.9 N/mm) was less than previously reported in children (162.4 N/mm (Waugh et al., 2012)) and male adults (234 N/mm (Shin et al., 2008), 2622 N/mm (Kongsgaard et al., 2011)). Modulus (average 674.9 MPa) was less than previously reported in adults (1160 MPa (Maganaris and Paul, 2002), ~1100 MPa (Arampatzis et al, 2007b)) and similar to that reported in children (8-10 years; 623.6 MPa (Waugh et al, 2012)).
The lack of difference in stiffness at 0 and 6 mos combined with the smaller CSA suggests that the AT material changed during the study to allow a smaller CSA to provide the same resistance to length change for a given change in FAT. Furthermore, the lack of difference in the AT strain indicates that although the AT was longer at 6 mos than at 0 mos (+ 1.6%) the peak AT deformation during the MIPF increased a proportional amount resulting in peak strains that were not statistically different.
Growth Rate
GR had a significant relationship with several of the parameters investigated. Peak AT stress, peak PF torque, and peak FAT were all smaller with faster GRs (−13.8%, −11.0%, and −11.3%, respectively). The decrease in both peak PF torque and peak FAT with a faster GR suggests that faster growing subjects did not maintain their PF strength. To our knowledge, similar studies and findings have not been previously reported in the literature. These findings raise several questions for further investigation including if the triceps surae muscles have become stretched and are acting on the descending portion of the force-deformation curve thereby decreasing the peak PF torque or if GR affected the actual muscle strength or neural control.
AT CSA decreased after 6 mos (−5.7%) but was not related to GR. This suggests in the study sample, CSA changed as a result of normal growth and development but not specifically based on the rate of growth. Previous studies have demonstrated greater AT CSA in older versus younger adults (Magnusson et al., 2003). The decrease in CSA reported here suggests that there may be a decrease in CSA prior to the increase in CSA that could not be identified in the prior cross-sectional design.
Interestingly, there was no relationship between GR and peak AT strain, stiffness, or modulus. No significant difference in the percent growth of the AT and of the shank is counter to previous studies that have anecdotally reported a greater GR in the shank than the tendon (Dalton, 1992; Micheli and Fehlandt, 1992; Micheli and Fehlandt, 1996; Scharfbillig et al., 2008). In our asymptomatic subjects, there was no relationship between GR and the peak strain or stiffness suggesting that in these growing subjects the AT properties adapted to the growth and development occurring in the leg. These results do not identify however, if the increased AT rest length was due to AT growth or if the AT was chronically being stretched.
Sex
Peak AT stress, peak AT strain, AT stiffness, and AT modulus did not differ between boys and girls. To our knowledge, no other known study has compared AT mechanical properties in biologically-aged matched youth between sex. The current findings differ from those reported by Kubo, et al. (2003) in adult AT properties, which were greater AT strain and stress, and lower tendon stiffness in women compared to men (Kubo et al., 2003). The lack of similar findings in the current study to those previously reported could be a result of the boys and girls being matched for biological age rather than chronological age or could be a result of hormonal differences in the girls of this study (which were not quantified) compared with older females in previous studies. At the cellular level, lower collagen synthesis rates in women compared to men have been reported to result from menstrual cycles in women (Magnusson et al., 2007) and higher levels of estrogen in women (Kjaer et al., 2009). Given the age of the subjects in this study, hormone levels are likely different that those of older females although they were not directly measured.
Physical activity level
Physical activity level (as measured by PAQ-C score) had a significant relationship with peak AT stress and AT rest length. With greater PAQ-C score, peak AT stress was greater (9.1%) and AT rest length was smaller (−2.0%). Increased physical activity levels would likely lead to greater triceps surae strength and therefore greater AT force. However, no statistical relationship between peak FAT, peak PF torque, or AT CSA with physical activity level was found. This suggests that physical activity results in a net increase in peak AT stress that is not related to a generalized AT CSA or peak FAT response, but varies among youth or perhaps in response to the type of activity the youth perform. These relationships raise the question as to why the AT would shorten in physically active kids. Future studies are needed to determine if the triceps surae muscle is lengthening or if the AT shortening is in response to the altered loading and stretching the muscle.
Previous studies identified differences in adult AT properties between specific types of activities, such as greater CSA in runners compared with non-runners (Kongsgaard et al., 2005; Rosager et al., 2002), smaller AT stress in runners compared with non-runners (Kongsgaard et al., 2005), and greater stiffness (triceps surae tendon and aponeurosis) in sprinters compared with endurance runners (Arampatzis et al., 2007a). Similar comparisons were not possible in this study because the subject’s overall physical activity level was assessed rather than the specific types of activities in which the subject’s participated. The PAQ-C provides an overall score rather than information about specific activities. The low variability of the PAQ-C scores (SD of 0.3 to 0.7) suggests that the subjects enrolled in the study were similar in their activity levels. This is likely a result of the somewhat limited geographical radius from which it was feasible to recruit subjects to visit the lab multiple times over 6 months. Future studies could enroll subjects specifically based on the overall activity level of the subjects as well as the specific types of activities to increase the variability of PA levels.
CONCLUSIONS
Peak AT strain and stiffness did not significantly differ between sexes matched for biological age or between the 0 and 6 month testing sessions. Peak AT strain and stiffness also had no significant relationship with GR or physical activity level. Peak AT stress was greater at 6 months compared to the beginning of the study, was greater in subjects with greater physical activity level, and was smaller in subjects with faster GRs. The results of this study are novel in reporting AT properties in youth over a period of growth.
Figure 2.
Force-deformation curve averaged across all subjects for 0 mos and 6 mos. Standard deviation bars are shown for both deformation and peak FAT. The passive force in the AT (e.g. at 0% strain) for a 90 degree ankle angle was 18.8 N (18.4 N standard deviation) and 23.1 N (24.7 N standard deviation) at 0 and 6 mos, respectively.
Figure 3.
Stress-strain curve averaged across all subjects for 0 mos and 6 mos. Standard deviation bars are shown for both strain and stress.\
ACKNOWLEDGEMENTS
This work was partially supported by funding from the University of California T32 Pre-Doctoral Clinical Research Training Program. Statistical advice was made possible by Grant Number UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The authors would like to thank Katie Reinhardt, Noelle Caron, Rosa Ferris, Heather Abbott, and Chloe McCloskey for their diligence in digitizing the ultrasound images.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT The authors have no conflict of interest to report.
REFERENCES
- CDC Injury Research Agenda. National Center for Injury Prevention and Control; Atlanta, GA: 2002. [Google Scholar]
- Almekinders LC, Vellema JH, Weinhold PS. Strain patterns in the patellar tendon and the implications for patellar tendinopathy. Knee Surgery, Sports Traumatology, Arthroscopy. 2002;10:2–5. doi: 10.1007/s001670100224. [DOI] [PubMed] [Google Scholar]
- Arampatzis A, Karamanidis K, Morey-Klapsing G, De Monte G, Stafilidis S. Mechanical properties of the triceps surae tendon and aponeurosis in relation to intensity of sport activity. Journal of Biomechanics. 2007a;40:1946–1952. doi: 10.1016/j.jbiomech.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Arampatzis A, Karamanidis K, Albracht K. Adaptational responses of the human Achilles tendon by modulation of the applied cyclic strain magnitude. The Journal of Experimental Biology. 2007b;210:2743–2753. doi: 10.1242/jeb.003814. [DOI] [PubMed] [Google Scholar]
- Berkey CS, Dockery DW, Wang X, Wypij D, Ferris B., Jr. Longitudinal height velocity standards for U.S. adolescents. Statistics in Medicine. 1993;12:403–414. doi: 10.1002/sim.4780120321. [DOI] [PubMed] [Google Scholar]
- Biddle SJ, Gorely T, Pearson N, Bull FC. An assessment of self-reported physical activity instruments in young people for population surveillance: Project ALPHA. International Journal of Behavioral Nutrition and Physical Activity. 2011;8:1–9. doi: 10.1186/1479-5868-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker PR, Bailey DA, Faulkner RA, Kowalski KC, McGrath R. Measuring general levels of physical activity: preliminary evidence for the Physical Activity Questionnaire for Older Children. Medicine & Science in Sports & Exercise. 1997;29:1344–1349. doi: 10.1097/00005768-199710000-00011. [DOI] [PubMed] [Google Scholar]
- Cuff S, Loud K, O’Riordan MA. Overuse injuries in high school athletes. Clinical Pediatrics. 2010;49:731–736. doi: 10.1177/0009922810363154. [DOI] [PubMed] [Google Scholar]
- Dalton SE. Overuse injuries in adolescent athletes. Sports Medicine. 1992;13:58–70. doi: 10.2165/00007256-199213010-00006. [DOI] [PubMed] [Google Scholar]
- Hawkins D, Lum C, Gaydos D, Dunning R. Dynamic creep and pre-conditioning of the Achilles tendon in-vivo. Journal of Biomechanics. 2009;42:2813–2817. doi: 10.1016/j.jbiomech.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Janz KF, Lutuchy EM, Wenthe P, Levy SM. Measuring activity in children and adolescents using self-report: PAQ-C and PAQ-A. Medicine & Science in Sports & Exercise. 2008;40:767–772. doi: 10.1249/MSS.0b013e3181620ed1. [DOI] [PubMed] [Google Scholar]
- Kjaer M, Langberg H, Heinemeier K, Bayer ML, Hansen M, Holm L, Doessing S, Kongsgaard M, Krogsgaard MR, Magnusson SP. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scandinavian Journal of Medicine & Science in Sports. 2009;19:500–510. doi: 10.1111/j.1600-0838.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- Kongsgaard M, Aagaard P, Kjaer M, Magnusson SP. Structural Achilles tendon properties in athletes subjected to different exercise modes and in Achilles tendon rupture patients. Journal of Applied Physiology. 2005;99:1965–1971. doi: 10.1152/japplphysiol.00384.2005. [DOI] [PubMed] [Google Scholar]
- Kongsgaard M, Nielsen CH, Hegnsvad S, Aagaard P, Magnusson SP. Mechanical properties of the human Achilles tendon, in vivo. Clinical Biomechanics. 2011;26:772–777. doi: 10.1016/j.clinbiomech.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Fukunaga T. Gender differences in the viscoelastic properties of tendon structures. European Journal of Applied Physiology. 2003;88:520–526. doi: 10.1007/s00421-002-0744-8. [DOI] [PubMed] [Google Scholar]
- Kubo K, Morimoto M, Komuro T, Tsunoda N, Kanehisa H, Fukunaga T. Age-related differences in the properties of the plantar flexor muscles and tendons. Medicine & Science in Sports & Exercise. 2007;39:541–547. doi: 10.1249/01.mss.0000247006.24965.74. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Paul JP. Tensile properties of the in vivo human gastrocnemius tendon. Journal of Biomechanics. 2002;35:1639–1646. doi: 10.1016/s0021-9290(02)00240-3. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Narici MV, Almekinders LC, Maffulli N. Biomechanics and pathophysiology of overuse tendon injuries: ideas on insertional tendinopathy. Sports Medicine. 2004;34:1005–1017. doi: 10.2165/00007256-200434140-00005. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Beyer N, Abrahamsen H, Aagaard P, Neergaard K, Kjaer M. Increased cross-sectional area and reduced tensile stress of the Achilles tendon in elderly compared with young women. Journal of Gerontology Series A. 2003;58:123–127. doi: 10.1093/gerona/58.2.b123. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Hansen M, Langberg H, Miller B, Haraldsson B, Westh EK, Koskinen S, Aagaard P, Kjaer M. The adaptability of tendon to loading differs in men and women. International Journal of Experimental Pathology. 2007;88:237–240. doi: 10.1111/j.1365-2613.2007.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson SP, Kjaer M. Region-specific differences in Achilles tendon cross-sectional area in runners and non-runners. European Journal of Applied Physiology. 2003;90:549–553. doi: 10.1007/s00421-003-0865-8. [DOI] [PubMed] [Google Scholar]
- Micheli LJ, Fehlandt A. Overuse injuries to tendons and apophyses in children and adolescents. Clinics in Sports Medicine. 1992;11:713–726. [PubMed] [Google Scholar]
- Micheli LJ, Fehlandt AF. Overuse tendon injuries in pediatric sports medicine. Sports Medicine and Arthroscopy Review. 1996;4:190–195. [Google Scholar]
- Philippaerts RM, Vaeyens R, Janssens M, Van Renterghem B, Matthys D, Craen R, Bourgois J, Vrijens J, Beunen G, Malina RM. The relationship between peak height velocity and physical performance in youth soccer players. Journal of Sports Science. 2006;24:221–230. doi: 10.1080/02640410500189371. [DOI] [PubMed] [Google Scholar]
- Rauch F, Bailey DA, Baxter-Jones A, Mirwald R, Faulkner R. The ‘muscle-bone unit’ during the pubertal growth spurt. Bone. 2004;34:771–775. doi: 10.1016/j.bone.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Rosager S, Aagaard P, Dyhre-Poulsen P, Neergaard K, Kjaer M, Magnusson SP. Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scandanavian Journal of Medicine & Science in Sports. 2002;12:90–98. doi: 10.1034/j.1600-0838.2002.120205.x. [DOI] [PubMed] [Google Scholar]
- Scharfbillig RW, Jones S, Scutter S. Sever’s disease: What does the literature really tell us? Journal of American Podiatric Medical Association. 2008;98:212–223. doi: 10.7547/0980212. [DOI] [PubMed] [Google Scholar]
- Shin D, Finni T, Ahn S, Hodgson J, Lee HD, Edgerton VR, Sinha S. In Vivo Estimation and Repeatability of Force–Length Relationship and Stiffness of the Human Achilles Tendon Using Phase Contrast MRI. Journal of Magnetic Resonance Imaging. 2008;28:1039–1045. doi: 10.1002/jmri.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh CM, Blazevich AJ, Fath F, Korff T. Age-related changes in mechanical properties of the Achilles tendon. Journal of Anatomy. 2012;220:144–155. doi: 10.1111/j.1469-7580.2011.01461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]