Abstract
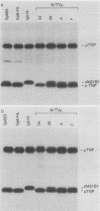
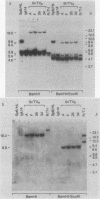
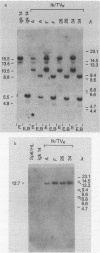
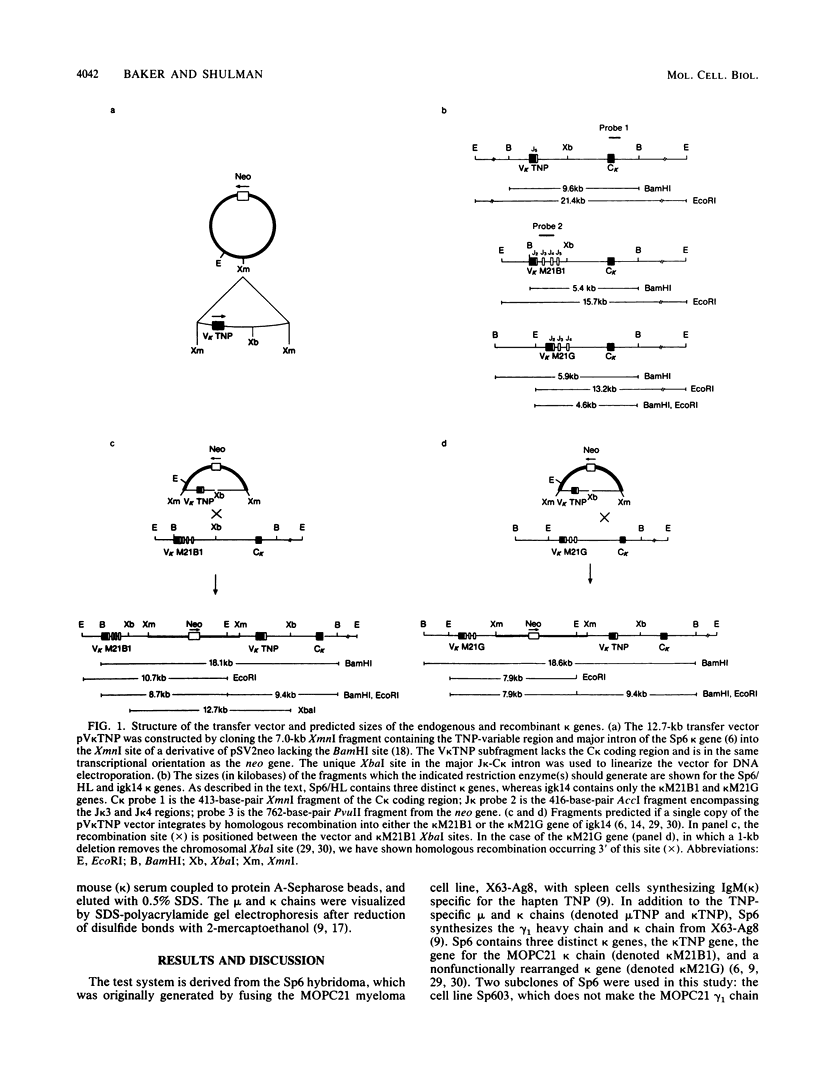
Homologous recombination between transferred and chromosomal DNAs provides a means of introducing well-defined, predetermined changes in the chromosomal genes. Here we report that this approach can be used to specifically modify the immunoglobulin genes in mouse hybridoma cells. The test system is based on the Sp6 hybridoma, which synthesizes immunoglobulin M (kappa) specific for the hapten 2,4,6-trinitrophenyl (TNP). As recipient cells, we used the Sp6-derived mutant hybridoma igk14, which has a deletion of the kappa TNP gene and consequently does not synthesize TNP-specific immunoglobulin M. igk14 retains the mu TNP gene and two additional rearranged kappa genes, denoted kappa M21B1 and kappa M21G. As a transfer vector, we used pSV2neo bearing the functionally rearranged TNP-specific V kappa segment. Following DNA transfer by electroporation, we isolated rare transformants which produced normal amounts of the functional kappa TNP chain. Analysis of the DNA of these transformants indicated that in all cases, a functional kappa TNP gene had been formed as the result of a homologous integrative recombination event with the igk14 kappa M21B1 gene. These results suggest that homologous recombination might be used for mapping and introducing immunoglobulin gene mutations and for more conveniently engineering specifically altered immunoglobulins.
Full text
PDF

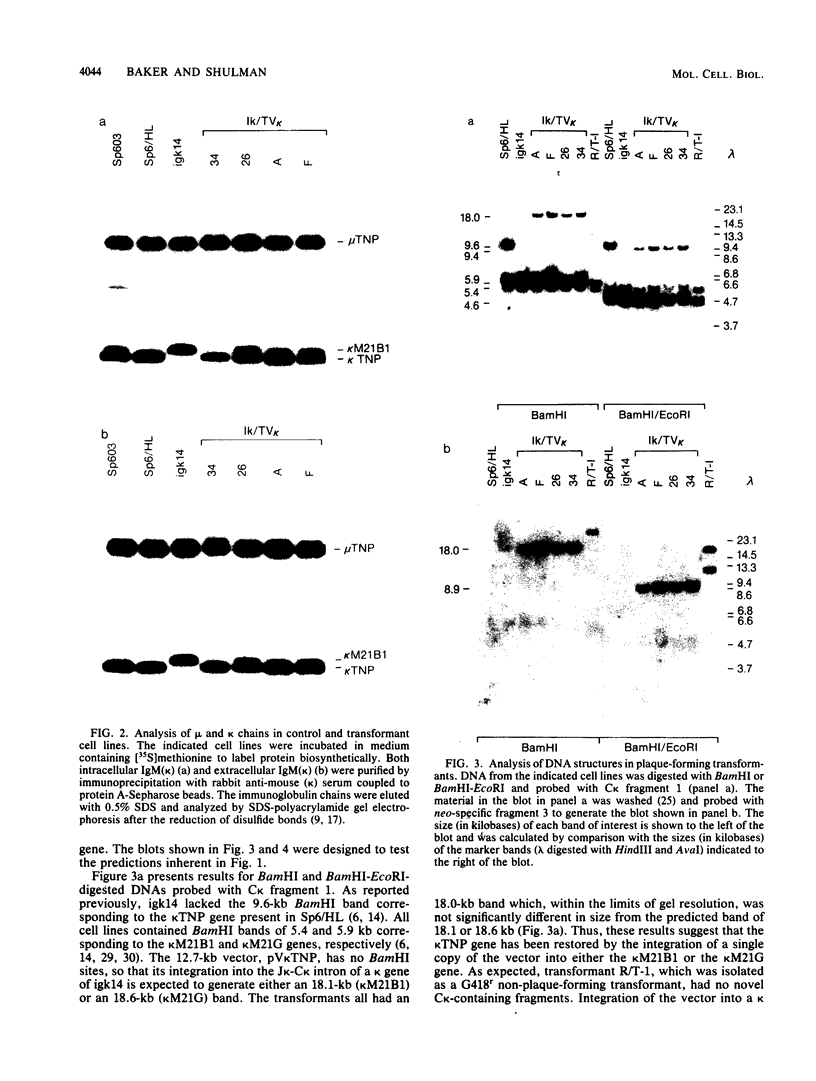



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulianne G. L., Hozumi N., Shulman M. J. Production of functional chimaeric mouse/human antibody. Nature. 1984 Dec 13;312(5995):643–646. doi: 10.1038/312643a0. [DOI] [PubMed] [Google Scholar]
- Boulianne G. L., Isenman D. E., Hozumi N., Shulman M. J. Biological properties of chimeric antibodies. Interaction with complement. Mol Biol Med. 1987 Feb;4(1):37–49. [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Doetschman T., Gregg R. G., Maeda N., Hooper M. L., Melton D. W., Thompson S., Smithies O. Targetted correction of a mutant HPRT gene in mouse embryonic stem cells. Nature. 1987 Dec 10;330(6148):576–578. doi: 10.1038/330576a0. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Hawley R. G., Shulman M. J., Murialdo H., Gibson D. M., Hozumi N. Mutant immunoglobulin genes have repetitive DNA elements inserted into their intervening sequences. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7425–7429. doi: 10.1073/pnas.79.23.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K., Henry C., Nordin A. A., Fuji H., Koros A. M., Lefkovits I. Plaque forming cells: methodology and theory. Transplant Rev. 1974;18:130–191. doi: 10.1111/j.1600-065x.1974.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Köhler G., Potash M. J., Lehrach H., Shulman M. J. Deletions in immunoglobulin mu chains. EMBO J. 1982;1(5):555–563. doi: 10.1002/j.1460-2075.1982.tb01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. L., Sperle K., Sternberg N. Model for homologous recombination during transfer of DNA into mouse L cells: role for DNA ends in the recombination process. Mol Cell Biol. 1984 Jun;4(6):1020–1034. doi: 10.1128/mcb.4.6.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. A., Nussbaum S. R., Eisen H. N. Hormone conjugated with antibody to CD3 mediates cytotoxic T cell lysis of human melanoma cells. Science. 1988 Jan 22;239(4838):395–398. doi: 10.1126/science.3257303. [DOI] [PubMed] [Google Scholar]
- Morrison S. L., Johnson M. J., Herzenberg L. A., Oi V. T. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6851–6855. doi: 10.1073/pnas.81.21.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger M. S., Williams G. T., Fox R. O. Recombinant antibodies possessing novel effector functions. Nature. 1984 Dec 13;312(5995):604–608. doi: 10.1038/312604a0. [DOI] [PubMed] [Google Scholar]
- Ochi A., Hawley R. G., Shulman M. J., Hozumi N. Transfer of a cloned immunoglobulin light-chain gene to mutant hybridoma cells restores specific antibody production. Nature. 1983 Mar 24;302(5906):340–342. doi: 10.1038/302340a0. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Morrison S. L., Herzenberg L. A., Berg P. Immunoglobulin gene expression in transformed lymphoid cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):825–829. doi: 10.1073/pnas.80.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack S. J., Jacobs J. W., Schultz P. G. Selective chemical catalysis by an antibody. Science. 1986 Dec 19;234(4783):1570–1573. doi: 10.1126/science.3787262. [DOI] [PubMed] [Google Scholar]
- Shulman M. J., Heusser C., Filkin C., Köhler G. Mutations affecting the structure and function of immunoglobulin M. Mol Cell Biol. 1982 Sep;2(9):1033–1043. doi: 10.1128/mcb.2.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M. J., Pennell N., Collins C., Hozumi N. Activation of complement by immunoglobulin M is impaired by the substitution serine-406----asparagine in the immunoglobulin mu heavy chain. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7678–7682. doi: 10.1073/pnas.83.20.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithies O., Gregg R. G., Boggs S. S., Koralewski M. A., Kucherlapati R. S. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985 Sep 19;317(6034):230–234. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- Song K. Y., Schwartz F., Maeda N., Smithies O., Kucherlapati R. Accurate modification of a chromosomal plasmid by homologous recombination in human cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6820–6824. doi: 10.1073/pnas.84.19.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Folger K. R., Capecchi M. R. High frequency targeting of genes to specific sites in the mammalian genome. Cell. 1986 Feb 14;44(3):419–428. doi: 10.1016/0092-8674(86)90463-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontano A., Janda K. D., Lerner R. A. Catalytic antibodies. Science. 1986 Dec 19;234(4783):1566–1570. doi: 10.1126/science.3787261. [DOI] [PubMed] [Google Scholar]
- Trimble W. S., Baker M. D., Boulianne G. L., Murialdo H., Hozumi N., Shulman M. J. Analysis of hybridoma mutants defective in synthesis of immunoglobulin M. Somat Cell Mol Genet. 1986 Sep;12(5):467–477. doi: 10.1007/BF01539918. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Fulton R. J., May R. D., Till M., Uhr J. W. Redesigning nature's poisons to create anti-tumor reagents. Science. 1987 Nov 20;238(4830):1098–1104. doi: 10.1126/science.3317828. [DOI] [PubMed] [Google Scholar]
- Walfield A. M., Storb U., Selsing E., Zentgraf H. Comparison of different rearranged immunoglobulin kappa genes of a myeloma by electronmicroscopy and restriction mapping of cloned DNA: implications for "allelic exclusion". Nucleic Acids Res. 1980 Oct 24;8(20):4689–4707. doi: 10.1093/nar/8.20.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walfield A., Selsing E., Arp B., Storb U. Misalignment of V and J gene segments resulting in a nonfunctional immunoglobulin gene. Nucleic Acids Res. 1981 Mar 11;9(5):1101–1109. doi: 10.1093/nar/9.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]