Abstract
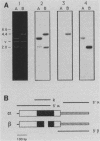
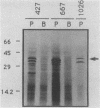
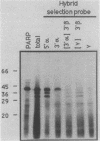
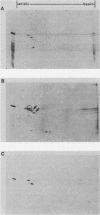
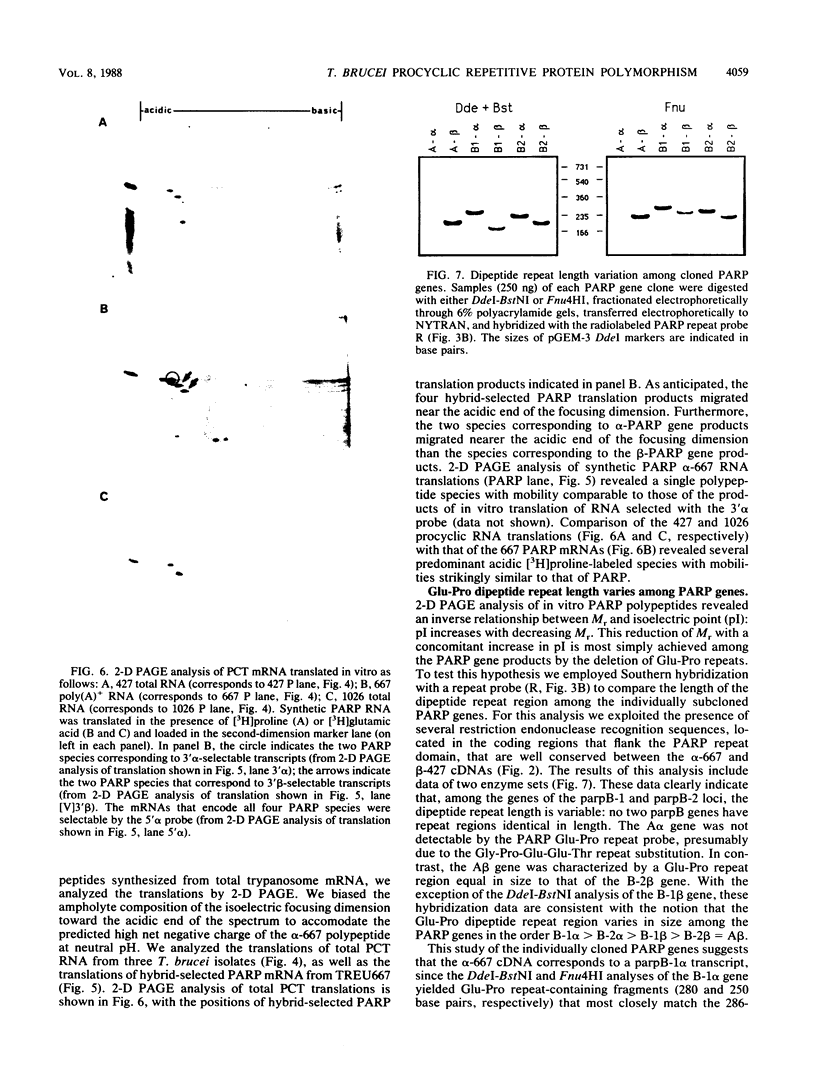
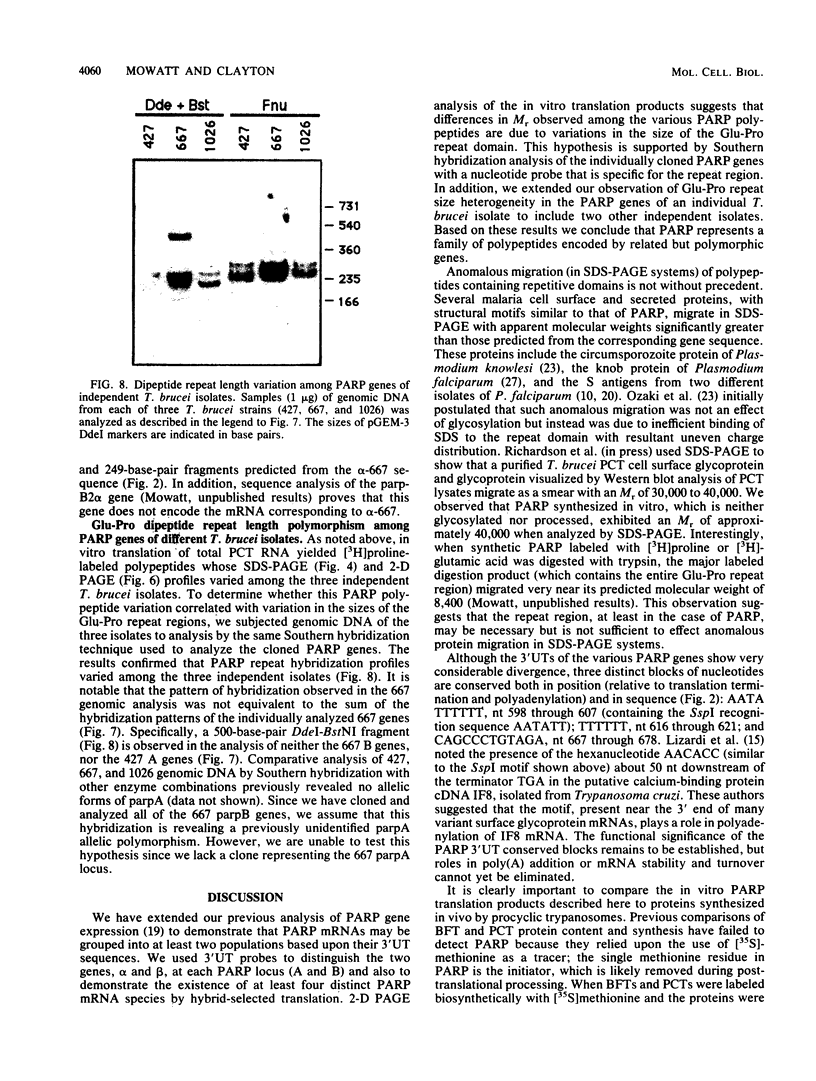
The expression of procyclic acidic repetitive protein (PARP) by Trypanosoma brucei is strongly induced during the transition of bloodstream form to cultured procyclic trypomastigotes in vitro. The membrane-associated protein is distinguished by a central domain consisting of tandemly repeated glutamate-proline dipeptides. The trypanosome genome contains eight PARP genes, at least four of which are expressed. A minimum of four distinct PARP mRNA species comprises two classes of PARP mRNA, based upon divergent 3' untranslated region sequences, and these mRNAs encode polypeptides that exhibited an inverse relation between molecular weight and isoelectric point. Comparative analysis of PARP gene structure indicated that these polypeptides differ by variation in size of the dipeptide repeat domain. Comparison of PARP genes and polypeptides of three independent T. brucei isolates suggested that PARP is not a homogeneous species but instead represents a family of polymorphic proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. L., Parish N. M., Richardson J. P., Pearson T. W. Comparison of African trypanosomes of different antigenic phenotypes, subspecies and life cycle stages by two-dimensional gel electrophoresis. Mol Biochem Parasitol. 1985 Sep;16(3):299–314. doi: 10.1016/0166-6851(85)90072-6. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Gene conversion: some implications for immunoglobulin genes. Cell. 1981 Jun;24(3):592–594. doi: 10.1016/0092-8674(81)90082-9. [DOI] [PubMed] [Google Scholar]
- Barry J. D., Vickerman K. Trypanosoma brucei: loss of variable antigens during transformation from bloodstream to procyclic forms in vitro. Exp Parasitol. 1979 Oct;48(2):313–324. doi: 10.1016/0014-4894(79)90114-0. [DOI] [PubMed] [Google Scholar]
- Bienen E. J., Hammadi E., Hill G. C. Trypanosoma brucei: biochemical and morphological changes during in vitro transformation of bloodstream- to procyclic-trypomastigotes. Exp Parasitol. 1981 Jun;51(3):408–417. doi: 10.1016/0014-4894(81)90128-4. [DOI] [PubMed] [Google Scholar]
- Bienen E. J., Hill G. C., Shin K. O. Elaboration of mitochondrial function during Trypanosoma brucei differentiation. Mol Biochem Parasitol. 1983 Jan;7(1):75–86. doi: 10.1016/0166-6851(83)90118-4. [DOI] [PubMed] [Google Scholar]
- Borst P. Discontinuous transcription and antigenic variation in trypanosomes. Annu Rev Biochem. 1986;55:701–732. doi: 10.1146/annurev.bi.55.070186.003413. [DOI] [PubMed] [Google Scholar]
- Brown R. C., Evans D. A., Vickerman K. Changes in oxidative metabolism and ultrastructure accompanying differentiation of the mitochondrion in Trypanosoma brucei. Int J Parasitol. 1973 Sep;3(5):691–704. doi: 10.1016/0020-7519(73)90095-7. [DOI] [PubMed] [Google Scholar]
- Clayton C. E. Import of fructose bisphosphate aldolase into the glycosomes of Trypanosoma brucei. J Cell Biol. 1987 Dec;105(6 Pt 1):2649–2654. doi: 10.1083/jcb.105.6.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman A. F., Saint R. B., Coppel R. L., Brown G. V., Anders R. F., Kemp D. J. Conserved sequences flank variable tandem repeats in two S-antigen genes of Plasmodium falciparum. Cell. 1985 Apr;40(4):775–783. doi: 10.1016/0092-8674(85)90337-x. [DOI] [PubMed] [Google Scholar]
- Evans D. A., Ellis D. S. Recent observations on the behaviour of certain trypanosomes within their insect hosts. Adv Parasitol. 1983;22:1–42. doi: 10.1016/s0065-308x(08)60460-1. [DOI] [PubMed] [Google Scholar]
- Feagin J. E., Stuart K. Developmental aspects of uridine addition within mitochondrial transcripts of Trypanosoma brucei. Mol Cell Biol. 1988 Mar;8(3):1259–1265. doi: 10.1128/mcb.8.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagin J. E., Stuart K. Differential expression of mitochondrial genes between life cycle stages of Trypanosoma brucei. Proc Natl Acad Sci U S A. 1985 May;82(10):3380–3384. doi: 10.1073/pnas.82.10.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelotti E. F., Hajduk S. L. Developmental regulation of trypanosome mitochondrial gene expression. J Biol Chem. 1987 Jan 15;262(2):927–932. [PubMed] [Google Scholar]
- Mowatt M. R., Clayton C. E. Developmental regulation of a novel repetitive protein of Trypanosoma brucei. Mol Cell Biol. 1987 Aug;7(8):2838–2844. doi: 10.1128/mcb.7.8.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls S. C., Hillman Y., Lockyer M. J., Odink K. G., Holder A. A. An S antigen gene from Plasmodium falciparum contains a novel repetitive sequence. Mol Biochem Parasitol. 1988 Feb;28(1):11–19. doi: 10.1016/0166-6851(88)90174-0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Overath P., Czichos J., Stock U., Nonnengaesser C. Repression of glycoprotein synthesis and release of surface coat during transformation of Trypanosoma brucei. EMBO J. 1983;2(10):1721–1728. doi: 10.1002/j.1460-2075.1983.tb01648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki L. S., Svec P., Nussenzweig R. S., Nussenzweig V., Godson G. N. Structure of the plasmodium knowlesi gene coding for the circumsporozoite protein. Cell. 1983 Oct;34(3):815–822. doi: 10.1016/0092-8674(83)90538-x. [DOI] [PubMed] [Google Scholar]
- Roditi I., Carrington M., Turner M. Expression of a polypeptide containing a dipeptide repeat is confined to the insect stage of Trypanosoma brucei. Nature. 1987 Jan 15;325(6101):272–274. doi: 10.1038/325272a0. [DOI] [PubMed] [Google Scholar]
- Shapiro S. Z., Kimmel B. E. Differential protein synthesis during the life cycle of the protozoan parasite Trypanosoma brucei. J Protozool. 1987 Feb;34(1):58–62. doi: 10.1111/j.1550-7408.1987.tb03132.x. [DOI] [PubMed] [Google Scholar]
- Sharma Y. D., Kilejian A. Structure of the knob protein (KP) gene of Plasmodium falciparum. Mol Biochem Parasitol. 1987 Nov;26(1-2):11–16. doi: 10.1016/0166-6851(87)90124-1. [DOI] [PubMed] [Google Scholar]
- Simpson A. M., Hughes D., Simpson L. Trypanosoma brucei: differentiation of in vitro-grown bloodstream trypomastigotes into procyclic forms. J Protozool. 1985 Nov;32(4):672–677. doi: 10.1111/j.1550-7408.1985.tb03100.x. [DOI] [PubMed] [Google Scholar]