Abstract
Background. Two antigenically distinct influenza B lineages have cocirculated since 2001, yet trivalent influenza vaccines (TIVs) contain 1 influenza B antigen, meaning lineage mismatch with the vaccine is frequent. We assessed a candidate inactivated quadrivalent influenza vaccine (QIV) containing both B lineages vs TIV in healthy children aged 3–17 years.
Methods. Children were randomized 1:1:1 to receive QIV or 1 of 2 TIVs (either B/Victoria or B/Yamagata lineage; N = 2738). Hemagglutination-inhibition assays were performed 28 days after 1 or 2 doses in primed and unprimed children, respectively. Immunological noninferiority of QIV vs TIV against shared strains, and superiority against alternate-lineage B strains was based on geometric mean titers (GMTs) and seroconversion rates. Reactogenicity and safety were also assessed (Clinicaltrials.gov NCT01196988).
Results. Noninferiority against shared strains and superiority against alternate-lineage B strains was demonstrated for QIV vs TIV. QIV was highly immunogenic; seroconversion rates were 91.4%, 72.3%, 70.0%, and 72.5% against A/H1N1, A/H3N2, B/Victoria, and B/Yamagata, respectively. Reactogenicity and safety of QIV was consistent with TIV.
Conclusions. QIV vs TIV showed superior immunogenicity for the additional B strain without interfering with immune responses to shared strains. QIV may offer improved protection against influenza B in children compared with current trivalent vaccines.
Keywords: pediatric, noninferiority, quadrivalent, seasonal influenza, superiority, trivalent
Children are at increased risk of influenza infection compared with the general population, and influenza is associated with relatively high rates of serious illness in children of preschool age [1–5]. Among influenza virus types, influenza A is generally considered to represent the greatest public health concern, yet the burden of influenza B in children is substantial. Influenza-related hospitalizations and complications such as myositosis are more common in children infected with influenza B than with influenza A [6–8].
Annual influenza vaccination is the most effective method for preventing influenza and associated complications, and trivalent influenza vaccines include 2 influenza A strains (A/H1N1, A/H3N2) and 1 influenza B strain, selected using surveillance-based forecasts [9]. Two antigenically distinct influenza B lineages (B/Yamagata and B/Victoria) emerged globally in humans in the early 1980s and have co-circulated in the United States since 2001[10]. However, trivalent influenza vaccines provide limited or no cross-reactive protection between the 2 influenza B lineages [11]. Moreover, in the United States, the seasonal trivalent vaccine was mismatched for the circulating influenza B lineage in 5 of 10 influenza seasons between 2001 and 2010 and in Europe was mismatched in 4 of 8 seasons between 2003 and 2010 [12–14]. Given the burden of influenza B infection and the rate of B-lineage mismatch with trivalent vaccines, in February 2012, the World Health Organization (WHO) recommended B strains from both lineages for inclusion in quadrivalent vaccines for use in the 2012/2013 season in the Northern Hemisphere [15].
Here we report a Phase III, randomized, double-blind study of a candidate inactivated quadrivalent split viron influenza vaccine (QIV) containing both B lineages compared with 2 inactivated trivalent influenza vaccines (TIV) in children aged 3–17 years. The purpose of the study was to test the immunologic noninferiority of QIV vs TIV against shared influenza A and B strains, and superiority against influenza B of QIV vs TIVs containing an alternate-lineage B strain. QIV was also assessed in children aged 6–35 months as a separate open-label group.
METHODS
Design and Subjects
This phase III, double-blind, randomized, multicenter study was conducted to assess the immunogenicity, reactogenicity, and safety of QIV vs TIV in children aged 3–17 years. There was also an open-label group to assess QIV in children aged 6–35 months. The study was conducted in the Czech Republic, France, Germany, the Philippines, and the United States (Clinicaltrials.gov NCT00287469).
Children were eligible for inclusion if they were aged 6 months to 17 years and were in stable health; children with chronic illness were eligible for inclusion unless there was evidence of significant pulmonary, cardiovascular, hepatic, or renal functional abnormalities. Children were excluded if they had received any registered or investigational seasonal influenza vaccination within 6 months or any investigational product within 30 days preceding the first study vaccine dose. Other exclusion criteria were history of Guillain-Barré syndrome within 6 weeks of a previous TIV, hypersensitivity to influenza vaccine or its components, immunosuppressed, and receipt of immunoglobulins or blood products within 3 months preceding vaccination.
Children who were considered “primed” received 1 dose of candidate or control vaccine, and those considered “unprimed” received 2 doses of candidate or control vaccine given 28 days apart; priming status was based on US Advisory Committee on Immunization Practices recommendations [16]. Children aged <9 years were considered primed if they had received at least 1 dose of an A/H1N1 2009 monovalent vaccine or had a laboratory-confirmed A/H1N1 2009 infection during the previous influenza season, and had also received 2 doses of a trivalent seasonal vaccine (at least 1 month apart) during the previous influenza season, or at least 1 trivalent seasonal vaccine dose prior to the previous influenza season. Children aged <9 years who did not fulfill the definition of primed were considered to be unprimed.
Written informed consent was obtained from parents/guardians of all children. The study was conducted in accordance with the Good Clinical Practice guidelines, the Declaration of Helsinki, and applicable local regulations. All study documents were approved by the appropriate Institutional Review Board.
Vaccines, Randomization, and Blinding
The QIV candidate contained influenza A/H1N1 (A/California/7/2009) and A/H3N2 (A/Victoria/210/2009) and B/Brisbane/60/2008 (B/Victoria lineage) as recommended by WHO for the 2010/2011 season in the Northern Hemisphere, and B/Brisbane/3/2007 (B/Yamagata lineage), which had been recommended for the 2008/2009 season. The TIVs contained the same influenza A strains as the QIV candidate, and either B/Brisbane/60/2008 (B/Victoria lineage) [Fluarix™] or B/Brisbane/3/2007 (B/Yamagata lineage). The inactivated split virion vaccines were thimerosal-free, contained 15 µg of each hemagglutinin antigen, and were manufactured by GlaxoSmithKline Vaccines in Dresden, Germany.
A randomization list was generated by GlaxoSmithKline Vaccines, Wavre, Belgium. Randomization in children aged 3–17 years was performed in a 1:1:1 ratio to QIV, TIV-B/Victoria (TIV-Vic), or TIV-B/Yamagata (TIV-Yam), and treatment allocation at each study site was performed using an internet-based system that balanced groups by age strata (3–8 years and 9–17 years). The randomization algorithm used a minimization procedure accounting for country, center, and previous receipt of influenza vaccine (priming status).
All vaccines were presented as colorless and slightly opalescent suspensions in prefilled glass syringes containing 1 dose (0.5 mL); in the randomized study, all participants/participants’ parents/guardians, investigators, and study personnel were blinded as to vaccine allocation. In the open-label group, vaccines were labeled as to contents. Serological data were not available during to investigators or study personnel; laboratory testing was blinded to the vaccine allocation, and codes linked subjects to each sample. Vaccines were administered intramuscularly in the anterolateral thigh (subjects aged <12 months) or in the deltoid.
Immunogenicity
Blood samples were collected before vaccination (day 0) and 28 days after the final vaccine dose, that is, day 28 in primed children who received 1 dose, and day 56 in unprimed children who received 2 doses. Antibody titers were assessed using hemagglutination-inhibition (HI) assay (cutoff titer ≥1:10), performed at GlaxoSmithKline Vaccines’ laboratory using standardized procedures [17].
Immunogenicity parameters calculated were geometric mean titer (GMT), seroprotection rate (SPR; proportion with postvaccination titer ≥1:40), seroconversion rate (SCR; proportion with antibody titer <1:10 at baseline and with postvaccination titer of ≥1:40, or prevaccination titer of ≥1:10 and a ≥4-fold post-vaccination increase in titer), and seroconversion factor (SCF; geometric mean of the ratio between prevaccination and postvaccination reciprocal HI titers). Subjects with HI antibody titers of ≥1:10 were considered to be seropositive.
Reactogenicity and Safety
Reactogenicity and safety was assessed in the randomized study (aged 3–17 years) and the open-label group (aged 6–35 months). The parents/guardians of subjects used diary cards to record solicited injection-site and general adverse events (AEs) for 7 days following vaccination. Injection-site AEs were pain, redness, and swelling, and general AEs were fever, irritability/fussiness, drowsiness, and loss of appetite (aged ≤5 years) or fever, headache, fatigue, gastrointestinal symptoms, joint pain, muscle aches, and shivering (aged >5 years). Fever was defined as an oral/axillary temperature ≥37.5°C or a rectal temperature ≥38°C. Intensity of solicited symptoms was graded (0–3); grade 1 symptoms were defined as not interfering with normal activities, and grade 3 symptoms were defined as preventing normal activities (grade 3 redness and swelling: diameter >50 mm; grade 3 fever: temperature >39°C).
Unsolicited AEs were recorded for 28 days after each vaccination, and serious adverse events (SAEs) and medically attended adverse events (MAEs) were recorded for 6 months postvaccination. Unsolicited AEs were coded using Medical Dictionary for Regulatory Activities. All solicited injection site symptoms were considered vaccination-related, and investigators provided causality assessments for solicited general AEs and unsolicited AEs.
Objectives
In the randomized study of children aged 3–17 years, the primary confirmatory objective was to evaluate the noninferiority of GMTs and SCRs 28 days after the final vaccine dose of vaccine for QIV vs TIV-Vic and TIV-Yam pooled against influenza A strains, and QIV vs TIV-Vic against B/Victoria, and QIV vs TIV-Yam against B/Yamagata (ie, shared strains). The secondary confirmatory objective was to evaluate the superiority of GMTs and SCRs 28 days after the last vaccine dose for QIV vs TIV-Vic against B/Yamagata, and QIV vs TIV-Yam against B/Victoria (ie, alternate-lineage B strains). Further secondary objectives were to describe GMTs, SCRs, SPRs, and SCFs 28 days after the final vaccine dose of each vaccine and to describe reactogenicity and safety of each vaccine.
The objective of the open-label group was to describe immunogenicity parameters, and reactogenicity and safety after vaccination with QIV in children aged 6–35 months.
Statistical Analyses
A sample size of 2700 children aged 3–17 years was estimated to provide 750 evaluable children per group, resulting in an overall power of >90% to demonstrate the primary objective of noninferiority of QIV vs TIV for shared strains, estimating using PASS, 1-sided 2-sample t-test for a difference of means for GMT ratio and a 1-sided t-test in the difference of proportions for SCRs (both 1-sided α = 2.5%). The target in the open-label group was 300 children aged 6–35 months to provide 255 evaluable children.
Adjusted GMTs were estimated using an ANCOVA model fitted on log10 transformed postvaccination HI titer including treatment as fixed effect and baseline titer as a covariate. The SCR difference and the 2-sided 95% CI of the SCR differences were computed after fitting a logistic regression on the SCR, including vaccine group as a fixed effect and baseline titer as a covariate. The confirmatory noninferiority and superiority objectives were analyzed sequentially: (1) Noninferior immunogenicity of QIV vs TIV for shared strains was demonstrated if the upper limit of the 2-sided 95% CI for the adjusted GMT ratio of TIV/QIV did not exceed 1.5, and the upper limit of the 2-sided 95% CI for the SCR difference (TIV minus QIV) did not exceed 10.0%; (2) Superior immunogenicity of QIV vs TIV for the alternate-lineage B strain was demonstrated if the lower limit of the 2-sided 95% CI on the adjusted GMT ratio (QIV/TIV-Vic and QIV/TIV-Yam) was >1.0, and the lower limit of the 2-sided 95% CI for the SCR difference (QIV minus TIV-Vic or TIV-Yam) was >0.0%.
In the randomized study, immunogenicity parameters were described by vaccine group, and GMTs were also described stratified by age (3–8 and 9–17 years) and priming status. In the open-label group, immunogenicity parameters were described after QIV vaccination. Immunogenicity outcomes were tabulated with 95% CIs. Immunogenicity analyses were performed on the per-protocol immunogenicity cohort including children who met the eligibility criteria, complied with the protocol, and for whom data were available at the evaluation time point.
Solicited and unsolicited AEs were tabulated with 95% CIs. Reactogenicity and safety analyses were performed on the total vaccinated cohort.
RESULTS
A total of 3027 children were enrolled, of which 915, 912, and 911 received double-blind QIV, TIV-Vic, or TIV-Yam, respectively, and 277 received open-label QIV; a total of 2933 children completed the study (Figure 1). The first child was enrolled on 4 October 2010, and the last study contact was on 15 June 2011. Demographics were balanced between the groups in the randomized study (Table 1). A review of the reported medical history revealed that a total of 160 children (5.3%) had chronic conditions, including asthma (n = 134), cardiovascular disease (n = 11), kidney disease (n = 4), Type I diabetes (n = 3), hematological disease (n = 5), and congenital syndromes (n = 3).
Figure 1.
Subject flow. Abbreviations: QIV, inactivated quadrivalent influenza vaccine; TIV-Vic, inactivated trivalent influenza vaccine Victoria lineage B strain; TIV-Yam, inactivated trivalent influenza vaccine Yamagata lineage B strain.
Table 1.
Demographic Characteristics in Children Aged 3–17 Years and Aged 6–35 Months in the Total Vaccinated Cohort
| 3–17 y |
6–35 mo |
|||
|---|---|---|---|---|
| QIV (N = 915) | TIV-Vic (N = 912) | TIV-Yam (N = 911) | QIV (N = 277) | |
| Mean age in months (SD; median; range) | 98.5 (44.40; 90.0; 36–215) | 98.2 (45.50; 87.0; 36–215) | 99.6 (44.20; 90.0; 35–213) | 22.1 (8.02; 23.0; 6–35) |
| Mean age in years (SD; median; range) | 7.8 (3.69; 7.0; 3–17) | 7.8 (3.78; 7.0; 3–17) | 7.8 (3.69; 7.0; 2–17) | 1.4 (0.71; 1.0; 0–2) |
| Male, n (%) | 472 (51.6) | 473 (51.9) | 471 (51.7) | 159 (57.4) |
| Female, n (%) | 443 (48.4) | 439 (48.1) | 440 (48.3) | 118 (42.6) |
| Age strata, n | ||||
| 3–8 y | 598 | 596 | 597 | – |
| 9–17 y | 317 | 316 | 313 | – |
| Priming status in children aged ≤8 y, n | ||||
| Primed | 89 | 89 | 88 | 15 |
| Unprimed | 509 | 507 | 509 | 262 |
| American Hispanic/Latino ethnicity, n (%) | 90 (9.8) | 85 (9.3) | 90 (9.9) | 0 (0.0) |
| Not American Hispanic/Latino ethnicity, n (%) | 825 (90.2) | 827 (90.7) | 821 (90.1) | 277 (100) |
| Heritage/race, n (%) | ||||
| European heritage/ Caucasian | 493 (53.9) | 478 (52.4) | 486 (53.3) | 193 (69.7) |
| African heritage/African American | 113 (12.3) | 121 (13.3) | 109 (12.0) | 6 (2.2) |
| Asian/Southeast heritage | 263 (28.7) | 265 (29.1) | 261 (28.6) | 56 (20.2) |
| Other | 46 (5.0) | 48 (5.2) | 55 (6.0) | 22 (7.6) |
Abbreviations: QIV, quadrivalent inactivated influenza vaccine; SD, standard deviation; TIV-Vic, trivalent inactivated influenza vaccine Victoria lineage B strain; TIV-Yam, trivalent inactivated vaccine Yamagata lineage B strain.
Immunogenicity
Confirmatory Analyses
The primary objective of noninferior HI antibody responses of QIV vs TIV for shared vaccine strains was demonstrated based on adjusted GMT ratio and SCR difference 28 days after the final dose of vaccine. For QIV vs TIV-Vic and TIV-Yam (pooled), the upper limits of the 95% CIs for the adjusted GMT ratio and SCR difference against A/H1N1 were 1.15% and 1.86%, respectively, and against A/H3N2 were 1.05% and 2.86%, respectively; the upper limits of the 95% CIs for the adjusted GMT ratio and SCR difference for QIV vs TIV-Vic against B/Victoria were 1.09% and 2.98%, respectively, and vs TIV-Yam against B/Yamagata were 1.18% and 2.65%, respectively.
HI antibody responses against alternate-lineage B strains were superior for QIV vs each TIV. The lower limits of the 95% CIs for the adjusted GMT and SCR difference for QIV vs TIV-Vic against B/Yamagata were 2.36% and 30.87%, respectively, and for QIV vs TIV-Yam for B/Victoria were 2.63% and 35.78%, respectively.
Descriptive Analyses
Each of the vaccines elicited strong immune responses against respective vaccine strains in children aged 3–17 years (Table 2). At 28 days after last vaccination in children aged 3–17 years in the QIV group, SPRs against A/H1N1 and A/H3N2 were 96.6% and 98.0%, respectively, and against B/Victoria and B/Yamagata were 97.3% and 99.2%, respectively. In the TIV groups, SPRs against A/H1N1 and A/H3N2 were 96.9%–97.1% and 96.5%–97.8%, respectively, and against B/Victoria were 96.6% (matched) and 79.8% (alternate-lineage), and against B/Yamagata were 99.6% (matched) and 94.4% (alternate-lineage). QIV elicited more than 2-fold higher mean HI antibody responses vs each TIV for the influenza B strain from the alternate lineage, which translated into an absolute SCR difference of at least 35.0%. The observed postvaccination GMTs against the vaccine strains in children aged 3–8 years were similar to those in children aged 9–17 years regardless of priming status, apart from the GMT for the B/Yamagata antigen, where children 9–17 years of age had marginally higher pre- and postvaccination titers (Figure 2). QIV was also immunogenic in children aged 6–35 months, although GMTs were lower than those observed in the older group; SPRs were ≥71.4%, SCRs were ≥68.1%, and SCFs were ≥9.7 (Table 2). There appeared to be no major differences in immune responses by sex or by priming status in the QIV and TIV groups.
Table 2.
Descriptive Immunogenicity Based on HI Antibody Titers in the per Protocol Cohort for Immunogenicity
| N | Vaccine strain |
||||||
|---|---|---|---|---|---|---|---|
| A/California/7/2009 (H1N1) | A/Victoria/210/2009 (H3N2) | B/Brisbane/60/2008 (Victoria) | B/Brisbane/3/2007 (Yamagata) | ||||
| GMT, Value (95% CI) | QIV | Pre | 790 | 21.6 (19.7–23.7) | 29.0 (26.6–31.6) | 30.9 (28.2–33.9) | 77.3 (70.0–85.3) |
| Post | 791 | 386.2 (357.3–417.4) | 228.8 (215.0–243.4) | 244.2 (227.5–62.1) | 569.6 (533.6–608.1) | ||
| TIV-Vic | Pre | 819 | 24.9 (22.8–27.3) | 31.4 (28.8–34.2) | 31.0 (28.2–34.0) | 77.2 (70.0–85.2) | |
| Post | 818 | 433.2 (401.0–468.0) | 227.3 (213.3–242.3) | 245.6 (229.2–263.2) | 224.7 (207.9–242.9) | ||
| TIV-Yam | Pre | 800 | 22.1 (20.1–24.2) | 31.2 (28.6–34.2) | 33.2 (30.2–36.6) | 84.7 (76.6–93.6) | |
| Post | 801 | 422.3 (390.5–456.5) | 234.0 (219.1–249.9) | 88.4 (81.5–95.8) | 643.3 (603.2–686.1) | ||
| QIV (6–35 m) | Pre | 232 | 12.3 (10.2–14.8) | 8.6 (7.4–9.9) | 9.0 (7.9–10.4) | 13.1 (11.4–15.2) | |
| Post | 234 | 140.0 (113.7–172.3) | 87.5 (73.8–103.7) | 86.4 (72.6–102.9) | 167.7 (144.1–195.3) | ||
| SPR, % (95% CI) | QIV | Pre | 790 | 43.4 (39.9–47.0) | 48.2 (44.7–51.8) | 48.2 (44.7–51.8) | 71.5 (68.2–74.6) |
| Post | 791 | 96.6 (95.1–97.7) | 98.0 (96.7–98.8) | 97.3 (96.0–98.3) | 99.2 (98.4–99.7) | ||
| TIV-Vic | Pre | 819 | 49.3 (45.9–52.8) | 50.3 (46.8–53.8) | 48.4 (44.9–51.8) | 70.2 (66.9–73.3) | |
| Post | 818 | 96.9 (95.5–98.0) | 97.8 (96.5–98.7) | 96.6 (95.1–97.7) | 94.4 (92.6–95.9) | ||
| TIV-Yam | Pre | 800 | 44.1 (40.6–47.6) | 51.1 (47.6–54.6) | 49.9 (46.4–53.4) | 74.1 (70.9–77.1) | |
| Post | 801 | 97.1 (95.7–98.2) | 96.5 (95.0–97.7) | 79.8 (76.8–82.5) | 99.6 (98.9–99.9) | ||
| QIV (6–35 m) | Pre | 232 | 25.9 (20.4–32.0) | 14.7 (10.4–19.9) | 12.1 (8.2–17.0) | 20.7 (15.7–26.5) | |
| Post | 234 | 79.9 (74.2–84.9) | 72.2 (66.0–77.9) | 71.4 (65.1–77.1) | 90.6 (86.1–94.0) | ||
| SCR, % (95% CI) | QIV | Post | 790 | 91.4 (89.2–93.3) | 72.3 (69.0–75.4) | 70.0 (66.7–73.2) | 72.5 (69.3–75.6) |
| TIV-Vic | Post | 818 | 89.9 (87.6–91.8) | 70.7 (67.4–73.8) | 68.5 (65.2–71.6) | 37.0 (33.7–40.5) | |
| TIV-Yam | Post | 800 | 91.6 (89.5–93.5) | 71.9 (68.6–75.0) | 29.6 (26.5–32.9) | 70.8 (67.5–73.9) | |
| QIV (6–35 m) | Post | 232 | 78.0 (72.1–83.2) | 68.5 (62.1–74.5) | 68.1 (61.7–74.1) | 82.3 (76.8–87.0) | |
| SCF, value (% CI) | QIV | Post | 790 | 18.0 (16.6–19.5) | 7.9 (7.3–8.6) | 7.9 (7.3–8.6) | 7.4 (6.8–8.0) |
| TIV-Vic | Post | 818 | 17.4 (16.0–18.8) | 7.2 (6.7–7.8) | 7.9 (7.2–8.6) | 2.9 (2.7–3.1) | |
| TIV-Yam | Post | 800 | 19.2 (17.7–20.9) | 7.5 (6.9–8.1) | 2.7 (2.5–2.9) | 7.6 (7.0–8.3) | |
| QIV (6–35 m) | Post | 232 | 11.7 (10.2–13.4) | 10.4 (9.0–11.9) | 9.7 (8.5–11.2) | 12.9 (11.0–15.3) | |
SPR defined as proportion of subjects with HI antibody titers ≥1:40; SCR defined as proportion of subjects with a prevaccination HI antibody titer <1:10 and postvaccination HI antibody titer ≥1:40, or subjects with at least a 4-fold increase in the postvaccination HI antibody titer; SCF defined as the geometric mean of the within subject ratios of reciprocal HI antibody titers for postvaccination vs prevaccination.
Abbreviations: CI, confidence interval; GMT, geometric mean titer; HI, hemagglutination-inhibition; M, months; QIV, quadrivalent inactivated influenza vaccine; SCF, seroconversion factor; SCR, seroconversion rate; SPR, seroprotection rate; TIV-Vic, trivalent inactivated influenza vaccine Victoria lineage B strain; TIV-Yam, trivalent inactivated vaccine Yamagata lineage B strain.
Figure 2.
HI antibody GMTs by age and priming status in the per-protocol cohort for immunogenicity. Unprimed children received 2 doses of vaccine 28 days apart and primed children received one dose of vaccine; GMTs were assessed pre-vaccination (day 0) and 28 days after the final dose (primed day 28, unprimed day 56). Abbreviations: CI, confidence interval; GMT, geometric mean titer; HI, hemagglutination-inhibition; QIV, inactivated quadrivalent influenza vaccine; TIV-Vic, inactivated trivalent influenza vaccine Victoria lineage B strain; TIV-Yam, inactivated trivalent influenza vaccine Yamagata lineage B strain.
Reactogenicity and Safety
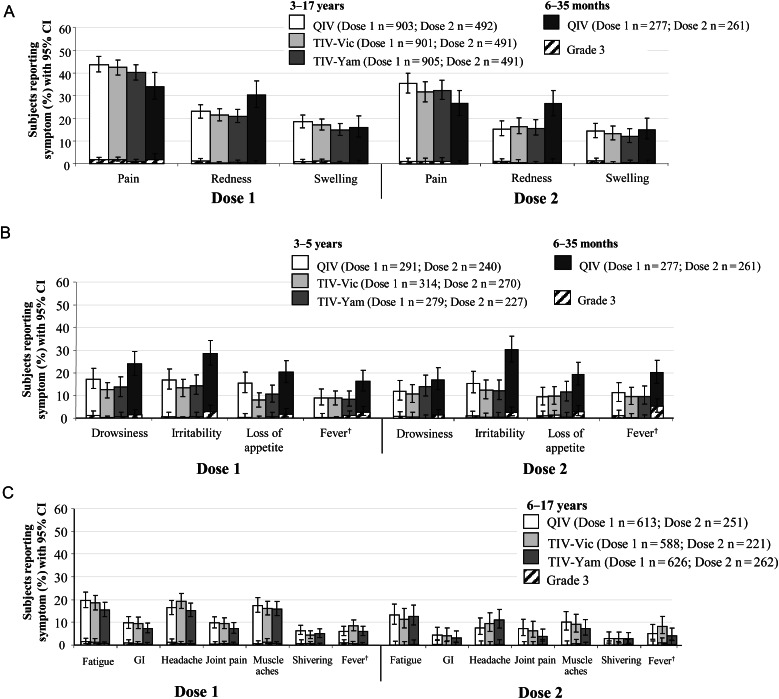
Solicited AEs are shown in Figure 3.
Figure 3.
Solicited injection-site symptoms (A) and general symptoms in children aged ≤5 years (B) and ≥6 years (C) in the Total Vaccinated Cohort. Unprimed children received 2 doses of vaccine 28 days apart and primed children received 1 dose of vaccine. †Axillary temperature ≥37.5°C; grade 3 >39°C. Abbreviations: CI, confidence interval; GI, gastrointestinal; QIV inactivated quadrivalent influenza vaccine; TIV-Vic, inactivated trivalent influenza vaccine Victoria lineage B strain; TIV-Yam, inactivated trivalent influenza vaccine Yamagata lineage B strain.
Children Aged 3–17 Years
During the 7-day postvaccination period after dose 1, injection site pain was the most frequent solicited injection-site AE with QIV (43.7%) and TIV (40.3%–42.4%). The most frequent solicited general AEs in children aged 3–5 years were drowsiness (QIV, 17.2%; TIV, 12.4%–13.6%) and irritability (QIV, 16.8%; TIV, 13.4%–14.3%), and in children aged 6–17 years, were fatigue (QIV, 19.7%; TIV 15.5%–18.5%), headache (QIV, 16.3%; TIV, 15.2%–19.2%), and muscle ache (QIV, 17.5%; TIV, 15.8%–16.0%). After the second dose of QIV or TIV, the frequency of solicited symptoms was similar to that observed after the first dose. After QIV or TIV grade 3 solicited injection-site (≤1.6% and ≤1.8%, respectively) and general (≤1.5% and ≤1.4%, respectively) symptoms were uncommon.
During the 28-day postvaccination periods in the QIV, TIV-Vic, and TIV-Yam groups, 284/915 (31.0%), 305/912 (33.4%), and 308/911 (33.8%) children, respectively, reported an unsolicited AE, which was most commonly nasopharyngitis in the QIV (5.4%), TIV-Vic (6.6%), and TIV-Yam (7.0%) groups. Over the 6-month follow-up, in the QIV, TIV-Vic, and TIV-Yam groups, 271 (29.6%), 278 (30.5%), and 303 (33.3%) children, respectively, experienced an MAE. Twenty-one children experienced 27 SAEs, including 8 (0.9%) children in the QIV group, and 6 (0.7%) and 7 (0.8%) children in the TIV groups. None of the SAEs were considered to be vaccine-related by the investigator. There was 1 death due to a motor vehicle accident.
Children Aged 6–35 Months
During the 7-day post-vaccination period after dose 1, the frequency of pain, redness, and swelling were 33.9%, 30.3%, and 15.9%, respectively, and the most frequent solicited general symptoms were irritability (28.5%), drowsiness (23.8%), and loss of appetite (20.2%); the frequency of solicited events was similar after the first and second dose apart from grade 3 fever at 2.5% and 5.4%, respectively. Apart from fever, the rate of grade 3 injection-site and general events was ≤1.8% and ≤2.9%, respectively.
During the 28-day postvaccination periods, 167/277 (60.3%) children reported an unsolicited AE, which was most commonly nasopharyngitis (13.4%). During the 6-month follow-up, 171 (61.7%) children experienced an MAE, including 2 cases of febrile seizures (one during a viral infection 16 days after dose 1, and 1 during an episode of otitis media 98 days after dose 2). Nine children (3.2%) experienced 18 SAEs, and none were considered to be vaccination-related by the investigator.
DISCUSSION
This Phase III, randomized, double-blind study of children aged 3–17 years showed that immunogenicity was noninferior for QIV vs TIV against shared vaccine strains and superior for QIV vs TIV for alternate-lineage B strains. In the open-label group, QIV was also immunogenic against all 4 vaccine strains in children aged 6–35 months. Reactogenicity and safety of QIV was consistent with the established profile of TIVs in younger and older children, suggesting that formulation with an additional 15 µg of influenza B antigen did not compromise safety. The noninferiority and superiority of the candidate QIV vs TIV against shared strains and an additional B strain, respectively, supports the use of QIV for vaccination against seasonal influenza in children aged more than 3 years as a strategy to potentially improve protection against influenza B.
There is a clear need for moving from a trivalent to a quadrivalent vaccine including both influenza B lineages as this could reduce the burden of seasonal influenza [18]. In children, influenza B is associated with substantial morbidity and hospitalization and is reported to be a disproportionate cause of influenza deaths [6, 19–24]. For example, in the United States during the 2010/2011 season, although influenza B accounted for only 26% of infections, 44 of 115 (38%) influenza-related deaths in children were associated with influenza B [25]. Moreover, a previous integrated analysis of trivalent live attenuated influenza vaccine (LAIV) studies in unprimed children aged 6 months to 6 years, showed that vaccine efficacy against influenza B was 86%, 55%, and 31% if the vaccine B strain vs the prevalent B strain was same lineage, same-lineage drift variant, or alternate lineage, respectively [11]. The results of our study suggest that the candidate inactivated QIV could address influenza B lineage mismatch and potentially improve protection. QIV was immunogenic in children aged 3–17 years, with SCRs of 91.4%, 72.3%, 70.0%, and 72.5% against A/H1N1, A/H3N2, B/Victoria, and B/Yamagata, respectively.
Influenza vaccines are only moderately immunogenic in children <3 years of age who have had limited previous exposure to vaccines and viruses, and 2 doses of vaccine are recommended in influenza vaccine-naive children aged 6 months to 8 years to achieve protective antibody titers [19]. Indeed, new vaccines and vaccination strategies are needed to improve protection against influenza in infants and toddlers. As such, the open-label arm of our study was conducted to generate pilot data of immunogenicity and safety of QIV in children aged 6–35 months prior to a confirmatory Phase III trial. We showed that QIV elicited robust immune responses against all 4 vaccine strains with SCRs of 78.0%, 68.5%, 68.1%, and 82.3%, against influenza A/H1N1, A/H3N2, B/Victoria, and B/Yamagata, respectively, thus supporting further development of QIV in very young children.
The addition of 15 µg of antigen to a trivalent vaccine has the potential to affect reactogenicity and safety. In our study, the reactogenicity profile of QIV was consistent with TIV in older children, and no major safety concerns were raised in infants. Injection site pain was the most frequent solicited AE in younger and older children with each vaccine. The rate of fever >39°C in children aged 6–35 months was 2.54% (dose 1) and 5.4% (dose 2), which was slightly more frequent than in children aged 3–5 years (0%–1.3%). Overall, grade 3 events were uncommon, and SAEs in the QIV group were consistent with the TIV group.
The main limitation of the study is that the immunogenicity data provides no information on the magnitude of protection against influenza B illness. Although QIV elicited antibody titers against the added B strain that were superior to TIV and that exceeded levels considered to be protective, the clinical benefits of QIV vs TIV remain to be established. After QIVs are used in annual vaccination programs, their impact on the prevention and control of influenza can be further evaluated in effectiveness trials conducted over multiple seasons.
Natural exposure to influenza viruses was a potential confounding factor as children were enrolled between early October and mid-December 2010, and blood samples were taken until mid-February 2011. In the United States and Europe, influenza activity increased in December 2010 and peaked in early February 2011, with A/California/7/2009 (A/H1N1) viruses predominating in Europe and A/H3N2 viruses predominating in the United States [26, 27]. Influenza B viruses, mainly from the B/Victoria lineage, also circulated widely in Europe and the United States [26, 27]. Natural exposure during the study cannot be excluded, yet the effect on the confirmatory immunogenicity endpoints was likely to be limited because the vast majority of blood samples (approximately 95%) were taken before peak season, the exposure risk was expected to be <10%, and the study was controlled, meaning that exposure would have been similar across both vaccine groups.
In summary, QIV provided noninferior immunogenicity against the shared strains, and superior immunogenicity against the additional B strain compared with TIV in children aged 3–17 years. In the randomized study, and the open-label group (children aged 6–35 months), QIV elicited robust antibody responses against all 4 vaccine strains. These results show that QIV could prevent influenza B lineage mismatch and potentially improve protection against influenza B. The immunogenicity and safety results support a switch from TIV to QIV in children aged 3–17 years.
Notes
Acknowledgments. All authors participated in the implementation of the study including substantial contributions to conception and design, the gathering of the data, or analysis and interpretation of the data. All authors were involved in the drafting of the article or revising it critically for important intellectual content and final approval of the manuscript.
The authors are indebted to the participating study volunteers and their parents, clinicians, nurses, and laboratory technicians at the study sites, as well as to the sponsor's project staff for their support and contributions throughout the study. In particular, we thank Drs Ulrich Behre, Marc Bonnefoy, Alain Boye, Daniel Drazan, Rolf Ebert, Brandon Essink, Earl Ruffin Franklin, Miriam Medero Eng, Andrea Kline Goldsmith, Lynn Rice, Shilpa Rajagopal, Ricardo Grillo-Paris, Darrell Herrington, Ursula Hoernlein, Robert Jeanfreau, Kaï Kassmann, Klaus Kindler, Karola Kirsten, Sandra Litao, Ralph Maier, Scott Meyers, Petr Pazdiora, Christian Petit, Ulrich Pfletschinger, Manfred Praun, Renata Ruzkova, Peter Soemantri, Philippe Tardif, Franck Thollot, Phu My Tran, Michael Vomstein, and Uta Walther. We are grateful to all teams of GSK Vaccines for their contribution to this study, especially Dr Olivier Godeaux for intellectual input during the development of the study report, Valerie Sengers for clinical study management, and Philippe Boutet from the clinical and serological laboratory teams, Wenjun Jiang (Clinical Safety Representative), and Vincent Dodeur for data management. Finally, the authors thank Annick Moon (Moon Medical Communications, UK) and Avishek Pal (GSK Vaccines) for providing medical writing services, and Jérémie Dedessus Le Moutier (Business and Decision Life Sciences, on behalf of GSK Vaccines) for editorial assistance and manuscript coordination.
Financial support. GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis (ClinicalTrials.gov NCT01196988). GlaxoSmithKline Biologicals SA also took in charge all costs associated with the development and the publishing of the present manuscript. All authors had full access to the data, and the corresponding author had final responsibility to submit for publication.
Potential conflicts of interest. All participating institutions received compensation for study involvement. Drs B. Innis, V. Jain, C. Claeys, M. Peeters, and Y. Feng are employees of GlaxoSmithKline group of companies. Drs C. Claeys, M. Peeters, B. Innis, and V. Jain report ownership of stock options. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bhat N, Wright JG, Broder KR, et al. Influenza-associated deaths among children in the United States, 2003–2004. N Engl J Med. 2005;353:2559–67. doi: 10.1056/NEJMoa051721. [DOI] [PubMed] [Google Scholar]
- 2.Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62. [PubMed] [Google Scholar]
- 3.Heikkinen T, Silvennoinen H, Peltola V, et al. Burden of influenza in children in the community. J Infect Dis. 2004;190:1369–73. doi: 10.1086/424527. [DOI] [PubMed] [Google Scholar]
- 4.Moore DL, Vaudry W, Scheifele DW, et al. Surveillance for influenza admissions among children hospitalized in Canadian immunization monitoring program active centers, 2003–2004. Pediatrics. 2006;118:e610–9. doi: 10.1542/peds.2005-2744. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien MA, Uyeki TM, Shay DK, et al. Incidence of outpatient visits and hospitalizations related to influenza in infants and young children. Pediatrics. 2004;113:585–93. doi: 10.1542/peds.113.3.585. [DOI] [PubMed] [Google Scholar]
- 6.Hite LK, Glezen WP, Demmler GJ, Munoz FM. Medically attended pediatric influenza during the resurgence of the Victoria lineage of influenza B virus. Int J Infect Dis. 2007;11:40–7. doi: 10.1016/j.ijid.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Hu JJ, Kao CL, Lee PI, et al. Clinical features of influenza A and B in children and association with myositis. J Microbiol Immunol Infect. 2004;37:95–8. [PubMed] [Google Scholar]
- 8.Peltola V, Ziegler T, Ruuskanen O. Influenza A and B virus infections in children. Clin Infect Dis. 2003;36:299–305. doi: 10.1086/345909. [DOI] [PubMed] [Google Scholar]
- 9.Ampofo WK, Baylor N, Cobey S, et al. Improving influenza vaccine virus selection: report of a WHO informal consultation held at WHO headquarters, Geneva, Switzerland, 14–16 June 2010. Influenza Other Respi Viruses. 2012;6:142–52. doi: 10.1111/j.1750-2659.2011.00277.x. e1–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Seasonal influenza activity surveillance reports: 2000–2001 to 2010–2011 seasons. http://www.cdc.gov/flu/weekly/pastreports.htm . Accessed 19 March 2013.
- 11.Belshe RB, Coelingh K, Ambrose CS, Woo JC, Wu X. Efficacy of live attenuated influenza vaccine in children against influenza B viruses by lineage and antigenic similarity. Vaccine. 2010;28:2149–56. doi: 10.1016/j.vaccine.2009.11.068. [DOI] [PubMed] [Google Scholar]
- 12.Ambrose CS, Levin MJ. The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother. 2012;8:81–88. doi: 10.4161/hv.8.1.17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belshe RB. The need for quadrivalent vaccine against seasonal influenza. Vaccine. 2010;28(Suppl 4):D45–53. doi: 10.1016/j.vaccine.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 14.United States Centers for Disease Control and Prevention. Seasonal influenza activity surveillance reports: 2000–2001 to 2010–2011 seasons. http://www.cdc.gov/flu/weekly/pastreports.htm . Accessed 19 March 2013.
- 15.World Health Organization. Recommended composition of influenza virus vaccines for use in the 2012–2013 northern hemisphere influenza season. 2012. http://www.who.int/influenza/vaccines/virus/recommendations/201202_recommendation.pdf . Accessed 19 March 2013.
- 16.Fiore AE, Uyeki TM, Broder K, et al. Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62. [PubMed] [Google Scholar]
- 17.Hehme N, Künzel W, Petschke F, et al. Ten years of experience with the trivalent split-influenza vaccine, Fluarix™. Clin Drug Invest. 2002;22:751–769. [Google Scholar]
- 18.Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine. 2012;30:1993–8. doi: 10.1016/j.vaccine.2011.12.098. [DOI] [PubMed] [Google Scholar]
- 19.Fiore AE, Shay DK, Broder K, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58:1–52. [PubMed] [Google Scholar]
- 20.Glezen WP, Couch RB, Taber LH, et al. Epidemiologic observations of influenza B virus infections in Houston, Texas, 1976–1977. Am J Epidemiol. 1980;111:13–22. doi: 10.1093/oxfordjournals.aje.a112865. [DOI] [PubMed] [Google Scholar]
- 21.Li WC, Shih SR, Huang YC, et al. Clinical and genetic characterization of severe influenza B-associated diseases during an outbreak in Taiwan. J Clin Virol. 2008;42:45–51. doi: 10.1016/j.jcv.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Olson DR, Heffernan RT, Paladini M, Konty K, Weiss D, Mostashari F. Monitoring the impact of influenza by age: emergency department fever and respiratory complaint surveillance in New York City. PLoS Med. 2007;4:e247. doi: 10.1371/journal.pmed.0040247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 24.Esposito S, Cantarutti L, Molteni CG, et al. Clinical manifestations and socio-economic impact of influenza among healthy children in the community. J Infect. 2011;62:379–87. doi: 10.1016/j.jinf.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC) Influenza-associated pediatric deaths – United States, September 2010–August 2011. MMWR Morb Mortal Wkly Rep. 2011;60(36):1233–8. [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. 2010–2011 Influenza Season Summary. http://www.cdc.gov/flu/weekly/weeklyarchives2010–2011/10-11summary.htm . Accessed 19 March 2013.
- 27.European Centre for Disease Prevention and Control. Weekly influenza surveillance overview. http://ecdc.europa.eu/en/publications/surveillance_reports/influenza/pages/weekly_influenza_surveillance_overview.aspx . Accessed 19 March 2013.