Abstract
Background. Systemic immune activation is a strong predictor of progression of human immunodeficiency virus type 1 (HIV-1) disease and a prominent feature of infection with Mycobacterium tuberculosis.
Objective. To understand the role of systemic immune activation and microbial translocation in HIV/tuberculosis dually infected patients over the full spectrum of HIV-1 immunodeficiency, we studied circulating sCD14 and lipopolysaccharide (LPS) and their relationship to HIV-1 activity.
Methods. Two cohorts of HIV/tuberculosis subjects defined by CD4 T-cell count at time of diagnosis of tuberculosis were studied: those with low (<350/μL) and those with high (≥350/μL) CD4 T-cell count. Circulating soluble CD14 (sCD14) and LPS were assessed.
Results. Levels of sCD14 were higher in HIV/tuberculosis with high (≥350/μL) as compared to low CD4 T-cell count (P < .001). Whereas sCD14 levels remained elevated in HIV/tuberculosis subjects with lower CD4 T-cell counts despite treatment of tuberculosis, in HIV/tuberculosis patients with higher CD4 T-cell count (≥350/μL), levels declined regardless of whether highly active antiretroviral therapy (HAART) was included with the anti-tuberculosis regimen. Circulating LPS levels in HIV/tuberculosis patients with CD4 T-cell count ≥350/μL were unaffected by treatment of tuberculosis with or without HAART.
Conclusion. During HIV/tuberculosis, systemic immune activation is dissociated from microbial translocation. Changes in circulating sCD14 and LPS are dependent on CD4 T-cell count.
Keywords: HIV-1, tuberculosis, LPS, soluble CD14
Tuberculosis is the most prevalent coinfection of HIV-1-infected subjects worldwide [1] and is associated with increased HIV-1-related morbidity and mortality [2–4]. At time of tuberculosis diagnosis, HIV-1 viral loads in HIV/tuberculosis dually infected subjects are higher compared to CD4-matched HIV-1-positive control subjects, regardless of CD4 T-cell counts [5]. However, in a majority of dually infected patients, high HIV-1 viral loads at diagnosis are not lowered upon treatment of tuberculosis alone [6, 7]. Systemic immune activation is a significant feature of infection with Mycobacterium tuberculosis in humans [8, 9] and is currently deemed to be a strong predictor of progression of HIV-1 disease [10]. Among the “drivers” of immune activation in HIV-1 disease, microbial translocation from the gut is believed to be prominent, even following successful antiretroviral therapy [11]. Whether systemic immune activation and microbial translocation are accentuated in HIV/tuberculosis dually infected subjects and their role in persistence of HIV-1 viral activity has not been investigated.
Recently, several markers of systemic immune activation have been studied intensively to identify their significance in the course of HIV-1 immunodeficiency. Circulating soluble CD14 (sCD14), which is secreted or shed from monocyte/macrophages upon immune activation [12], has been found to correlate with progression of HIV-1 disease [13]. In HIV/tuberculosis patients with pulmonary tuberculosis plasma, sCD14 levels were extremely high in the majority of patients; however, reductions in plasma sCD14 levels upon completion of tuberculosis treatment did not correlate with HIV-1 viral load [6]. Of note, expression of CD14 is not limited to monocyte/macrophages, as it is found on neutrophils, and at very low levels on epithelial and endothelial cells and even fibroblasts [14]. More specific to monocyte/macrophage activation is the hemoglobin scavenger receptor molecule sCD163, which is shed upon Toll-like receptor (TLR) ligation [15]. Plasma levels of sCD163 are increased in HIV-1-infected subjects during both early and chronic phases of HIV infection [16].
A direct correlation between systemic immune activation and bacterial components originating from damaged gastrointestinal (GI) tract lamina propria has been established in both human and animal models of HIV-1 disease [17]. In HIV-1-infected subjects plasma levels of bacterial DNA and lipopolysaccharide (LPS) have been found to correlate with both sCD14 levels and the degree of immunological reconstitution subsequent to highly active antiretroviral therapy (HAART) [18, 19]. Studies from Africa have reported contrasting results; a study from Uganda found no association between HIV-1 disease progression and microbial translocation [20, 21], whereas a study from South Africa showed higher levels of microbial translocation in HIV-infected subjects with opportunistic infection (OI) compared to those without OI [22]. The effect of microbial translocation and its association with systemic immune activation during HIV/tuberculosis is largely unknown.
Here we assessed circulating sCD14 and LPS and their relationship to HIV-1 activity in dually infected subjects over the full spectrum of HIV-1 immunodeficiency. Two cohorts of HIV/tuberculosis subjects as defined by CD4 T-cell count at time of diagnosis of tuberculosis, those with lower (<350/μL) and those with higher (≥350/μL) CD4 T-cell count were studied. We found a very different profile of systemic immune activation and microbial translocation in these cohorts. Whereas plasma sCD14 remained unchanged in HIV/tuberculosis subjects with lower CD4 T-cell counts (<350/μL) despite treatment of tuberculosis, in HIV/tuberculosis patients with higher CD4 T-cell count (≥350/μL), it resolved regardless of inclusion of HAART with anti-tuberculosis therapy. Circulating LPS levels remained higher than those of respective CD4-matched HIV-1-infected control groups regardless of HAART.
METHODS
Study Subjects
Between October 2004 and September 2008 HIV-1-infected and -uninfected subjects with pulmonary tuberculosis were recruited from the Tuberculosis Clinic at Mulago Hospital in Kampala, Uganda. Diagnosis of tuberculosis was based on sputum culture positivity for M. tuberculosis. Two cohorts of HIV/tuberculosis patients with pulmonary tuberculosis were studied (Table 1).
Table 1.
Clinical Characteristics of HIV/Tuberculosis Patients
| Cohort II |
|||
|---|---|---|---|
| Characteristic | Cohort I | No HAART (Group A) | HAART (Group B) |
| No. | 28 | 18 | 19 |
| Age, mean ± SEM | 35 ± 7.2 | 31 ± 6.0 | 32 ± 5.6 |
| Male, % | 42 | 63 | 52 |
| CD4 T-cell count, cells/μL (median and range) | 196 (70–347) | 534 (407–532) | 517 (414–640) |
| Viral load,(log (median and range) | 5.4 (4.5–5.0) | 4.7 (4.0–5.7) | 4.6 (4.1–5.1) |
Abbreviations: HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; SEM, standard error of the mean.
Cohort I includes HIV/tuberculosis subjects with pulmonary tuberculosis and CD4 T-cell count <350 cells/μL. These subjects were treated with short course (6 months) anti-tuberculosis treatment alone as detailed elsewhere [23] and were followed through the end of tuberculosis treatment only.
Cohort II consisted of a subset of HIV/tuberculosis patients with pulmonary tuberculosis with CD4 T-cell count of ≥350 cells/μL enrolled in a larger open-label randomized clinical trial entitled “Randomized clinical trial of a 6-month punctuated course of antiretroviral therapy (PART) in Uganda” [7]. In this study, eligible HIV/tuberculosis patients were started on anti-tuberculosis treatment and randomized 2 weeks (W2) later to receive HAART (Trizivir, GlaxcoSmithKline) (group B) or not (group A). At 6 months (M6), anti-tuberculosis chemotherapy and HAART (in group B) were terminated, and both groups were followed for an additional 6 months (M12).
HIV-1-positive healthy subjects matched by CD4 T-cell count (±50) to HIV/tuberculosis patients with pulmonary tuberculosis in either Cohort I or II were recruited during the same time period (their demographics are provided in Table 2).
Table 2.
Clinical Characteristics of HIV-Infected Healthy Control Subjects
| Characteristic | HIV+ subjects (CD4 <350 cells/μL) | HIV+ subjects (CD4 ≥350 cells/μL) |
|---|---|---|
| No. | 20 | 25 |
| Age (mean ± SEM) | 29 ± 5.0 | 31 ± 6.0 |
| Male, % | 42 | 50 |
| CD4 T-cell count (cells/μL) (median and range) | 208 (50–326) | 501 (350–793) |
| Viral load, log (median and range) | 4.8 (4.5–5.0) | 4.3 (4.0–4.7) |
Abbreviations: HIV, human immunodeficiency virus; SEM, standard error of the mean.
HIV-negative subjects with or without pulmonary tuberculosis age-matched (±5 years) to HIV/tuberculosis or HIV-positive subjects were also recruited.
Written informed consent approved by the institutional review boards from Makerere University (Kampala, Uganda) and Case Western Reserve University/University Hospitals (Cleveland, US) was obtained from all subjects.
Immunoassays for Cytokines and Immune Activation Markers
Enzyme-linked immunosorbent assays (ELISA) for sCD14, sCD163, and high-sensitivity interleukin 6 (IL-6) were from R&D Systems (Minneapolis, MN). Detection limits for these assays were 125 pg/mL, 58 pg/mL, and 0.1 pg/mL, respectively.
LPS and LBP Assays
LPS measurement was by Pyrogene (Recombinant Factor C Endotoxin Detection Assay) from Lonza/Cambrex (Wakersville, MD). Detection limits are 0.01 to 10 of Endotoxin Unit (EU)/mL. One EU is equivalent to 100 pg of LPS. In side-by-side comparison, this assay was found to highly correlate with the chromogenic limulus amebocyte lysate (LAL) from the same company [24]. However, this assay is considerably easier to perform than the more commonly used (LAL QCL-1000).
Measurement of LPS binding protein (LBP) was by ELISA from Cell Sciences (Canton, MA). The sensitivity range of this assay is 5–50 ng/mL. In healthy donors, serum LBP is 5–15 μg/mL.
Statistical Analysis
Data sets were analyzed by student t-test, paired t-test, Wilcoxon 2 sample test, Kruskall Wallis test, and Spearman rank order correlation, as appropriate. A P value of ≤ .05 was considered significant.
RESULTS
Elevated sCD14 Characterizes Tuberculosis Regardless of CD4 Immunodeficiency in HIV/Tuberculosis Patients
First, we assessed sCD14 in plasma samples obtained at diagnosis of tuberculosis from HIV/tuberculosis patients in Cohort I (CD4 T-cell count <350/μL), Cohort II (CD4 T-cell count ≥350/μL; Table 1), and HIV-1 singly infected subjects CD4-matched to each Cohort of HIV/tuberculosis patients (Table 2).
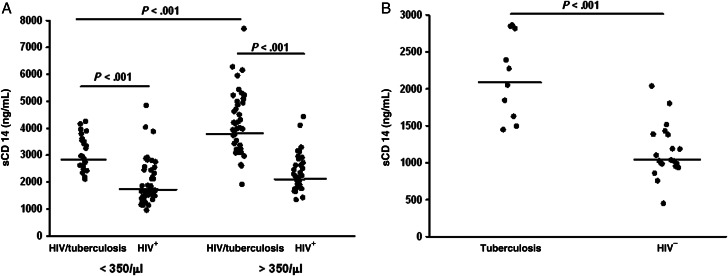
Results are shown in Figure 1A. Plasma sCD14 levels in HIV/tuberculosis subjects from either cohort were significantly higher (P < .001) than those from their respective CD4-matched HIV-positive control groups. Interestingly plasma sCD14 levels in HIV/tuberculosis subjects with CD4 T-cell count ≥350/μL were significantly higher (P < .001) than those in the cohort with CD4 T-cell count <350/μL (Figure 1A). Plasma sCD14 levels in HIV-uninfected subjects with tuberculosis were higher than healthy subjects (Figure 1B; P < .001). Levels of sCD14 were higher in healthy HIV-1-infected subjects (Figure 1A) when compared to HIV-1-negative healthy subjects (Figure 1B; P < .001). Levels of sCD14 were significantly higher among patients with HIV/tuberculosis (Figure 1A) when compared to patients with tuberculosis alone (Figure 1B; P < .001). Interestingly, sCD14 levels in HIV-1 singly infected subjects were only minimally and insignificantly lower than in HIV-1-uninfected tuberculosis subjects, implicating a comparable effect of single infection (HIV or tuberculosis) on immune activation.
Figure 1.
Circulating soluble (s) CD14 in dual HIV/tuberculosis-infected subjects with CD4 <350/μL) and CD4 ≥350/μL) at the time of diagnosis of tuberculosis. (A) sCD14 in plasma from HIV/tuberculosis subjects and CD4 T-cell counts <350/μL (Cohort I) (left) and CD4 ≥350/μL (Cohort II) (right), and respective CD4-matched HIV-infected control subjects are shown. (B) Plasma sCD14 at time of diagnosis of tuberculosis in HIV-uninfected subjects with tuberculosis and healthy HIV-negative subjects are shown. Line shown in each group represents median sCD14 activity. Abbreviations: HIV, human immunodeficiency virus.
In neither Cohort I nor Cohort II HIV/tuberculosis subjects was a correlation between plasma sCD14 and either CD4 T-cell count or plasma viral load found.
However, in both HIV-positive control groups, a significant correlation between sCD14 and viral load was seen. In HIV-1-infected subjects with CD4 T-cell <350/μL, plasma sCD14 correlated with their viral load (r = 0.55, P < .005). And in HIV-1-infected healthy subjects with CD4 T-cell >350/μL plasma sCD14 was found (r = 0.34, P < .05).
Circulating LPS in HIV/tuberculosis Patients With Low CD4 T-cell Counts
During HIV infection, immune activation is presumably, at least in part, based on microbial translocation from the GI tract. First we investigated microbial translocation as measured by circulating LPS in HIV/tuberculosis patients from Cohort I (CD4 <350/μL).
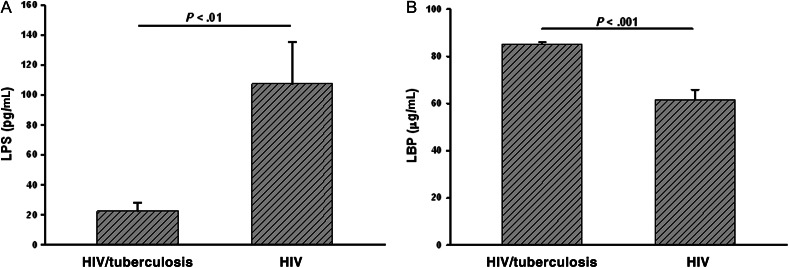
Plasma LPS levels measured at time of diagnosis of HIV/tuberculosis and levels in CD4 matched HIV-positive controls are shown in Figure 2A. Surprisingly, circulating LPS levels were significantly lower in HIV/tuberculosis patients as compared to levels in HIV-1-infected control subjects (P < .01).
Figure 2.
Circulating LPS levels in dual HIV/tuberculosis-infected subjects with low CD4 T-cell count (<350/μL) at diagnosis of tuberculosis. LPS and LBP were assessed in plasma from HIV/tuberculosis subjects at time of diagnosis of tuberculosis. (A) LPS levels at time of diagnosis of HIV/tuberculosis and in CD4-matched healthy HIV-infected subjects are shown. (B) LBP levels in the same samples as in panel A are shown. Abbreviations: HIV, human immunodeficiency virus; LBP,LPS binding protein; LPS, lipopolysaccharide.
We rationalized that LPS levels may be low due to high circulating LPS binding protein(s). The predominant LPS binding molecule in plasma is LBP, levels of which are increased during active tuberculosis (regardless of HIV infection) and resolve upon treatment of tuberculosis [25]. Here, we found that LBP levels in plasma samples from dually infected HIV/tuberculosis patients were significantly higher than levels in healthy HIV- infected subjects (P < .001; Figure 2B). However, a negative correlation between low plasma LPS and high LBP was not found. These data implicate effect(s) from additional LPS binding proteins (unrelated to LBP) in the circulation.
Course of Plasma LPS and sCD14 in HIV/tuberculosis Patients With Low CD4 T-cell Counts
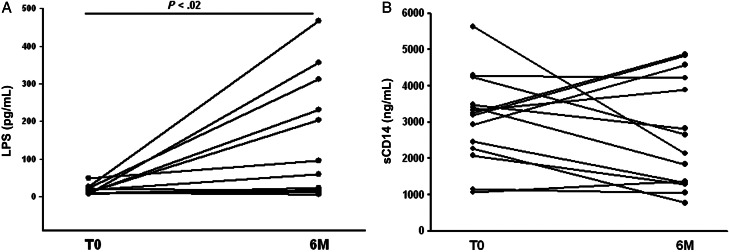
To assess changes in plasma LPS and sCD14 in HIV/tuberculosis patients upon treatment of tuberculosis, we assessed these indices in paired samples at time of diagnosis of tuberculosis (T0) and at 6 months (M6) in a subgroup (n = 12) of HIV/tuberculosis patients from Cohort I (CD4 T-cell count <350/μL).
As compared to baseline, levels of LPS were increased at the end of tuberculosis therapy in 10 of 12 subjects (Figure 3A) (P < .02). In these subjects, high baseline (t0) LBP levels were found to be reduced by half at M6 (P < .01; data not shown).
Figure 3.
Course of plasma LPS and sCD14 in HIV/tuberculosis subjects with CD4 T-cell count <350/μL (Cohort I) upon treatment of tuberculosis. (A) Changes in plasma LPS in a subgroup (n = 12) of HIV/tuberculosis patients measured at T0 and by end of treatment (M6) are shown. (B) Changes in plasma sCD14 in the same subgroup of HIV/tuberculosis patients measured at T0 and by end of treatment (M6) are shown. Abbreviations: HIV, human immunodeficiency virus; LPS, lipopolysaccharide; T0, time of diagnosis of tuberculosis.
Plasma sCD14 levels did not change significantly between t0 and M6 (Figure 3B): Plasma sCD14 levels were lower at M6 compared to t0 (1.6–2.9-fold) in only 6 subjects and either remained unchanged or increased in the rest.
Similar to sCD14 levels, no change in plasma sCD163 levels were found in samples from t0 and M6 (data not shown).
Therefore, in dually infected HIV/tuberculosis subjects with low CD4 T-cell counts (<350/μL), treatment of tuberculosis alone does not resolve immune activation as measured by sCD14 or sCD163.
There was no correlation of LPS levels and sCD14 at the end of tuberculosis therapy in HIV/tuberculosis patients.
Course of Immune Activation in HIV/tuberculosis Patients With High CD4 T-cell Count
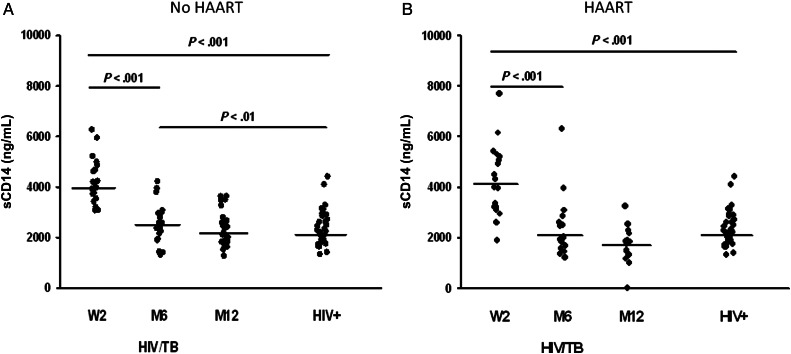
Next, we assessed the course of changes in plasma sCD14 in HIV/tuberculosis patients from Cohort II, that is, patients with more preserved CD4 T-cell counts (≥350/μL). As noted, Cohort II, is a placebo controlled study of HIV/tuberculosis patients with CD4 T-cell counts ≥350 cells/μL randomized to receive punctuated HAART, 2 weeks after initiation of anti-tuberculosis chemotherapy (group B) or not (group A) [7]. Plasma samples from HIV/tuberculosis patients in each group were assessed at time of HAART randomization (W2), at 6 months, and at 12 months. A group of HIV-infected subjects who had CD4 T-cell counts ≥350/μL was compared to either group. Plasma samples from the control group were analyzed only once.
Figure 4A and 4B show plasma sCD14 levels in HIV/tuberculosis patients in group A and group B, respectively; sCD14 levels were significantly higher in either group at W2 than levels in the HIV+ control group (P < .001). There was a dramatic lowering of sCD14 levels in HIV/tuberculosis subjects for either group with treatment of tuberculosis (±HAART); at M6 sCD14 levels were significantly lower than those at W2 (P < .001) in either group. However, at the end of tuberculosis therapy (M6), levels of sCD14 in HIV/tuberculosis subjects who did not receive HAART, that is, group A (Figure 4A), were still higher than levels in the HIV-positive control group (P < .01). This difference resolved at M12. On the other hand, in the HAART-treated group (group B) (Figure 4B), sCD14 levels were no different than levels in the HIV-positive control group at M6. Notably, by M12, sCD14 levels in HAART-treated HIV/tuberculosis patients (group B) were lower than those who did not receive HAART (group A) (P < .01).
Figure 4.
Circulating soluble (s) CD14 in dual HIV/tuberculosis-infected subjects with high CD4 T-cell count (≥350/μL). Plasma from HIV/tuberculosis subjects who were treated with 6 months of anti-tuberculosis treatment alone (A) or who received HAART in addition to anti-tuberculosis treatment (B) were assessed for sCD14 at time of HAART randomization (W2), at 6 months (M6), and at 12 months (M12). Plasma sCD14 in a group of healthy CD4-matched HIV-positive subjects (HIV+) is shown for comparison. Line shown in each group represents median sCD14 activity. Abbreviations: HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus.
Only at M6 did HIV viral load from HIV-1/tuberculosis in either group correlate with plasma sCD14 levels (r = 0.51, P < .02).
We also measured plasma IL-6 and sCD163 in both groups of HIV/tuberculosis patients in Cohort II. Plasma IL-6 levels in HIV/tuberculosis patients dropped significantly by end of tuberculosis treatment alone. In group A, IL-6 levels at t0 were (10.06 pg/mL ± 1.5) and at M6 (3.8 pg/mL ± 1.1) (P < .001). In group B changes in IL-6 were similar.
On the other hand, the drop in sCD163 upon tuberculosis treatment alone (group A) was only significant by M12; sCD163 at t0 (1148 pg/mL ± 88.30) and at M12 (969 pg/mL ± 72.3) (P < .02). However, in group B who received HAART in addition to anti-tuberculosis therapy, sCD163 levels were significantly lower at M6 (852.5 pg/mL ± 68) as compared to t0 (1260 pg/mL ± 99.1) (P < .02). At M6, sCD163 levels were significantly lower in group B compared to levels in group A (P < .01).
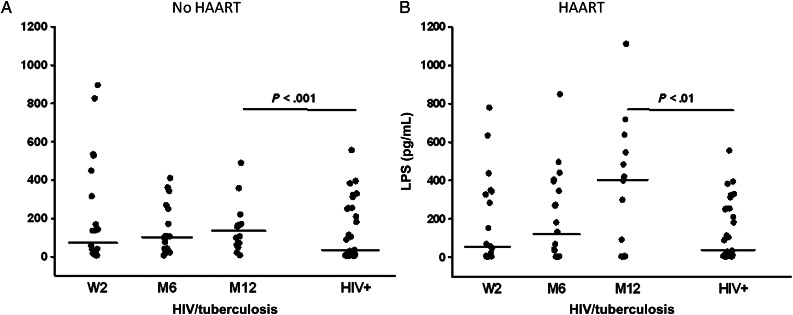
Microbial Translocation in HIV/tuberculosis Patients With High CD4 T-cell Count
Next, circulating LPS in plasma samples from HIV/tuberculosis patients with CD4 T-cell counts ≥350/μL were assessed. In group A who received tuberculosis treatment alone, LPS levels at W2 were higher than levels in the control group (P < .001) and remained elevated at all timepoints (M6, M12) (Figure 5A). There was no difference in LPS levels in group A HIV/tuberculosis patients at any time point tested.
Figure 5.
Plasma LPS in dual HIV/tuberculosis-infected subjects with high CD4 T-cell count (≥350/μL). Plasma from HIV/tuberculosis subjects who were treated with 6 months of anti-tuberculosis treatment alone (A) or who received HAART in addition to anti-tuberculosis treatment (B) were assessed for LPS at time of HAART randomization (W2), at 6 months (M6), and at 12 months (M12). Plasma LPS in a group of healthy CD4-matched HIV-positive subjects (HIV+) are shown for comparison. Line shown in each group represents median LPS level. Abbreviations: HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; LPS, lipopolysaccharide.
Plasma LPS levels in HIV/tuberculosis patients who were HAART treated (group B) (Figure 5B), however, were distinct from levels in group A. Again, LPS levels were significantly higher at all timepoints (W2, M6, M12) in HIV/tuberculosis patients compared to levels in HIV+ control subjects. There was an increase in LPS levels in this group at M12 (6 months after termination of anti-tuberculosis + HAART) compared to levels in these patients at the end of tuberculosis treatment (M6). At 12 months (M12), LPS levels in group B were significantly higher than levels in HIV + control subjects (P < .01), and higher than levels in HIV/tuberculosis patients who received tuberculosis treatment alone (group A) (Figure 5A) (P < .02).
In Cohort II, LBP levels in plasma of HIV/tuberculosis patients were similar to HIV-1 control subjects (data not shown).
DISCUSSION
Immune activation is a concomitant of chronic infections including both HIV-1 and tuberculosis. Immune activation is particularly accentuated in HIV/tuberculosis dually infected patients, as is shown in this study. Levels of sCD14 in the plasma of HIV/tuberculosis subjects were significantly higher than levels in subjects with single HIV-1 infection (regardless of their CD4 T-cell count) or M. tuberculosis infection (Figure 1). Significantly higher sCD14 levels were found in HIV/tuberculosis subjects from Cohort II with more preserved CD4 T-cell counts compared to patients with low CD4 T-cell counts (<350/μL) (Cohort I). However, in Cohort I HIV/tuberculosis patients, sCD14 elevation was prolonged and remained un-resolved by end of tuberculosis treatment. These data confirm our previous findings where reduction of sCD14 upon tuberculosis treatment was only observed in 60% of HIV/tuberculosis subjects with low CD4 T-cell counts [6]. As we found here, another recent study from Uganda of HIV-1-infected subjects with low CD4 T-cell counts did not find an association of HIV-1 disease progression and plasma sCD14 levels [20].
Changes in circulating LPS levels, which reflect microbial translocation from the intestinal lumen during HIV-1 disease, were different in HIV/tuberculosis patients in Cohort I when compared to levels in healthy CD4 matched HIV-infected subjects. LPS levels were significantly lower in HIV/tuberculosis patients at time of tuberculosis diagnosis (t0) than in control subjects, and increased upon treatment of tuberculosis at 6 months (Figure 3A). On the other hand, LBP levels were significantly higher than healthy HIV + subjects at t0 (Figure 3B). Thus, at the time of tuberculosis diagnosis, circulating LPS may be masked by very high LBP and thus not measurable. Alternatively other circulating LPS binding proteins may account for low LPS levels. Whereas at low concentrations of LBP, its complex with LPS may affect immune cell activation, at high concentrations of LBP, LPS induced activation of immune cells appears to be inhibited [26]. Therefore, the contribution of circulating LPS-LBP complex on immune activation here is unclear. Plasma LBP decreased significantly by the end of tuberculosis treatment (M6), a possible explanation for the recovery of LPS levels. However, a correlation between LPS and sCD14 levels at M6 was not found. Collectively, in HIV/tuberculosis patients with low CD4 T-cell count (<350/μL), a role for microbial translocation in persistence of immune activation remains unclear.
In HIV/tuberculosis patients with preserved CD4 T-cell counts (≥350/μL) (Cohort II), sCD14 levels were significantly higher at diagnosis of tuberculosis than were levels in CD4 matched control subjects, and even higher than sCD14 levels in samples from Cohort I patients (Figure 1). In the group of Cohort II patients who received tuberculosis treatment alone (group A), sCD14 levels decreased significantly by M6, however, still remained significantly higher than that in the control group (Figure 4A). Another study of the same Cohort of HIV/tuberculosis patients (received no HAART) recruited in the randomized clinical trial of HAART in Uganda (PART) [7], also found resolution of immune activation assessed by levels of CD8+CD38+ T cells upon treatment of tuberculosis alone [27]. Of note the overlap of patients in this latter study with group A studied here was minimal (one patient). In patients who received HAART in addition to anti-tuberculosis treatment (group B), sCD14 levels were reduced to levels measured in HIV+ control subjects more rapidly, at M6. (Figure 4B). In group B, sCD163 levels also decreased faster as compared to group A. Collectively, these data implicate an additional effect of HAART on resolution of immune activation in HIV/tuberculosis dual infection. Here, heightened circulating sCD14 levels in Cohort II HIV/tuberculosis patients at diagnosis of tuberculosis, did not correlate with viral load. Only at time of termination of anti-tuberculosis treatment with or without HAART (M6) did plasma sCD14 correlate with viral load. Therefore, an impact of immune activation on HIV-1 activity is only seen upon resolution of active tuberculosis infection. Resolution of immune activation in HIV/HCV dually infected subjects was also found upon treatment of Hepatitis C [28]. Therefore, a predominant role of active coinfection with M. tuberculosis on viral activity (likely at sites of tuberculosis), rather than tuberculosis-associated systemic immune activation, appears to be operational at diagnosis of tuberculosis. In support of this contention, we have found extremely high sCD14 levels at pleural sites of tuberculosis, that is, in pleural fluid of tuberculosis patients regardless of HIV co-infection (Toossi, unpublished). Here, patients who received HAART for 6 months (group B), had significantly lower sCD14 levels at 12 months than patients who received tuberculosis treatment alone. These data implicate that concomitant use of HAART with anti-tuberculosis treatment leads to resolution of immune activation to a greater degree (than treatment of tuberculosis alone) in HIV/tuberculosis patients.
In Cohort II, in both HAART treated (group B) and untreated (group A) HIV/tuberculosis patients, circulating LPS levels increased by end of therapy (M6) and at follow-up (M12). LPS levels were significantly higher at all timepoints (W2, M6, and M12) than levels in healthy HIV-infected subjects. This implicates that microbial translocation is unaffected by anti-tuberculosis chemotherapy with or without HAART. Among HAART treated patients (group B), LPS levels increased significantly by 12 months (ie, 6 months after discontinuation of HAART) compared to LPS levels in patients from group A, who received anti-tuberculosis treatment alone. The increase in LPS in group B at M12 may be attributable to microbial translocation unleashed subsequent to discontinuation of HAART. This finding supports the already well-known contention that structured interruption of HAART (as in group B here) is ineffective and may even be damaging in HIV-1 disease at any CD4 T-cell count [29]. Continuous HAART has been found to be critically important in containment of HIV viral activity and improvement of mortality in HIV/tuberculosis patients [30].
Of note, 6 months after resolution of tuberculosis in HIV/tuberculosis patients in Cohort II, high plasma LPS levels were not associated with high sCD14. It is possible that microbial translocation is coupled to compartmentalized immune activation in gut associated lymphoid tissue (GALT) only not reflected systemically. Early institution of HAART was not effective in reversing GALT associated immunological changes as assessed by rectal biopsies in HIV-infected subjects [31]. As genomic HIV RNA induces innate immune responses [32], it is possible that HIV activity in GALT sustains local immune activation and microbial translocation.
In summary, tuberculosis associated immune activation in HIV/tuberculosis is dependent on levels of CD4 T-cell immunodeficiency. Immune activation remains unresolved in subjects with lower CD4 T-cell counts after tuberculosis treatment. In HIV/tuberculosis subjects with preserved CD4 T-cell count, active tuberculosis, and not microbial translocation may be a major driver of sCD14 levels, since high plasma sCD14 levels resolve with anti-tuberculosis chemotherapy. However, in this group of HIV/tuberculosis patients microbial translocation is unaffected by treatment of tuberculosis with or without HAART, despite resolution of tuberculosis associated immune activation. In these HIV/tuberculosis subjects, a recent (6-month) history of resolved infection is characterized by a higher degree of GALT associated microbial translocation. Continuous HAART is necessary in HIV/tuberculosis coinfection to control microbial translocation from this compartment effectively.
Notes
Acknowledgments. We wish to recognize the contribution of all patients who participated in this study.
We acknowledge Drs W. H. Boom and D. V. Havlir as coinvestigators of the controlled study of punctuated antiretroviral treatment of HIV/tuberculosis in Uganda, and Dr Michael Lederman at Case Western Reserve University for his helpful comments.
Financial support. These studies were supported by Tuberculosis Research Unit (AI 70022) and Center for AIDS Research at Case Western Reserve University, and funding from NHLBI (HL-05636) and NIAID (AI 080313).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lawn SD, Wood R, Wilkinson RJ. Changing concepts of “latent tuberculosis infection” in patients living with HIV infection. Clin Dev Immunol. 2011 doi: 10.1155/2011/980594. doi:10.1155/2011/980594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daley CL, Small PM, Schecter GF, et al. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus: an analysis using restriction-fragment-length polymorphisms. N Engl J Med. 1992;326:231–5. doi: 10.1056/NEJM199201233260404. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Gatell H, Cole SR, Margolick JB, et al. Effect of tuberculosis on the survival of HIV-infected men in a country with low tuberculosis incidence. AIDS. 2008;22:1869–73. doi: 10.1097/QAD.0b013e32830e010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med. 1995;151:129–35. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]
- 5.Toossi Z. Virological and immunological impact of tuberculosis on human immunodeficiency virus type 1 disease. J Infect Dis. 2003;188:1146–55. doi: 10.1086/378676. [DOI] [PubMed] [Google Scholar]
- 6.Kizza HM, Rodriguez B, Quinones-Mateu M, et al. Persistent replication of human immunodeficiency virus type 1 despite treatment of pulmonary tuberculosis in dually infected subjects. Clin Diagn Lab Immunol. 2005;12:1298–304. doi: 10.1128/CDLI.12.11.1298-1304.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nanteza MW, Mayanja-Kizza H, Charlebois E, et al. A randomized trial of punctuated antiretroviral therapy in Ugandan HIV-seropositive adults with pulmonary tuberculosis and CD4 T-cell counts of ≥350 cells/μL. J Infect Dis. 2011;204:884–92. doi: 10.1093/infdis/jir503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hertoghe T, Wajja A, Ntambi L, et al. T cell activation, apoptosis and cytokine dysregulation in the (co)pathogenesis of HIV and pulmonary tuberculosis (TB) Clin Exp Immunol. 2000;122:350–7. doi: 10.1046/j.1365-2249.2000.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goletti D, Weissman D, Jackson RW, et al. Effect of Mycobacterium tuberculosis on HIV replication; role of immune activation. J Immunol. 1996;157:1271–8. [PubMed] [Google Scholar]
- 10.Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–8. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 11.Butler SL, Valdez H, Westby M, et al. Disease-modifying therapeutic concepts for HIV in the era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2011;58:297–303. doi: 10.1097/QAI.0b013e31822ccfcc. [DOI] [PubMed] [Google Scholar]
- 12.Wright SD. CD14 and innate recognition of bacteria. J Immunol. 1995;155:6–8. [PubMed] [Google Scholar]
- 13.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–90. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anas A, van der Poll T, de Vos AF. Role of CD14 in lung inflammation and infection. Crit Care. 2010;14:209. doi: 10.1186/cc8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weaver LK, Hintz-Goldstein KA, Pioli PA, et al. Pivotal advance: activation of cell surface Toll-like receptors causes shedding of the hemoglobin scavenger receptor CD163. J Leukocyte Biol. 2006;80:26–35. doi: 10.1189/jlb.1205756. [DOI] [PubMed] [Google Scholar]
- 16.Burdo TH, Lentz MR, Autissier P, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after antiretroviral therapy. J Infect Dis. 2011;204:154–63. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 18.Jiang W, Lederman MM, Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177–85. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchetti G, Bellistri GM, Borghi E, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008;22:2035–8. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]
- 20.Redd AD, Dabitao D, Bream JH, et al. Microbial translocation, the innate cytokine response, and HIV-1 disease progression in Africa. Proc Natl Acad Sci USA. 2009;106:6718–23. doi: 10.1073/pnas.0901983106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redd AD, Gray RH, Quinn TC. Is microbial translocation a cause or consequence of HIV disease progression? J Infect Dis. 2011;203:744–5. doi: 10.1093/infdis/jiq107. author reply 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cassol E, Malfeld S, Mahasha P, et al. Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis. 2010;202:723–33. doi: 10.1086/655229. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch CS, Toossi Z, Johnson JL, et al. Augmentation of apoptosis and interferon-gamma production at sites of active Mycobacterium tuberculosis infection in human tuberculosis. J Infect Dis. 2001;183:779–88. doi: 10.1086/318817. [DOI] [PubMed] [Google Scholar]
- 24.Alwis KU, Milton DK. Recombinant factor C assay for measuring endotoxin in house dust: comparison with LAL, and (1–>3)-beta-D-glucans. Am J Ind Med. 2006;49:296–300. doi: 10.1002/ajim.20264. [DOI] [PubMed] [Google Scholar]
- 25.Juffermans NP, Verbon A, van Deventer SJ, et al. Serum concentrations of lipopolysaccharide activity-modulating proteins during tuberculosis. J Infect Dis. 1998;178:1839–42. doi: 10.1086/314492. [DOI] [PubMed] [Google Scholar]
- 26.Gutsmann T, Muller M, Carroll SF, MacKenzie RC, Wiese A, Seydel U. Dual role of lipopolysaccharide (LPS)-binding protein in neutralization of LPS and enhancement of LPS-induced activation of mononuclear cells. Infect Immun. 2001;69:6942–50. doi: 10.1128/IAI.69.11.6942-6950.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahan CS, Walusimbi M, Johnson DF, et al. Tuberculosis treatment in HIV infected Ugandans with CD4 counts >350 cells/mm reduces immune activation with no effect on HIV load or CD4 count. PloS ONE. 2010;5:e9138. doi: 10.1371/journal.pone.0009138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchetti G, Nasta P, Bai F, et al. Circulating sCD14 is associated with virological response to pegylated-interferon-α/ribavirin treatment in HIV/HCV coinfected patients. PLoS ONE. 2012;7:e32028. doi: 10.1371/journal.pone.0032028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 30.Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365:1492–501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tincati C, Biasin M, Bandera A, et al. Early initiation of highly active antiretroviral therapy fails to reverse immunovirological abnormalities in gut-associated lymphoid tissue induced by acute HIV infection. Antivir Ther. 2009;14:321–30. [PubMed] [Google Scholar]
- 32.Berg RK, Melchjorsen J, Rintahaka J, et al. Genomic HIV RNA induces innate immune responses through RIG-I-dependent sensing of secondary-structured RNA. PloS ONE. 2012;7:e29291. doi: 10.1371/journal.pone.0029291. [DOI] [PMC free article] [PubMed] [Google Scholar]