Abstract
The natural history of chlamydia is variable and may include persisting asymptomatic infection, complications, or spontaneous resolution before treatment. Reinfection is common. We evaluated whether spontaneous resolution was associated with decreased reinfection in women returning for treatment of a positive chlamydia screening test. At enrollment, participants were tested for chlamydia, treated with azithromycin, and scheduled for a 6-month follow-up visit for repeat testing. Two hundred participants returned 1 to 12 months after treatment. Spontaneous resolution at enrollment was demonstrated in 44 (22.0%). Reinfection at follow-up occurred in 33 (16.5%), being more frequent in those with persisting infection at enrollment versus spontaneous resolution (31 of 156 [19.9%] vs 2 of 44 [4.5%]; P = .016). Adjusting for age, the odds of reinfection was 4 times higher for participants with persisting infection at enrollment (odds ratio 4.0, 95% confidence interval, 1.1–25.6; P = .034). Chlamydia treatment may attenuate protective immunity in some patients.
Keywords: chlamydia, spontaneous, resolution, reinfection, recurrence, immunity, treatment
(See the editorial commentary by Brunham on pages 1796–7.)
Genital Chlamydia trachomatis infection (chlamydia) is a major public health burden. Chlamydia is the most common bacterial sexually transmitted infection (STI) in the United States and worldwide [1–3]. Over 1.3 million chlamydia cases were reported to the US Centers for Disease Control and Prevention (CDC) in 2010, with a calculated rate of 426 cases per 100 000 population [1]; this represents the highest rate of any reportable infection in the United States recorded by CDC surveillance [4]. However, reported cases underestimate the true burden of infection. It has been previously estimated that the annual number of new chlamydial infections worldwide is approximately 92 million [3] and for the United States is approximately 3 million [2]. Chlamydia is most common among adolescents and young adults [1]. Untreated chlamydia is associated with serious complications in women, including pelvic inflammatory disease, ectopic pregnancy, and infertility; chlamydia is a leading preventable cause of infertility worldwide. Despite chlamydia prevention and control efforts (including routine annual chlamydia screening in young sexually active women, prompt chlamydia treatment, partner treatment, and retesting for repeat infection), chlamydia prevalence in the United States has not significantly declined [1, 5].
The natural history of chlamydia in humans is variable. Untreated chlamydia may persist without symptoms for long periods, may progress to cause complications, or may resolve spontaneously without treatment (“self-cure”). Reinfection is common after therapy, occurring in about 10%–20% of patients within 12 months [6–8]; this suggests chlamydia does not elicit protective immunity in some patients. On the other hand, there is also evidence suggesting that host immune responses can control chlamydia naturally under certain circumstances. We and others have observed that about 20% of chlamydia-infected patients without signs of infection, who are diagnosed through screening, experience spontaneous resolution of their infection in the interval between their initial chlamydia screening test and returning for treatment for the positive test [9–11]. Neither the host factors contributing to spontaneous resolution of chlamydia in humans nor the clinical implications of spontaneous resolution have been fully elucidated. However, murine models of chlamydia demonstrate that when primary chlamydial infection resolves in the absence of therapy, it results in immunity against subsequent reinfection [12]. We hypothesized that spontaneous resolution of chlamydia in humans would be associated with a decreased risk for reinfection, and undertook a prospective study in women returning for treatment of a positive chlamydia screening test to evaluate the influence of spontaneous resolution on the risk for chlamydia reinfection. If the hypothesis is supported by the study findings, then this would suggest chlamydia treatment before natural resolution of infection could attenuate development of protective immunity in some patients, and stresses the need for an effective chlamydia vaccine to improve chlamydia prevention efforts.
MATERIALS AND METHODS
Study Design and Study Population
We prospectively collected data on the natural history of untreated chlamydia in the interval between testing and treatment and the epidemiology of chlamydia reinfection in women enrolled between January 2008 and June 2011. The study was approved by the University of Alabama at Birmingham (UAB) and Jefferson County Department of Health (JCDH) institutional review boards. Written informed consent was obtained from all patients who participated in the study. The study population was comprised of women ≥16 years of age presenting to the JCDH Sexually Transmitted Disease (STD) Clinic in Birmingham, Alabama, for treatment of a positive chlamydia screening test. A 60-day maximum time interval between screening and returning for treatment was chosen to minimize confounding factors such as unreported interval treatment or exposure to a new chlamydial infection. The chlamydia test used during the study period was the Gen-Probe Aptima Combo 2 (GP AC2; Gen-Probe, San Diego, CA) nucleic acid amplification test, performed according to the manufacturer's protocol. Although many women tested for chlamydia in the clinic are empirically treated at the time of initial evaluation for a chlamydia-associated syndrome or as a recent sexual contact to a potentially chlamydia-infected partner, only women who had not received antibiotics effective for C. trachomatis at the time of screening or in the interval between screening and returning to the clinic for treatment were eligible for study inclusion. We excluded women who were pregnant or had a prior hysterectomy.
Study Participant Recruitment and Follow-Up
Women were recruited for the study at the time they presented to the clinic for treatment of a positive chlamydia screening test (all screening tests had been performed on cervical swab specimens). At enrollment, participants were interviewed by either a research study coordinator or an investigator and had their clinic record reviewed. The following data were then recorded on a data collection form: demographic characteristics, sexual history (including STI history and risk factors), hormonal contraception use, condom use, antibiotic use, and reported partner treatment. Urine was collected for a pregnancy test, and a pelvic examination was performed to obtain a vaginal swab specimen for a wet mount and a cervical swab specimen for chlamydia and gonorrhea testing by GP AC2. All participants received directly observed therapy with azithromycin 1 g (consisting of 2 azithromycin 500 mg tablets) and were encouraged to refer all sexual partners for treatment if not already treated. A follow-up visit was scheduled for approximately 6 months. We included data for determining reinfection status from any follow-up visit between 1 to 12 months after treatment if this was the time the patient returned to the study staff at the clinic. Follow-up earlier than 1 month was not deemed evaluable for reinfection because of concern that a false positive GP AC2 result could occur from insufficient time to permit clearance of residual C. trachomatis nucleic acids from nonviable organisms from the genital tract [13–15]. At the follow-up visit, participants were interviewed and had their clinic record reviewed to obtain the same information collected at enrollment, other than demographic characteristics, and also were asked about partner treatment, new sexual partners since treatment, and occurrence of any interim visits in which antibiotics may have been taken or STI testing was performed and, if tested, the results of the tests (an unscheduled visit occurred in about 20% of participants). Cervical swabs were again collected for chlamydia and gonorrhea testing by GP AC2.
Statistical Analysis
Patients were classified as either having spontaneous resolution of chlamydia or persisting chlamydial infection at the time of enrollment (time of treatment) based on negative or positive chlamydia test results, respectively, from the GP AC2 performed at that visit. Univariate associations between spontaneous resolution status and other participant characteristics with reinfection between 1–12 months after treatment were investigated using Pearson's χ2 or Fisher's exact test for categorical characteristics and the Wilcoxon rank sum test for continuous variables (ie, length of follow-up). Variables that were significant at the α = 0.10 level were candidates for inclusion in a multivariable logistic regression model, and variables maintaining significance at the α = 0.10 level were retained in the model. Model fit was assessed using deviance and Pearson goodness of fit tests. Given the association among predictor variables, likelihood ratio P values were computed. To characterize bias associated with being lost to follow-up, univariate analyses of participant baseline characteristics according to follow-up status were performed. All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
Of 245 women enrolled, 200 (81.6%) returned for a follow-up visit. Study participant characteristics at enrollment for those who returned for a follow-up visit can be found in Table 1. The study population was predominantly African American (93.5%) with a median (range) age of 22 (16–54) years. The majority (56.0%) had a history of prior chlamydia (by patient report and/or review of prior laboratory testing). Bacterial vaginosis was frequently diagnosed at enrollment (31.0%), but other genital infections occurred more rarely. Spontaneous resolution of chlamydia was demonstrated in 44 participants (22.0%) at enrollment (the time when patients returned for treatment of a positive chlamydia screening test), with a median interval between screening and enrollment of 15 days (range 6–47 days).
Table 1.
Study Participant Characteristics at Enrollment (n = 200)
| Characteristic | n (%) |
|---|---|
| Age (years) | |
| <25 | 129 (64.5) |
| ≥25 | 71 (35.5) |
| Race/ethnicity | |
| Non–African American/ non-Hispanica | 13 (6.5) |
| African American/ non-Hispanic | 187 (93.5) |
| Chlamydia status | |
| Spontaneous resolution | 44 (22.0) |
| Persisting infection | 156 (78.0) |
| Prior chlamydia | |
| No | 88 (44.0) |
| Yes | 112 (56.0) |
| Hormonal contraception use | |
| No | 118 (59.0) |
| Yes | 82 (41.0) |
| Reported condom use | |
| No | 82 (41.0) |
| Yes | 118 (59.0) |
| Sexual partners prior 6 mo | |
| Median | 2 |
| Range | 0–10 |
| Concomitant gonorrhea | |
| No | 195 (97.5) |
| Yes | 5 (2.5) |
| Concomitant bacterial vaginosis | |
| No | 138 (69.0) |
| Yes | 62 (31.0) |
| Concomitant vaginal candidiasis | |
| No | 174 (87.0) |
| Yes | 26 (13.0) |
| Concomitant trichomoniasis | |
| No | 191 (95.5) |
| Yes | 9 (4.5) |
a One non–African American participant was Asian; the remaining were white.
To characterize the bias due to participants being lost to follow-up, participant characteristics were compared according to follow-up status. There were no significant differences in terms of spontaneous resolution of chlamydia at enrollment, which occurred in 22% of participants in those with and without follow-up visits. There were also no significant differences by age, race, or history of prior chlamydia. There was a lower median number of sexual partners in the prior 6 months for those lost to follow-up as compared to those who were not, although this was not statistically significant (median [range] was 1 [1–5] vs 2 [0–10]; P = .058).
At follow-up, 96.0% (192/200) reported being sexually active since treatment and 71.0% (142/200) reported condom use since treatment. Also, 76.6% (147/192) reported partners receiving treatment and 40.6% (78/192) reported new partners. There were 9.5% (19/200) diagnosed with an STI other than chlamydia in the interim (12 with trichomoniasis, 6 with gonorrhea, and 1 with both trichomoniasis and gonorrhea). Posttreatment behaviors did not differ significantly by chlamydia resolution status at the time of enrollment; however, a higher proportion of those with persistent infection at enrollment reported to have new partners post treatment as compared to those with spontaneous resolution at enrollment (42.9% vs 27.3%; P = .062).
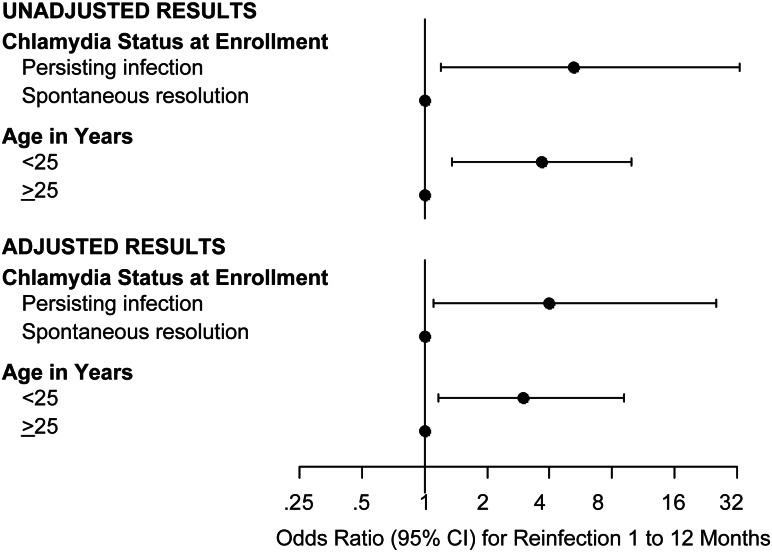
Chlamydia reinfection occurred in 33 (16.5%) participants with a follow-up study visit. The median time from enrollment to the follow-up study visit was 183 days, and duration of follow-up did not differ in those without versus those with reinfection (184 days [range 35–365] vs 175 days [range 42–312]; P = .22). Reinfection rates according to participant characteristics are shown in Table 2. Spontaneous resolution status at enrollment and age were each significantly associated with reinfection status in univariate analyses (Table 2 and Figure 1). Compared to participants with spontaneous resolution of chlamydia demonstrated at enrollment, a higher proportion of those with persisting infection had reinfection at the follow-up study visit (31 of 156 [19.9%] vs 2 of 44 [4.5%]; odds ratio [OR] 5.2, 95% confidence interval [CI], 1.2–33.0; P = .016). Compared to participants aged ≥25 years, a higher proportion of those <25 years had reinfection (28 of 129 [21.7%] vs 5 of 71 [7.0%]; OR 3.7, 95% CI, 1.4–10.0; P = .008). There was a lower reinfection rate among women reporting that their partners had received chlamydia treatment as compared to those whose partners were not known to have been treated, although this was not statistically significant (19 of 147 [12.9%] vs 11 of 45 [24.4%]; OR 0.5, 95% CI, .2–1.1; P = .063).
Table 2.
Chlamydia Reinfection Rates According to Participant Characteristics (n = 200)
| Characteristic | Chlamydia reinfection at follow-up % (n) | Unadjusted OR | Unadjusted P valuea | Adjusted OR | Adjusted 95% CI | Adjusted P valueb |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| ≥25 | 7.0% (5/71) | … | … | … | … | … |
| <25 | 21.7% (28/129) | 3.7 | .008 | 3.0 | 1.2–9.2 | .022 |
| Race/Ethnicity | ||||||
| Non–African American/ non-Hispanic | 7.7% (1/13) | … | … | … | … | … |
| African American/ non-Hispanic | 17.1% (32/187) | 2.5 | .70 | … | … | … |
| Prior chlamydia | ||||||
| No | 17.0% (15/88) | … | … | … | … | … |
| Yes | 16.1% (18/112) | 0.9 | .85 | … | … | … |
| Hormonal contraception use | ||||||
| No | 14.4% (17/118) | … | … | … | … | … |
| Yes | 19.5% (16/82) | 1.4 | .34 | … | … | … |
| Chlamydia status | ||||||
| Spontaneous resolution | 4.5% (2/44) | … | … | … | … | … |
| Persisting infection | 19.9% (31/156) | 5.2 | .016 | 4.0 | 1.1–25.6 | .034 |
| Partner treated | ||||||
| No/don't knowc | 24.4% (11/45) | … | … | … | … | … |
| Yes | 12.9% (19/147) | 0.5 | .063 | … | … | … |
| Sexually active since treatment | ||||||
| No | 25.0% (2/8) | … | … | |||
| Yes | 16.1% (31/192) | 0.6 | .62 | … | … | … |
| New partners since treatment | ||||||
| No/not applicabled | 14.2% (17/120) | … | … | … | … | … |
| Yes | 19.2% (15/78) | 1.4 | .34 | … | … | … |
| Condom use since treatment | ||||||
| Yes | 16.9% (24/142) | … | … | … | … | … |
| No | 15.5% (9/58) | 0.9 | .81 | … | … | … |
| STI other than CT at follow-up | ||||||
| No | 15.5% (28/181) | … | … | … | … | … |
| Yes | 26.3% (5/19) | 2.0 | .32 | … | … | … |
Adjusted ORs are shown for those variables entered and retained in the final logistic regression model (P < .10).
Abbreviations: CI, confidence interval; CT, chlamydia; OR, odds ratio; STI, sexually transmitted infection.
a P value for χ2 or Fisher's exact test (univariate analysis).
b P value for multivariable logistic regression model.
c Data missing for 8 women; 19 women reported Don't know.
d Data missing for 2 women; not applicable for 8 women who were not sexually active since treatment.
Figure 1.
Forest plot of odds ratios and 95% CI for chlamydia reinfection at follow-up based on chlamydia resolution status at enrollment (time of treatment) and age. Estimates to the right of the vertical line indicate reinfection is more likely, and those to the left indicate reinfection is less likely. When the 95% confidence interval does not cross the vertical line, then the odds ratio is significantly different from 1. Unadjusted results are from separate univariate analyses for chlamydia resolution status at enrollment (the time of treatment) and age. Adjusted results are estimates from a multivariable model, including chlamydia resolution status at enrollment and age as independent variables. Abbreviation: CI, confidence interval.
Spontaneous chlamydia resolution status at enrollment, age, and reported partner treatment met the multivariate model inclusion criteria, but reported partner treatment did not meet the retention criteria after adjusting for resolution status and age (P = .123). After adjusting for age, the odds of chlamydia reinfection was 4 times higher for participants with persisting infection demonstrated at enrollment compared with those with spontaneous resolution (OR 4.0, 95% CI, 1.1–25.6; P = .034) (Figure 1). Also, the odds of reinfection was 3.0 times higher for participants aged <25 years as compared to those who were older (OR 3.0, 95% CI, 1.2–9.2; P = .022). The goodness-of-fit tests were not rejected, indicating adequate model fit.
DISCUSSION
An understanding of the natural history of chlamydia in humans and the potential for development of protective immunity is important both for chlamydia screening recommendations and for efforts to develop chlamydial vaccines. We and others have observed that about 20% of chlamydia-infected patients experience spontaneous resolution of infection in the interval between chlamydia screening and returning for treatment of a positive test [9–11], providing evidence that host immune responses can eradicate chlamydia in the absence of antimicrobial therapy. Murine models of genital chlamydia have demonstrated that resolution of untreated infection leads to development of protection against reinfection [12], but do humans develop protection against reinfection after spontaneous resolution? Our data suggest that the answer is yes. Our study is the first to demonstrate an association of natural resolution of genital chlamydial infection in humans with a decreased risk for reinfection. Other than the prior human ocular challenge experiments and vaccine trials with trachoma (ocular infection due to unique C. trachomatis serovars) demonstrating resistance to repeat trachoma infection [16, 17], our study provides some of the most direct evidence to date supporting protective immunity to C. trachomatis reinfection in humans.
Indirect evidence from studies using either documented prior chlamydia or older age in populations at risk for chlamydia as surrogates for protective immunity to chlamydia also support the notion that humans develop protective immunity. For example, a study of STD clinic attendees demonstrated that prior documented chlamydia was associated with a reduced risk of reinfection, although resistance to reinfection appeared to be of limited duration [18]. Another example includes a study of female sex workers that found that longer duration of prostitution was associated with reduced risk for chlamydia [19]. In our study, we found that older age (≥25 years) was associated with a lower risk for reinfection, but prior chlamydia was not associated with reinfection. Data from US CDC chlamydial surveillance [1], from a US managed care population database [20], and from a nationally representative survey [5] have all demonstrated low chlamydia prevalence rates in women ≥25 years of age, and such data are used in determining the age cutoff for chlamydia screening recommendations in women [13, 21]. While lower chlamydia prevalence in older age groups may, in part, reflect lower behavioral risk, it may also reflect protective immunity that developed from repeated chlamydia exposures in a subset of the older population. Although we collect self-reported data and review patients’ medical records to assess for a history of prior chlamydia, we still cannot exclude the possibility that subjects without a reported history of chlamydia still might have had prior chlamydia. It is likely that prior chlamydia is underrecognized in populations with a high chlamydia prevalence (eg, an STD clinic population such as in our study), and this could have led to misclassification of prior chlamydia in our study.
Our study reaffirms that up to 20% of women who test chlamydia-positive at the time of treatment will have subsequent reinfection within a year of treatment for infection. This relatively high rate of chlamydia reinfection after therapy may suggest that some infected individuals do not develop protective immunity. Duration of infection and timing of treatment may modify resistance to future infection. In a murine chlamydia model, antibiotic treatment given before immune responses were fully developed impaired development of protective immunity [22]. Our study did not have sufficient information on previous sexual behaviors and chlamydia testing that would allow us to determine the duration of infection prior to therapy (ie, time between infection acquisition and treatment). Recent population-based studies from Canada have reported high chlamydia reinfection rates [23, 24]; it has been suggested that this may be, in part, due to early treatment of chlamydia through a chlamydia control program leading to attenuated development of immunity and, as a result, increased susceptibility to reinfection (“the arrested immunity hypothesis”) [25]. Although chlamydia control efforts with increases in chlamydia screening and treatment appear beneficial in decreasing the incidence of chlamydia complications [20, 26–29], this could be leading to more chlamydia reinfections due to attenuated immunity, and stresses the need for a chlamydia vaccine to further assist in chlamydia prevention efforts. Until a safe, effective chlamydia vaccine is available, it will be important to continue to provide chlamydia treatment to minimize the risk for complications, many of which are subclinical or silent.
Our study is not without other limitations. The study population was women, mostly African American, evaluated in an STD clinic setting. It is unknown whether our study findings will be applicable to populations with different demographics and/or clinical settings. The race distribution of our study population is representative of the population of women routinely seen at the JCDH STD Clinic. Men evaluated at STD clinics are difficult to enroll in such a natural history study design because they are often empirically treated for urethritis before chlamydia test results are known, prohibiting the opportunity to evaluate for spontaneous resolution before returning for treatment of a positive test. One may question whether the initial chlamydia screening test was a false-positive and that subjects classified as spontaneously resolved may have not been infected. This is highly unlikely for most subjects considering the high specificity (99%) of GP AC2 on cervical specimens and the high positive predictive value (PPV) in our clinic population (PPV 94% using an approximate chlamydia prevalence of 11% for our population) [30]. The CDC does not recommend a confirmatory nucleic acid amplification test in populations with ≥90% PPV [31]. While we believe most repeat positive tests at follow-up were due to reinfection (from a current or new partner), we cannot exclude the possibility that a small number of these could have been persisting infection due to azithromycin treatment failure. A recent study reported a high rate of azithromycin treatment failure in men with symptomatic chlamydia urethritis [32], although other recent studies suggest most chlamydia-infected women treated with azithromycin are cured [33, 34]. We also cannot exclude the possibility that C. trachomatis genotype differences could influence risk for chlamydia persistence and/or reinfection, and future chlamydia natural history studies aimed at understanding these outcomes should consider incorporating C. trachomatis genotyping. The duration of follow-up for up to 12 months in our study only provides the opportunity to evaluate short-term immunity, and it is possible as has been suggested [18, 19] that human immunity to chlamydia may only be short-lived. Longer prospective follow-up in an STD clinic population can be challenging, as evidenced by the already 18% lost to follow-up in our study with follow-up of up to 12 months. The lack of significant differences in spontaneous resolution rate and most other patient characteristics in those who were versus were not lost to follow-up provides some reassurance that study outcomes were not greatly biased by participants lost to follow-up. Finally, understanding how the course of untreated chlamydia may be modified by host immune responses requires further in-depth investigation. While murine studies indicate CD4+ T-helper type 1 immune responses are essential for chlamydia resolution and protection against reinfection [12], effector immune mechanisms may differ in humans.
In summary, we present the first evidence in humans that spontaneous resolution of genital chlamydial infection is associated with decreased risk for reinfection. A challenge ahead of us will be to understand the immune mechanisms that contribute to resolution of infection and development of protective immunity. It will also be important to identify genetic determinants that modulate the immune responses. Such knowledge could provide correlates of protective immunity necessary for the development of an effective chlamydia vaccine.
Notes
Acknowledgments. We thank staff from the UAB STD Program and the JCDH STD Clinic for their valuable contributions.
Financial support. This work was supported by the National Institutes of Health [NIH K23AI069505 to W. M. G.].
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Atlanta: U.S. Department of Health and Human Services; 2011. Sexually Transmitted Disease Surveillance 2010. [Google Scholar]
- 2.Cates W., Jr Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. Sex Transm Dis. 1999;26:S2–7. doi: 10.1097/00007435-199904001-00002. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Global prevalence and incidence of selected curable sexually transmitted infections: overview and estimates. http://www.who.int/hiv/pub/sti/who_hiv_aids_2001.02.pdf. Accessed 19 October 2012. [PubMed]
- 4.Centers for Disease Control and Prevention. Summary of notifiable diseases—United States, 2010. MMWR. 2012;59(No. RR-53):1–116. [PubMed] [Google Scholar]
- 5.Datta SD, Torrone E, Kruszon-Moran D, et al. Chlamydia trachomatis trends in the United States among persons 14 to 39 years of age, 1999–2008. Sex Transm Dis. 2012;39:92–6. doi: 10.1097/OLQ.0b013e31823e2ff7. [DOI] [PubMed] [Google Scholar]
- 6.Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis. 2009;36:478–89. doi: 10.1097/OLQ.0b013e3181a2a933. [DOI] [PubMed] [Google Scholar]
- 7.Xu F, Stoner BP, Taylor SN, et al. Use of home-obtained vaginal swabs to facilitate rescreening for Chlamydia trachomatis infections: two randomized controlled trials. Obstet Gynecol. 2011;118(2 Pt 1):231–9. doi: 10.1097/AOG.0b013e3182246a83. [DOI] [PubMed] [Google Scholar]
- 8.Dunne EF, Chapin JB, Rietmeijer CA, et al. Rate and predictors of repeat Chlamydia trachomatis infection among men. Sex Transm Dis. 2008;35:S40–4. doi: 10.1097/OLQ.0b013e31817247b2. [DOI] [PubMed] [Google Scholar]
- 9.Geisler WM, Wang C, Morrison SG, Black CM, Bandea CI, Hook EW., 3rd The natural history of untreated Chlamydia trachomatis infection in the interval between screening and returning for treatment. Sex Transm Dis. 2008;35:119–23. doi: 10.1097/OLQ.0b013e318151497d. [DOI] [PubMed] [Google Scholar]
- 10.Joyner JL, Douglas JM, Jr, Foster M, Judson FN. Persistence of Chlamydia trachomatis infection detected by polymerase chain reaction in untreated patients. Sex Transm Dis. 2002;29:196–200. doi: 10.1097/00007435-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Rogers SM, Miller WC, Turner CF, et al. Concordance of Chlamydia trachomatis infections within sexual partnerships. Sex Transm Infect. 2008;84:23–8. doi: 10.1136/sti.2007.027029. [DOI] [PubMed] [Google Scholar]
- 12.Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect Immun. 2002;70:2741–51. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. MMWR. 2010;59(No. RR-12)):44–7. [PubMed] [Google Scholar]
- 14.Workowski KA, Lampe MF, Wong KG, Watts MB, Stamm WE. Long-term eradication of Chlamydia trachomatis genital infection after antimicrobial therapy. Evidence against persistent infection. JAMA. 1993;270:2071–5. [PubMed] [Google Scholar]
- 15.Renault CA, Israelski DM, Levy V, Fujikawa BK, Kellogg TA, Klausner JD. Time to clearance of Chlamydia trachomatis ribosomal RNA in women treated for chlamydial infection. Sex Health. 2011;8:69–73. doi: 10.1071/SH10030. [DOI] [PubMed] [Google Scholar]
- 16.Jawetz E, Rose L, Hanna L, Thygeson P. Experimental inclusion conjunctivitis in man: measurements of infectivity and resistance. JAMA. 1965;194:620–32. [PubMed] [Google Scholar]
- 17.Grayston JT, Wang SP, Yeh LJ, Kuo CC. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985;7:717–25. doi: 10.1093/clinids/7.6.717. [DOI] [PubMed] [Google Scholar]
- 18.Katz BP, Batteiger BE, Jones RB. Effect of prior sexually transmitted disease on the isolation of Chlamydia trachomatis. Sex Transm Dis. 1987;14:160–4. doi: 10.1097/00007435-198707000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Brunham RC, Kimani J, Bwayo J, et al. The epidemiology of Chlamydia trachomatis within a sexually transmitted diseases core group. J Infect Dis. 1996;173:950–6. doi: 10.1093/infdis/173.4.950. [DOI] [PubMed] [Google Scholar]
- 20.Scholes D, Satterwhite CL, Yu O, Fine D, Weinstock H, Berman S. Long-term trends in Chlamydia trachomatis infections and related outcomes in a US managed care population. Sex Transm Dis. 2012;39:81–8. doi: 10.1097/OLQ.0b013e31823e3009. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Preventive Services Task Force. Screening for chlamydial infection: U.S. preventive services task force recommendation statement. Ann Intern Med. 2007;147:128–34. doi: 10.7326/0003-4819-147-2-200707170-00172. [DOI] [PubMed] [Google Scholar]
- 22.Su H, Morrison R, Messer R, Whitmire W, Hughes S, Caldwell HD. The effect of doxycycline treatment on the development of protective immunity in a murine model of chlamydial genital infection. J Infect Dis. 1999;180:1252–8. doi: 10.1086/315046. [DOI] [PubMed] [Google Scholar]
- 23.Brunham RC, Pourbohloul B, Mak S, White R, Rekart ML. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J Infect Dis. 2005;192:1836–44. doi: 10.1086/497341. [DOI] [PubMed] [Google Scholar]
- 24.Généreux M, Leclerc P, Bédard L, Allard R. Upsurge of chlamydial reinfection in a large Canadian city: an indication of suboptimal chlamydia screening practices? Can J Public Health. 2010;101:420–4. doi: 10.1007/BF03404865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunham RC, Rekart ML. The arrested immunity hypothesis and the epidemiology of chlamydia control. Sex Transm Dis. 2008;35:53–4. doi: 10.1097/OLQ.0b013e31815e41a3. [DOI] [PubMed] [Google Scholar]
- 26.Moss NJ, Ahrens K, Kent CK, Klausner JD. The decline in clinical sequelae of genital Chlamydia trachomatis infection supports current control strategies. J Infect Dis. 2006;193:1336–8. doi: 10.1086/503114. [DOI] [PubMed] [Google Scholar]
- 27.Sutton MY, Sternberg M, Zaidi A, St Louis ME, Markowitz LE. Trends in pelvic inflammatory disease hospital discharges and ambulatory visits, United States, 1985–2001. Sex Transm Dis. 2005;32:778–84. doi: 10.1097/01.olq.0000175375.60973.cb. [DOI] [PubMed] [Google Scholar]
- 28.Chen MY, Fairley CK, Donovan B. Discordance between trends in chlamydia notifications and hospital admission rates for chlamydia related diseases in New South Wales, Australia. Sex Transm Infect. 2005;81:318–22. doi: 10.1136/sti.2004.012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rekart ML, Gilbert M, Meza R, et al. Chlamydia public health programs and the epidemiology of pelvic inflammatory disease and ectopic pregnancy. J Infect Dis. 2013;207:30–8. doi: 10.1093/infdis/jis644. [DOI] [PubMed] [Google Scholar]
- 30.Van Der Pol B, Liesenfeld O, Williams JA, et al. Performance of the cobas CT/NG test compared to the Aptima AC2 and Viper CTQ/GCQ assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2012;50:2244–9. doi: 10.1128/JCM.06481-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Screening tests to detect Chlamydia trachomatis and Neisseria gonorrhoeae infections—2002. MMWR. 2002;51(No. RR-15):1–40. [PubMed] [Google Scholar]
- 32.Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: emphasizing emerging pathogens—a randomized clinical trial. Clin Infect Dis. 2011;52:163–70. doi: 10.1093/cid/ciq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batteiger BE, Tu W, Ofner S, et al. Repeated Chlamydia trachomatis genital infections in adolescent women. J Infect Dis. 2010;201:42–51. doi: 10.1086/648734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golden MR, Whittington WL, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med. 2005;352:676–85. doi: 10.1056/NEJMoa041681. [DOI] [PubMed] [Google Scholar]