Abstract
Background. Correlates of immune protection in patients with human immunodeficiency virus (HIV)–associated cryptococcal meningitis are poorly defined. A clearer understanding of these immune responses is essential to inform rational development of immunotherapies.
Methods. Cryptococcal-specific peripheral CD4+ T-cell responses were measured in 44 patients with HIV-associated cryptococcal meningitis at baseline and during follow-up. Responses were assessed following ex vivo cryptococcal mannoprotein stimulation, using 13-color flow-cytometry. The relationships between cryptococcal-specific CD4+ T-cell responses, clinical parameters at presentation, and outcome were investigated.
Results. Cryptococcal-specific CD4+ T-cell responses were characterized by the production of macrophage inflammatory protein 1α, interferon γ (IFN-γ), and tumor necrosis factor α (TNF-α). Conversely, minimal interleukin 4 and interleukin 17 production was detected. Patients surviving to 2 weeks had significantly different functional CD4+ T-cell responses as compared to those who died. Patients with a response predominantly consisting of IFN-γ or TNF-α production had a 2-week mortality of 0% (0/20), compared with 25% (6/24) in those without this response (P = .025). Such patients also had lower fungal burdens (10 400 vs 390 000 colony-forming units/mL; P < .001), higher cerebrospinal fluid lymphocyte counts (122 vs 8 cells/μL; P < .001), and a trend toward faster rates of clearance of infection.
Conclusions. The phenotype of the peripheral CD4+ T-cell response to Cryptococcus was associated with disease severity and outcome in HIV-associated cryptococcal meningitis. IFN-γ/TNF-α–predominant responses were associated with survival.
Keywords: HIV, cryptococcus neoformans, TB, CMV, memory T cells, flow cytometry
(See the editorial commentary by Williamson on pages 1793–5.)
Cryptococcal meningitis is a leading cause of mortality in human immunodeficiency virus (HIV)–infected patients in the developing world [1]. A clear understanding of the underlying immune response to Cryptococcus neoformans is essential to help elucidate the causes of mortality and inform development of immune-based therapies. Unfortunately, correlates of immune protection in patients with HIV-associated cryptococcal meningitis remain poorly defined.
Most data regarding the immune response to C. neoformans comes from animal models and in vitro work. Cryptococcus evades killing by the innate immune system [2], and adaptive CD4+ T-cell responses are critical for immune control and clearance [3–6]. In mouse models, a dichotomy exists between T-helper 1 (Th1)–type T-cell responses and T-helper 2 (Th2)–type responses. Th1-type responses are protective and associated with proinflammatory cytokine responses; production of interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), and interleukin 12; and effective intracellular killing of C. neoformans by classically activated macrophages [3, 4, 7–20]. Th2-type responses appear to be detrimental and are characterized by production of interleukin 10 (IL-10), interleukin 4 (IL-4), and interleukin 13; alternative macrophage activation; increased C. neoformans proliferation; and impaired killing by innate effector cells [7, 16–28]. T-helper 17 (Th17)–type cytokine production has also been associated with reduced fungal burdens and effective resolution of infection [29–32].
Human data are limited. The epidemiology of cryptococcal disease clearly demonstrates that CD4+ T-cell depletion is the key predisposing factor [33]. Cryptococcal meningitis nearly exclusively affects patients with profound defects in cell-mediated immunity. In HIV-infected patients who develop cryptococcal meningitis, adverse clinical and microbiological outcomes are associated with lower CD4+ T-cell counts and poor inflammatory responses in the cerebrospinal fluid (CSF) [34–36], but the phenotype of the immune response in HIV-infected patients with cryptococcal meningitis is not well described. HIV disease progression has been associated with a loss of Th1-type responses and a switch to Th2-weighted CD4+ T-cell and cytokine responses [37–39], although very few data are available that directly examine the functional phenotypes of CD4+ T cells in HIV-infected patients with advanced disease.
To explore the host response to cryptococcal infection in patients with HIV-associated cryptococcal meningitis, both at the site of infection in the central nervous system and systemically, CSF cytokine levels and Cryptococcus-specific peripheral CD4+ T-cell responses were measured in an exploratory analysis of 44 patients with acute cryptococcal meningitis enrolled in a trial of adjuvant interferon gamma immunotherapy. The phenotype of the peripheral CD4+ T-cell responses to Cryptococcus was compared to better characterized antigen-specific cytomegalovirus (CMV)– and Mycobacterium tuberculosis–specific responses [40, 41], both to act as internal validation of the performance of the experiments and to enable a comparison between the CD4+ T-cell responses to mycobacterial, fungal, and viral opportunistic infections. The relationships between Cryptococcus-specific peripheral CD4+ T-cell responses and clinical parameters and outcomes were explored.
METHODS
Study Subjects
Subjects were participants in a randomized controlled trial examining the effect of short-course adjuvant interferon gamma immunotherapy for the treatment of HIV-associated cryptococcal meningitis. The trial was performed in Cape Town, South Africa, between 2007 and 2010 and has been described elsewhere (Supplementary Methods) [42]. Written informed consent was obtained. The study was approved by the research ethics committees of the University of Cape Town and St. George's University of London.
CSF Cytokine Analysis
CSF samples were collected at baseline, prior to receipt of antifungal therapy, and centrifuged, and the supernatant was frozen at −80°C for subsequent quantification of cytokine concentrations. Levels of interleukin 2 (IL-2), IL-4, interleukin 6 (IL-6), IL-10, interleukin 17 (IL-17), IFN-γ, TNF-α, RANTES, macrophage inflammatory protein 1α (MIP-1α), monocyte chemotactic protein 1 (MCP-1), and granulocyte-macrophage colony-stimulating factor (GM-CSF) were measured using Luminex multiplex cytokine analysis (Bio-plex kits, Bio-Rad Laboratories).
Peripheral Blood Mononuclear Cell (PBMC) Flow Cytometry Analysis
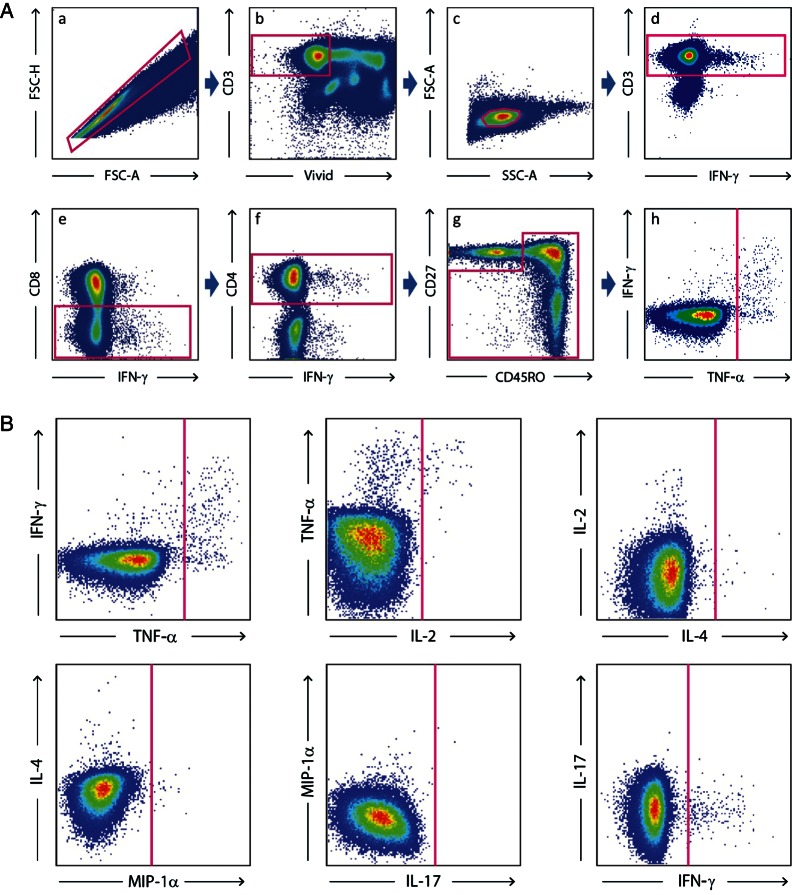
PBMCs were obtained from 30 mL of venous blood taken at study admission (prior to any therapy), study day 14, and after 1 month of antiretroviral therapy (ART). Cryptococcal antigen (CRAG) stimulations were performed using purified cryptococcal mannoproteins [43]. CMV stimulations were performed using pp65 peptides, and M. tuberculosis stimulations were performed using a mix of purified protein derivative, ESAT-6, and CFP-10. Cell stimulation and staining were performed using a modification of the method described by Betts et al [44]. Cells were analyzed using a modified LSRII (BD Immunocytometry Systems). Analytic gating of the flow cytometry data was performed using FlowJo (version 9.0.1; TreeStar). For polychromatic analysis, all CD4+ T cells were identified in the same manner, and standard cytokine gates were applied to all samples. The memory T-cell population was defined as CD3+CD8−CD4+ cells that were not CD27+CD45RO−. Cytokine gating for IFN-γ, IL-2, IL-4, IL-17, MIP-1α, and TNF-α was done on the memory-cell population (Supplementary Methods and Figure 1).
Figure 1.
A, Analytic gating of the flow cytometry data. a, Singlet cells were sorted from aggregates on the basis of forward-scatter height (FSC-H) and forward-scatter area (FSC-A). b, Dead cells, B-cells, and monocytes were excluded by staining with live/dead violet viability/vitality dye staining and CD14 and CD19 staining. c, The small lymphocyte population was selected. d, CD3+ cells were selected. CD3+CD8− cells (e) and then CD3+CD4+ cells (f) were sequentially selected. g, The memory T-cell population was defined as CD3+CD8−CD4+ cells that were not CD27+CD45RO−. h, Cytokine gating for interferon γ (IFN-γ), interleukin 2 (IL-2), interleukin 4 (IL-4), interleukin 17 (IL-17), macrophage inflammatory protein 1α (MIP-1α) and tumor necrosis factor α (TNF-α) was done on the memory cell population. B, Analytic gating of the flow cytometry data is shown. Cytokine gating for IFN-γ, IL-2, IL-4, IL-17, MIP-1α, and TNF-α was done on the memory cell population.
Statistical Analysis
Data were analyzed using Stata, version 11.0 (StataCorp); Prism, version 5a (GraphPad Software); and Spice, version 5.2 (NIAID, NIH, Bethesda, MD). Variables were compared across groups, using the Mann–Whitney U, Kruskal–Wallis, χ2, or Fisher exact tests. Comparisons of paired groups were made using the Wilcoxon matched pairs test. The Spearman correlation coefficient was used to examine associations between continuous variables, and an adjusted linear regression model compared early fungicidal activity by treatment group. Patterns of cytokine production between response phenotypes were compared by permutation analysis, using a 1 000 000-iteration Monte Carlo simulation model described in detail elsewhere [45]. Because this was an exploratory study to highlight potentially novel findings and avenues for future research, adjustment for multiple comparisons was not made, because use of stringent adjustment markedly increases the probability of type 2 errors. For all analyses, the 2-dose and 6-dose IFN-γ treatment groups were considered as a single “IFN-γ–treated” group. Statistical significance was defined as P value of ≤ .05.
RESULTS
PBMCs were collected from 44 HIV-infected patients at presentation with cryptococcal meningitis. The median age was 32 years, 43% were male, and the median CD4+ T-cell count was 24 cells/μL (Table 1). Eighteen patients received standard antifungal therapy, and 26 received standard therapy plus interferon gamma. Two-week mortality was 14%. For patients who survived, ART was initiated after a median of 23 days of antifungal therapy. None of the patients had clinically apparent CMV disease at the time of sample collection or developed CMV end-organ disease during the first year of ART. Thirty-four percent of patients (15) were being treated for tuberculosis at the time of sample collection, a further 23% (10) had a history of treated tuberculosis, and 5% (2) developed tuberculosis during the 1-year follow-up period. Additional PBMC samples were collected from 37 of the 38 surviving patients 2 weeks after the initial sample was collected, following completion of induction-phase antifungal therapy but prior to ART initiation, and from 16 surviving patients 1 month following ART initiation.
Table 1.
Baseline Characteristics of the Cohort
| Variable | Overall | Alive at 2 wk | Died at 2 wk |
|---|---|---|---|
| Age, y | 32 (28–38) | 32 (27–38) | 32 (28–34) |
| Male sex | 43 (19) | 45 (17) | 33 (2) |
| CD4+ T-cell count, cells/μL | 24 (15–50) | 26 (16–63) | 15 (9–39) |
| HIV load, log10 copies/mL | 4.99 (4.4–5.4) | 4.94 (4.4–5.4) | 5.32 (5.0–5.7) |
| CSF lymphocyte count, ×106 cells/L | 15 (1–60) | 23 (1–100) | 2 (0–5) |
| Baseline fungal burden, log10 CFU/mL | 5.29 (4.0–5.9) | 5.27 (4.0–5.8) | 5.9 (3.3–6.1) |
| Abnormal mental status | 34 (15) | 32 (12) | 50 (3) |
| 2-wk mortality | 14 (6) | … | … |
Data are median (interquartile range) or % (no.) of subjects.
Abbreviations: CFU, colony-forming units; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus.
Frequency and Magnitude of Antigen-Specific CD4+ T-Cell Responses
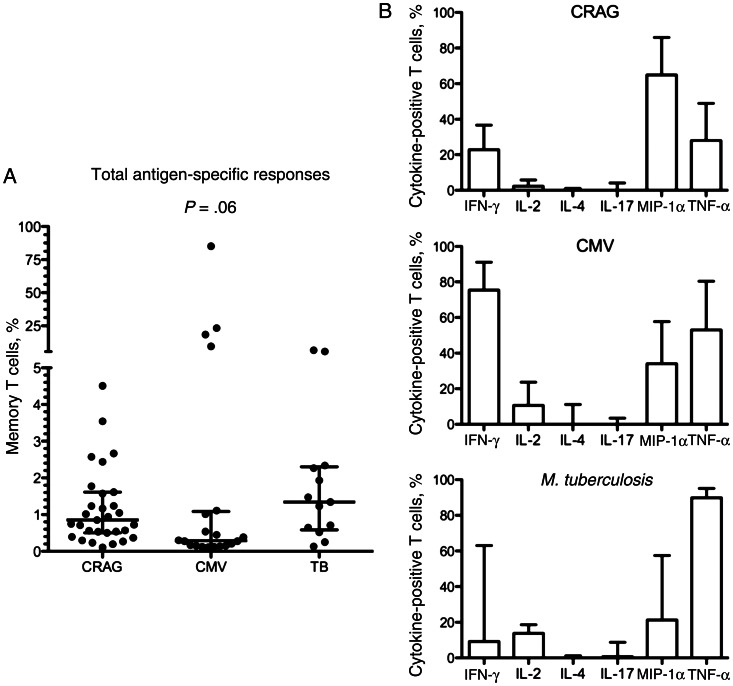
At baseline, CRAG-specific memory CD4+ T-cell responses were present in 70% of patients (31/44), CMV-specific responses were present in 77% (20/26), and M. tuberculosis–specific responses were present in 68% (13/19). The median frequencies of CRAG-, CMV- and M. tuberculosis–specific memory CD4+ T cells in patients with detectable responses were 0.86% (interquartile range [IQR], 0.5%–1.6%), 0.29% (IQR, 0.15%–1.1%), and 1.34% (IQR, 0.6%–2.3%), respectively (Figure 2A). Despite no clinical evidence of CMV disease, a number of patients had extremely large CMV-specific responses: in one patient, 85% of memory CD4+ T cells were CMV specific. M. tuberculosis–specific responses were present in 100% of patients (4/4) with active tuberculosis, 75% (3/4) with previous tuberculosis, and 55% (6/11) with no history of tuberculosis, none of whom developed active tuberculosis during the 1-year follow-up period.
Figure 2.
A, The magnitude of cryptococcal antigen (CRAG)–, cytomegalovirus (CMV)–, and Mycobacterium tuberculosis–specific CD4+ T-cell responses in patients with HIV-associated cryptococcal meningitis. The frequency of CRAG-, CMV-, and M. tuberculosis–specific CD4+ memory T-cell responses in patients with a detectable antigen-specific response at baseline is shown. The frequency of cytokine-producing cells in each individual is shown as a percentage of their total memory CD4+ T-cell population. Wide bars represent the median percentage, with error bars showing the interquartile range. A detectable total CD4+ T-cell response was defined as at least 0.1% of cells cytokine positive after subtraction of background, with at least 10 cytokine-positive events over background. Statistical comparison between groups was performed using the Kruskal-Wallis test. In the 4 patients with large magnitude CMV responses, 10%, 18%, 23%, and 85% of CD4+ memory T cells were CMV specific. In the 2 patients with large-magnitude M. tuberculosis responses, 6% and 7% of CD4+ memory T cells were M. tuberculosis specific. B, Differences in cytokine production in response to CRAG, CMV, and M. tuberculosis. The proportion of CRAG-, CMV-, and M. tuberculosis–specific CD4+ memory T cells producing interferon γ (IFN-γ), interleukin 2 (IL-2), interleukin 4 (IL-4), interleukin 17 (IL-17), macrophage inflammatory protein 1α (MIP-1α), and tumor necrosis factor α (TNF-α; as determined by flow cytometry after background subtraction) in patients with a detectable antigen specific response is shown. Bars are to the median, with error bars to the 75th percentile. The proportion of IFN-γ–producing cells was highest in the CMV-specific responses, while M. tuberculosis–specific responses had the highest proportions of IL-2 and TNF-α production, and MIP-1α production was highest in CRAG-specific responses.
Phenotype of Response
Maturational and functional characteristics were examined in pathogen-specific CD4+ T cells at baseline. Cellular maturation was studied using the differentiation markers CD27, CD45RO, and CD57 to distinguish between naive (CD27+CD45RO−), CD27+ memory (CD27+CD45RO+), effector-memory (CD27–CD45RO+), and terminally differentiated (CD57+) CD4+ T cells [46]. M. tuberculosis–specific CD4+ T cells had the least mature functional profile, with 18% having a CD27+ memory phenotype, 75% having an effector-memory phenotype, and 7% having a terminally differentiated phenotype. Of the CRAG-specific CD4+ T cells, 13% had a CD27+ memory phenotype, 67% had an effector-memory phenotype, and 18% had a terminally differentiated phenotype. CMV-specific CD4+ T cells had the most mature phenotype, with 14% having a CD27+ memory phenotype, 33% having an effector-memory phenotype, and 53% having a terminally differentiated phenotype.
To further assess baseline functional response, expression of IL-2, IL-4, IL-17, MIP-1α, IFN-γ, and TNF-α was measured. Significantly different patterns of cytokine production were detected in the CRAG-, CMV- and M. tuberculosis–specific T-cell responses (Figure 2B). A large proportion of CRAG-specific CD4+ T cells produced MIP-1α (median, 65%), with slightly less IFN-γ and TNF-α production (median, 37% and 28%, respectively). Negligible IL-2, IL-4, and IL-17 production was observed. In contrast, CMV-specific T cells primarily produced IFN-γ (median, 75%) and TNF-α (median, 53%), with lower proportions of MIP-1α (median, 34%) and IL-2 (median, 11%). M. tuberculosis–specific responses were characterized by a very high percentage of TNF-α–producing cells (median, 90%), along with a lower proportion of IFN-γ– (median, 9%), MIP-1α–, and IL-2–producing cells. Very little antigen-specific IL-4 or IL-17 production was detected with either CMV or M. tuberculosis stimulation.
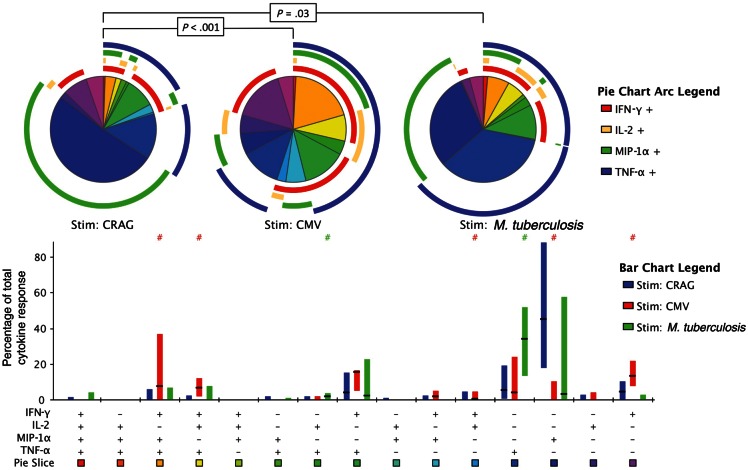
Baseline polyfunctional phenotypes were assessed throughout the 64 different possible combinations of the 6 cytokines (data not shown); however, as IL-4 and IL-17 production was minimal, phenotypic analysis of a 4-function panel (IFN-γ, IL-2, MIP-1α, and TNF-α) yielded results that were comparable to those for the full 6-function panel. In keeping with the overall cytokine production presented above, significantly differing functional phenotypes were seen in the different antigen-specific T-cell responses (CRAG vs CMV, P < .001; CRAG vs M. tuberculosis, P = .03; Figure 3). CRAG-specific responses were characterized by a predominance of single-function MIP-1α–producing cells (median, 42%; IQR, 23%–77%), with single-function IFN-γ–producing cells (median, 6%; IQR, 1%–11%) and single-function TNF-α–producing cells (median, 4%; IQR, 0%–15%) the next most frequent phenotypes. Very little polyfunctionality was observed, with a relatively small proportion of dual-function cells producing TNF-α and IFN-γ (median, 4%; IQR, 0%–15%) and a very low proportion of cells producing any other combination of ≥2 cytokines. In contrast, CMV-specific responses were characterized by a notable proportion of triple-function cells producing either TNF-α, IFN-γ, and MIP-1α (median, 10%; IQR, 1%–27%) or TNF-α, IFN-γ, and IL-2 (median, 6%; IQR, 2–12), along with a large proportion of dual-function cells producing TNF-α and IFN-γ (median, 16%; IQR, 6%–17%). M. tuberculosis–specific responses were less polyfunctional and were characterized by large proportions of single-function TNF-α–producing cells (median, 34%; IQR, 15%–49%).
Figure 3.
The functional phenotype of cryptococcal antigen (CRAG)–, cytomegalovirus (CMV)–, and Mycobacterium tuberculosis–specific CD4+ memory T-cell responses at baseline. Peripheral blood mononuclear cells from human immunodeficiency virus–positive subjects with cryptococcal meningitis were stimulated with cryptococcal mannoprotein, CMV pp65, or M. tuberculosis ESAT-6, CFP-10, and PPD. Flow cytometry of interferon γ (IFN-γ), interleukin 2 (IL-2), macrophage inflammatory protein 1α (MIP-1α), and tumor necrosis factor α (TNF-α) production within pathogen-specific CD4+ memory T cells is shown. The bar chart shows each of the 15 possible response profiles on the x-axis. The percentage of the total cytokine response is shown on the y-axis, with the filled bar representing the interquartile range and a line at the median. CRAG-specific responses are shown in blue, CMV-specific responses are in red, and M. tuberculosis–specific responses are in green. Statistically significant differences (P < .05) by rank-sum testing are indicated by the pound sign. The pie charts show the fractions according to the pie-slice colors shown at the bottom of the bar chart, with color-coded circles indicating the contributions of IFN-γ (red), IL-2 (yellow), MIP-1α (green), and TNF-α (blue) to the 4-, 3-, 2-, and 1-function responses. Statistical comparisons of the overall responses by permutation testing are shown in the pie category test result chart.
Temporal Changes in Response
Between baseline and day 14, there were no significant changes in the magnitude or phenotype of antigen-specific T-cell responses to CRAG, CMV, or M. tuberculosis. Following 1 month of ART, there was no significant change in the overall median frequency of CRAG-specific CD4+ T cells, but significant decreases of 90% in the frequency of IFN-γ–producing cells (P = .03) and 49% in the frequency of TNF-α–producing cells (P = .02) were observed. Conversely, there was an increase of 49% in the frequency of CMV-specific T cells (P = .09), which was primarily accounted for by an increase in the frequency of IFN-γ–producing cells. There was also a very large increase of >10-fold (1116%) in the frequency of IFN-γ–producing M. tuberculosis–specific T cells following 1 month of ART (P = .03), along with a 98% increase in frequency of TNF-α–producing M. tuberculosis–specific T cells (P = .03), the same pattern seen both in those with and those without active tuberculosis.
Associations Between CRAG-Specific T-Cell Responses and Clinical Presentation and Outcome of Cryptococcal Meningitis
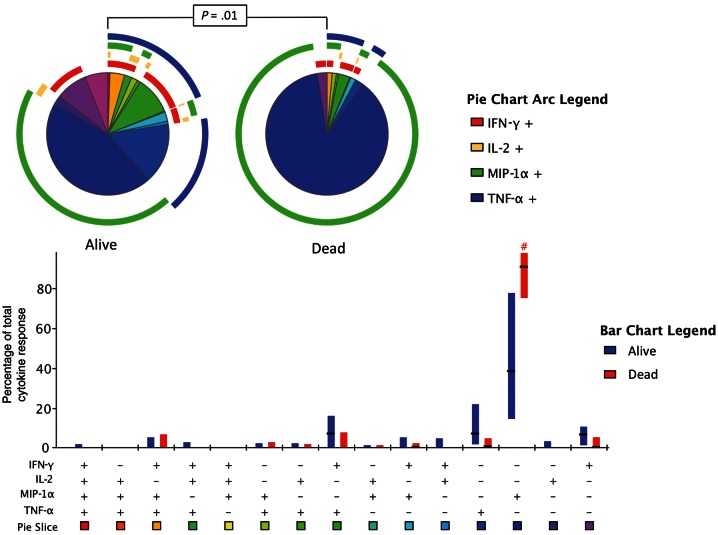
Associations between the CRAG-specific T-cell responses and the baseline fungal burden, rate of clearance of infection, and mortality at 2 weeks were first examined according to the presence or absence of a detectable antigen-specific response (hereafter referred to as “total response”). There were no significant differences in either baseline fungal burden or rate of clearance of infection in patients with and patients without CRAG-specific T-cell responses, and mortality in the 2 groups was similar (13% vs 15%; P = 1.0). However, the functional phenotype of the response differed significantly between those who survived and those who died (P = .01; Figure 4). Those who died had a significantly higher proportion of single-function MIP-1α–producing cells than those who survived (80% vs 38%; P = .02). Survivors tended to have higher percentages of single-function IFN-γ-producing cells (7% vs 0%; P = .19) and single-function TNF-α–producing cells (6% vs 1%; P = .13) and a larger proportion of polyfunctional dual-function IFN-γ– plus TNF-α–producing cells (7% vs 0%; P = .35) than those who died.
Figure 4.
Differences in functional phenotype of the cryptococcal antigen (CRAG)–specific responses at baseline between subjects who survived and subjects who died. Results of flow cytometry of interferon γ (IFN-γ), interleukin 2 (IL-2), macrophage inflammatory protein 1α (MIP-1α), and tumor necrosis factor α (TNF-α) production within CRAG-specific CD4+ memory T cells at baseline for patients who survived to 2 weeks and those who died are shown. The bar chart shows each of the 15 possible response profiles on the x-axis. The percentage of the total cytokine response is shown on the y-axis, with the filled bar representing the interquartile range and a line at the median. CRAG-specific responses in survivors are shown in blue, and response for those who died are in red. Statistically significant differences (P < .05) on rank-sum testing are indicated by the pound sign. The pie charts show the fractions according to the pie-slice colors shown at the bottom of the bar chart, with color-coded circles indicating the contributions of IFN-γ (red), IL-2 (yellow), MIP-1α (green), and TNF-α (blue) to the 4-, 3-, 2-, and 1-function responses. Statistical comparisons of the overall responses in those who survived versus those who died by permutation testing are shown in the pie category test result chart.
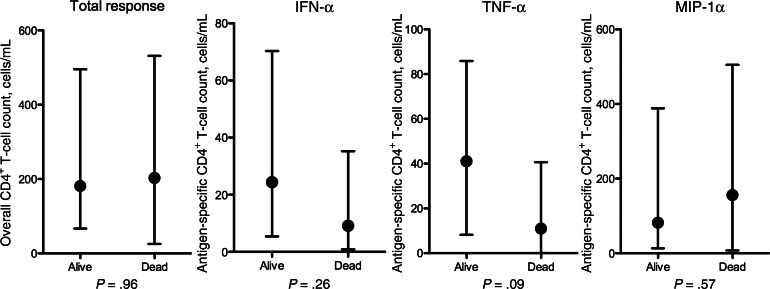
To further examine associations between the antigen-specific CD4+ T-cell response phenotype and clinical outcome, the absolute numbers of CRAG-specific T cells (ie, total response) and IFN-γ–, TNF-α–, and MIP-1α–producing T cells were calculated according to mortality (Figure 5). The magnitude of the total response was similar in survivors and nonsurvivors, but IFN-γ–producing and TNF-α–producing CRAG-specific CD4+ T-cell counts tended to be higher in survivors than nonsurvivors (24 cells/mL vs 9 cells/mL [P = .26] and 41 cells/mL vs 11 cells/mL [P = .09]).
Figure 5.
The absolute cryptococcal antigen (CRAG)–specific cytokine–producing, interferon γ (IFN-γ)–producing, tumor necrosis factor α (TNF-α)–producing and macrophage inflammatory protein 1α (MIP-1α)–producing CD4+ T-cell counts at baseline in subjects who survived and subjects who died. Absolute numbers of antigen-specific CD4+ T cells were calculated by multiplying the proportion of cytokine-positive CD4+ T cells by the total CD4+ T-cell count. Responses are shown according to 2-week mortality outcome. Statistical comparisons were made using the Mann–Whitney U test.
Given this dichotomous association, patients were then analyzed according to whether they had an IFN-γ– and/or TNF-α–producing CRAG-specific T-cell response at baseline (hereafter referred to as “IFN-γ/TNF-α response”). Twenty patients (45%) had detectable IFN-γ– and/or TNF-α–producing CRAG-specific CD4+ T-cell responses. Overall, patients with an IFN-γ/TNF-α response were significantly more likely to survive than those without this response (2-week mortality, 0% [0 patients] vs 25% [6 patients]; P = .025). Even in an analysis restricted to patients with a CRAG-specific total response at baseline, with responses split into MIP-1α–predominant responses (>50% MIP-1α producing) or IFN-γ/TNF-α–predominant responses (<50% MIP-1α producing), those with the IFN-γ/TNF-α–predominant response had lower mortality at 2 weeks (0% vs 19%; P = .16), significantly lower baseline fungal burdens (10 400 colony-forming units [CFU]/mL vs 390 000 CFU/mL; P < .001) and more rapid clearance of cryptococcal infection from the CSF (early fungicidal activity, −0.64 vs −0.52 log10 CFU/mL/day; P = .16).
Correlations Between CRAG-Specific CD4+ T-Cell Responses and the CSF Immune Response
We examined relationships between CRAG-specific CD4+ T-cell responses and 12 CSF immune parameters (CSF lymphocyte count and IL-2, IL-4, IL-6, IL-10, IL-17, IFN-γ, TNF-α, RANTES, MIP-1α, MCP-1, and GM-CSF concentrations). Absolute CRAG-specific CD4+ T-cell counts (total response and IFN-γ–, TNF-α–, and MIP-1α–producing cells) were correlated with CSF lymphocyte counts and log10 CSF cytokine/chemokine concentrations, using the Spearman correlation coefficient. The total CRAG-specific CD4+ T-cell count was not significantly correlated with the CSF lymphocyte count or any of the cytokines or chemokines. However, as with the clinical associations, it appeared that the phenotype, rather than the magnitude of the peripheral T-cell response, was most relevant. The IFN-γ–producing CRAG-specific CD4+ T-cell count was significantly associated with an increasing CSF lymphocyte count (Spearman rho, 0.34; P = .02), and a similar although nonsignificant association was found between the TNF-α–producing CRAG-specific CD4+ T-cell count and an increasing CSF lymphocyte count (Spearman rho, 0.26; P = .09). IFN-γ– and TNF-α–producing CRAG-specific CD4+ T-cell counts were also positively associated with CSF IL-10 and IL-17 concentrations (Spearman rho, 0.29 for IFN-γ and IL-10 [P = .05], 0.27 for TNF-α and IL-10 [P = .08], 0.21 for IFN-γ and IL-17 [P = .2], and 0.42 for TNF-α and IL-17 [P = .005]) and negatively associated with MCP-1 concentrations (Spearman rho, −0.35 for IFN-γ and MCP-1 [P = .02] and −0.27 for TNF-α and MCP-1 [P = .08]).
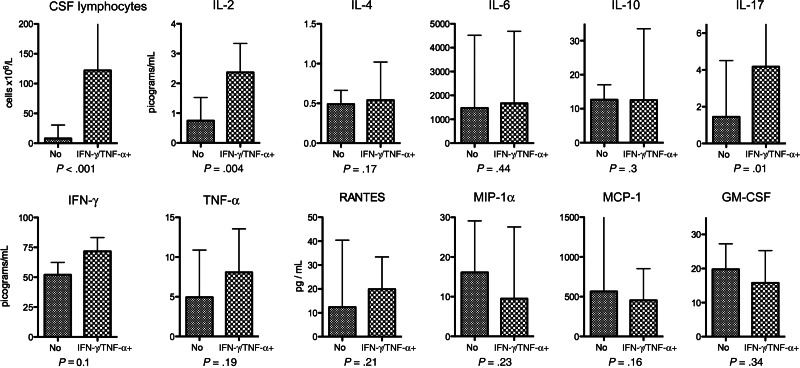
To examine the influence of CD4+ T-cell response phenotype independently from magnitude, an analysis restricted to patients with a CRAG-specific total response at baseline was performed, with responses classified as MIP-1α predominant or IFN-γ/TNF-α predominant as described above. Patients with IFN-γ/TNF-α–predominant CRAG-specific CD4+ T-cell responses had higher levels of proinflammatory cytokines in the CSF and lower levels of MIP-1α, MCP-1, and GM-CSF (Figure 6).
Figure 6.
Cerebrospinal fluid (CSF) immune parameters in subjects with and subjects without an interferon γ (IFN-γ)/tumor necrosis factor α (TNF-α)–predominant cryptococcal antigen (CRAG)–specific CD4+ T-cell response. CSF cytokine concentrations in patients with a detectable CRAG-specific CD4+ memory T-cell response are shown, divided into patients with an IFN-γ/TNF-α–predominant response or a macrophage inflammatory protein 1α (MIP-1α)–predominant response. A detectable total CD4+ T-cell response was defined as at least 0.1% of cells cytokine positive after subtraction of background, with at least 10 cytokine-positive events over background. A MIP-1α–predominant response was defined as >50% of CRAG-specific cells producing MIP-1α, and a IFN-γ/TNF-α–predominant response was defined as <50% CRAG-specific cells producing MIP-1α. Statistical comparisons were made using the Mann–Whitney U test. Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; MCP-1, monocyte chemotactic protein 1.
Effects of Exogenous Interferon Gamma on Systemic Immune Responses
The 44 patients in this study are a subset of a 90-patient cohort enrolled in a clinical trial assessing the effect of adjuvant interferon gamma administration on the rate of clearance of cryptococcal infection from the CSF of patients with HIV-associated cryptococcal meningitis. Administration of interferon gamma with standard antifungal therapy led to significantly faster rates of clearance of infection than standard therapy alone [42]. When examined according to baseline antigen-specific CD4+ T-cell responses, the effects of adjunctive interferon gamma therapy were found to be most marked in patients who had no CRAG-specific IFN-γ/TNF-α–producing CD4+ T-cell response at baseline. In the 24 patients without an IFN-γ/TNF-α–producing CD4+ T-cell response at baseline, the addition of interferon gamma led to an increase of 0.25 log10 CFU/mL/day (95% confidence interval [CI], .07–.43) in the rate of clearance (P = .01), compared with an increase of 0.15 log10 CFU/mL/day (95% CI, −.05–.35) in the rate among those with a CRAG-specific IFN-γ/TNF-α–producing CD4+ T-cell response (P = .14), following adjustment for absolute CD4+ T-cell count and baseline fungal burden.
To determine whether administration of adjuvant interferon gamma had an effect on the CRAG-specific T-cell response, the change in magnitude of the total CRAG-specific CD4+ T-cell response over the initial 2 weeks of antifungal treatment was examined with respect to interferon gamma exposure. Overall, interferon gamma treatment was not found to have a statistically significant impact on either the change in magnitude of the total CRAG-specific response between days 1 and 14 or the phenotype of the CD4+ T-cell response on day 14 (Supplementary Figure 1A). Interferon gamma–treated patients had proportionally less single-function MIP-1α–producing CD4+ T cells than controls on day 14 (24% vs 67%) and higher percentages of single-positive IFN-γ– and TNF-α–producing cells (4% vs 1% and 13% vs 4%, respectively) and dual-function IFN-γ/TNF-α–producing cells (11% vs 6%).
DISCUSSION
This is the first study characterizing antigen-specific peripheral CD4+ T-cell responses to Cryptococcus in patients with HIV-associated cryptococcal meningitis. Although this work is primarily a descriptive, or “discovery,” study examining a relatively small and heterogeneous patient population, clear trends are evident when the results are taken as a whole, providing novel data and unique insights. The magnitude of CRAG-specific CD4+ T-cell responses in patients with active cryptococcal infection was similar to the magnitude of CMV-specific and M. tuberculosis–specific responses, even though none of these patients had clinically apparent CMV disease and only one-third had evidence of active tuberculosis. CRAG-specific CD4+ T-cell responses differed markedly from M. tuberculosis–specific and CMV-specific responses in terms of maturational and functional profiles. M. tuberculosis–specific CD4+ T cells were the least differentiated and were primarily TNF-α producing. CMV-specific CD4+ T cells exhibited a highly differentiated effector-memory phenotype, primarily produced IFN-γ and TNF-α, and included large numbers of polyfunctional cells. CRAG-specific CD4+ T cells had a more differentiated maturational profile than M. tuberculosis–specific CD4+ cells but were less differentiated than CMV-specific cells. A large proportion of CRAG-specific cells produced MIP-1α, with slightly lower proportions producing IFN-γ or TNF-α, and very little polyfunctionality observed. Following ART initiation, CRAG-specific responses decreased or remained constant, in keeping with a reduction in antigen burden during effective antifungal therapy. In contrast, the magnitude of both M. tuberculosis– and CMV-specific responses increased following ART initiation, particularly markedly in the case of M. tuberculosis–specific responses. From low levels of M. tuberculosis–specific IFN-γ production at baseline (when TNF-α predominated), there was a 10-fold increase in the frequency of IFN-γ–producing CD4+ T cells after 1 month of ART. This observation is consistent with the observed poor performance of IFN-γ release assays in the diagnosis of tuberculosis in highly immune-suppressed populations [47].
Although CD4+ T-cell depletion is the key predisposing factor for development of HIV-associated cryptococcal meningitis, in this cohort of patients with cryptococcal meningitis the functional phenotype of the CRAG-specific CD4+ T-cell response was associated with disease severity and clinical outcome, unlike the overall magnitude of response. Survivors had higher proportions of IFN-γ– and TNF-α–producing cells and significantly lower proportions of MIP-1α–producing cells. When analyzed according to the presence or absence of an IFN-γ/TNF-α–producing CRAG-specific CD4+ T-cell response, those with such a response were found to have higher CSF lymphocyte counts, a more proinflammatory CSF cytokine response, significantly lower baseline fungal burdens, a trend to more rapid clearance of infection from the CSF, and a significantly lower 2-week mortality.
No Th2- or Th17-type antigen-specific cytokine production was seen in response to mannoprotein stimulation. Given the widely reported role of Th2-type responses in animal models of cryptococcal meningitis [16, 22, 23, 28], and especially in light of the described Th2-weighting of immune responses in late-stage HIV-infection [37], this may seem surprising. However this finding could be related to the use of mannoprotein rather than whole-organism or other capsular components in cell stimulations. Cryptococcal mannoproteins have previously been shown to elicit delayed-type hypersensitivity and Th1-type cytokines [48, 49], while other capsular components, notably GXM, appear to inhibit these responses, promoting detrimental Th2-type immunity [50]. It is possible that, rather than Th2-type CD4+ T-cell responses to C. neoformans, an absolute lack of CD4+ T cells may lead to detrimental Th2-type cytokine production by monocytes/macrophages or glial cells in HIV-associated cryptococcal meningitis. However, we found no inverse correlation between either total or IFN-γ/TNF-α–producing CRAG-specific peripheral CD4+ T-cell counts and Th2-type CSF cytokine levels. The only inverse correlation seen was between the number of IFN-γ/TNF-α–producing CRAG-specific peripheral CD4+ T cells and the chemokine MCP-1, suggesting that an adequate peripheral CD4+ T-cell response leads to downregulation of CNS chemokine production.
In terms of determining the mechanism by which exogenous IFN-γ leads to more rapid clearance of cryptococcal infection from the CSF, our results are inconclusive. The benefits of exogenous IFN-γ were most marked in patients with no IFN-γ/TNF-α–producing CRAG-specific peripheral CD4+ T-cell response. However, this did not reach statistical significance, possibly because our study was underpowered for such comparisons, particularly for subgroup analyses and in situations where there was much heterogeneity of responses. As the biological effects of exogenous IFN-γ may primarily result from direct activation of innate effector cells such as monocytes/macrophages and microglial cells, a limitation of this study is that innate effector-cell function was not directly examined. Further work is required to better characterize the subgroup of patients who have poor baseline immune responses to Cryptococcus, in whom adjuvant immunotherapy may be of most benefit.
In conclusion, the presence of an IFN-γ/TNF-α–producing CRAG-specific peripheral CD4+ T-cell response has been shown to correlate with favorable microbiological and clinical outcomes in patients with HIV-associated cryptococcal meningitis. Mannoprotein stimulation of peripheral CD4+ T cells did not lead to Th2-cytokine production even in these patients with advanced HIV. Unlike animal models, we found no evidence of a dichotomous Th1/Th2 response in this cohort of patients with advanced HIV infection and cryptococcal meningitis. Rather, an IFN-γ/TNF-α–producing CRAG-specific peripheral CD4+ T-cell response was associated with increased concentrations of cytokines at the site of infection, including classical Th1 cytokines, as well as IL-10 and IL-17, and the presence of this combined inflammatory response appears to be beneficial, while its absence is detrimental.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Chrono Lee, for technical assistance purifying the cryptococcal mannoprotein; Nonzwaki Bangani, for assistance with PBMC isolation and storage; G. Ntombomzi Williams, for providing clinical care to the patients; and Robert J. Wilkinson, for providing laboratory assistance in Cape Town.
Financial support. This work was supported by the Wellcome Trust (training fellowship WT081794 to J. N. J.) and the National Institutes of Health (grant RO1 AI025780 to S. M. L.). Flow cytometry was supported by funding from the Intramural Research Program of the Vaccine Research Center, National Institutes of Allergy and Infectious Disease, National Institutes of Health. S. D. L. is funded by the Wellcome Trust (grant 088590).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–30. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Casadevall A, Steenbergen JN, Nosanchuk JD. ‘Ready made’ virulence and ‘dual use’ virulence factors in pathogenic environmental fungi—the Cryptococcus neoformans paradigm. Curr Opin Microbiol. 2003;6:332–7. doi: 10.1016/s1369-5274(03)00082-1. [DOI] [PubMed] [Google Scholar]
- 3.Huffnagle GB, Yates JL, Lipscomb MF. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J Exp Med. 1991;173:793–800. doi: 10.1084/jem.173.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huffnagle GB, Lipscomb MF, Lovchik JA, Hoag KA, Street NE. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J Leukoc Biol. 1994;55:35–42. doi: 10.1002/jlb.55.1.35. [DOI] [PubMed] [Google Scholar]
- 5.Hill JO, Aguirre KM. CD4+ T cell-dependent acquired state of immunity that protects the brain against Cryptococcus neoformans. J Immunol. 1994;152:2344–50. [PubMed] [Google Scholar]
- 6.Buchanan KL, Doyle HA. Requirement for CD4(+) T lymphocytes in host resistance against Cryptococcus neoformans in the central nervous system of immunized mice. Infect Immun. 2000;68:456–62. doi: 10.1128/iai.68.2.456-462.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoag KA, Lipscomb MF, Izzo AA, Street NE. IL-12 and IFN-gamma are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am J Respir Cell Mol Biol. 1997;17:733–9. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]
- 8.Wozniak KL, Ravi S, Macias S, et al. Insights into the mechanisms of protective immunity against Cryptococcus neoformans infection using a mouse model of pulmonary cryptococcosis. PLoS One. 2009;4:e6854. doi: 10.1371/journal.pone.0006854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawakami K, Kohno S, Kadota J, et al. T cell-dependent activation of macrophages and enhancement of their phagocytic activity in the lungs of mice inoculated with heat-killed Cryptococcus neoformans: involvement of IFN-gamma and its protective effect against cryptococcal infection. Microbiol Immunol. 1995;39:135–43. doi: 10.1111/j.1348-0421.1995.tb02180.x. [DOI] [PubMed] [Google Scholar]
- 10.Milam JE, Herring-Palmer AC, Pandrangi R, McDonald RA, Huffnagle GB, Toews GB. Modulation of the pulmonary type 2 T-cell response to Cryptococcus neoformans by intratracheal delivery of a tumor necrosis factor alpha-expressing adenoviral vector. Infect Immun. 2007;75:4951–8. doi: 10.1128/IAI.00176-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wormley FL, Jr., Perfect JR, Steele C, Cox GM. Protection against cryptococcosis by using a murine gamma interferon-producing Cryptococcus neoformans strain. Infect Immun. 2007;75:1453–62. doi: 10.1128/IAI.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herring AC, Lee J, McDonald RA, Toews GB, Huffnagle GB. Induction of interleukin-12 and gamma interferon requires tumor necrosis factor alpha for protective T1-cell-mediated immunity to pulmonary Cryptococcus neoformans infection. Infect Immun. 2002;70:2959–64. doi: 10.1128/IAI.70.6.2959-2964.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawakami K, Tohyama M, Teruya K, Kudeken N, Xie Q, Saito A. Contribution of interferon-gamma in protecting mice during pulmonary and disseminated infection with Cryptococcus neoformans. FEMS Immunol Med Microbiol. 1996;13:123–30. doi: 10.1016/0928-8244(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 14.Huffnagle GB, Toews GB, Burdick MD, et al. Afferent phase production of TNF-alpha is required for the development of protective T cell immunity to Cryptococcus neoformans. J Immunol. 1996;157:4529–36. [PubMed] [Google Scholar]
- 15.Kawakami K, Qureshi MH, Zhang T, et al. Interferon-gamma (IFN-gamma)-dependent protection and synthesis of chemoattractants for mononuclear leucocytes caused by IL-12 in the lungs of mice infected with Cryptococcus neoformans. Clin Exp Immunol. 1999;117:113–22. doi: 10.1046/j.1365-2249.1999.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arora S, Olszewski MA, Tsang TM, McDonald RA, Toews GB, Huffnagle GB. Effect of cytokine interplay on macrophage polarization during chronic pulmonary infection with Cryptococcus neoformans. Infect Immun. 2011;79(5):1915–25. doi: 10.1128/IAI.01270-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu Y, Davis MJ, Dayrit JK, et al. Immune modulation mediated by cryptococcal laccase promotes pulmonary growth and brain dissemination of virulent Cryptococcus neoformans in mice. PLoS One. 2012;7:e47853. doi: 10.1371/journal.pone.0047853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardison SE, Herrera G, Young ML, Hole CR, Wozniak KL, Wormley FL., Jr Protective immunity against pulmonary cryptococcosis is associated with STAT1-Mediated classical macrophage activation. J Immunol. 2012;189:4060–8. doi: 10.4049/jimmunol.1103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He X, Lyons DM, Toffaletti DL, et al. Virulence factors identified by Cryptococcus neoformans mutant screen differentially modulate lung immune responses and brain dissemination. Am J Pathol. 2012;181:1356–66. doi: 10.1016/j.ajpath.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wozniak KL, Hardison S, Olszewski M, Wormley FL., Jr Induction of protective immunity against cryptococcosis. Mycopathologia. 2012;173:387–94. doi: 10.1007/s11046-011-9505-8. [DOI] [PubMed] [Google Scholar]
- 21.Decken K, Kohler G, Palmer-Lehmann K, et al. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun. 1998;66:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koguchi Y, Kawakami K. Cryptococcal infection and Th1-Th2 cytokine balance. Int Rev Immunol. 2002;21:423–38. doi: 10.1080/08830180213274. [DOI] [PubMed] [Google Scholar]
- 23.Blackstock R, Buchanan KL, Adesina AM, Murphy JW. Differential regulation of immune responses by highly and weakly virulent Cryptococcus neoformans isolates. Infect Immun. 1999;67:3601–9. doi: 10.1128/iai.67.7.3601-3609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller U, Stenzel W, Kohler G, et al. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J Immunol. 2007;179:5367–77. doi: 10.4049/jimmunol.179.8.5367. [DOI] [PubMed] [Google Scholar]
- 25.Blackstock R, Murphy JW. Role of interleukin-4 in resistance to Cryptococcus neoformans infection. Am J Respir Cell Mol Biol. 2004;30:109–17. doi: 10.1165/rcmb.2003-0156OC. [DOI] [PubMed] [Google Scholar]
- 26.Huffnagle GB, Chen GH, Curtis JL, McDonald RA, Strieter RM, Toews GB. Down-regulation of the afferent phase of T cell-mediated pulmonary inflammation and immunity by a high melanin-producing strain of Cryptococcus neoformans. J Immunol. 1995;155:3507–16. [PubMed] [Google Scholar]
- 27.Almeida GM, Andrade RM, Bento CA. The capsular polysaccharides of Cryptococcus neoformans activate normal CD4(+) T cells in a dominant Th2 pattern. J Immunol. 2001;167:5845–51. doi: 10.4049/jimmunol.167.10.5845. [DOI] [PubMed] [Google Scholar]
- 28.Muller U, Piehler D, Stenzel W, et al. Lack of IL-4 receptor expression on T helper cells reduces T helper 2 cell polyfunctionality and confers resistance in allergic bronchopulmonary mycosis. Mucosal Immunol. 2012;5:299–310. doi: 10.1038/mi.2012.9. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Wang F, Tompkins KC, et al. Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. Am J Pathol. 2009;175:2489–500. doi: 10.2353/ajpath.2009.090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleinschek MA, Muller U, Brodie SJ, et al. IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12. J Immunol. 2006;176:1098–106. doi: 10.4049/jimmunol.176.2.1098. [DOI] [PubMed] [Google Scholar]
- 31.Szymczak WA, Sellers RS, Pirofski LA. IL-23 dampens the allergic response to Cryptococcus neoformans through IL-17-independent and -dependent mechanisms. Am J Pathol. 2012;180:1547–59. doi: 10.1016/j.ajpath.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wozniak KL, Hardison SE, Kolls JK, Wormley FL. Role of IL-17A on resolution of pulmonary C. neoformans infection. PLoS One. 2011;6:e17204. doi: 10.1371/journal.pone.0017204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jarvis JN, Harrison TS. HIV-associated cryptococcal meningitis. AIDS. 2007;21:2119–29. doi: 10.1097/QAD.0b013e3282a4a64d. [DOI] [PubMed] [Google Scholar]
- 34.Bicanic T, Muzoora C, Brouwer AE, et al. Independent association between rate of clearance of infection and clinical outcome of HIV-associated cryptococcal meningitis: analysis of a combined cohort of 262 patients. Clin Infect Dis. 2009;49:702–9. doi: 10.1086/604716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bicanic T, Jarvis JN, Loyse A, et al. Determinants of acute outcome and long-term survival in HIV-associated cryptococcal meningitis: results from a combined cohort of 523 patients [abstract P-123]. 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 27 February–2 March 2011. [Google Scholar]
- 36.Siddiqui AA, Brouwer AE, Wuthiekanun V, et al. IFN-gamma at the site of infection determines rate of clearance of infection in cryptococcal meningitis. J Immunol. 2005;174:1746–50. doi: 10.4049/jimmunol.174.3.1746. [DOI] [PubMed] [Google Scholar]
- 37.Altfeld M, Addo MM, Kreuzer KA, et al. T(H)1 to T(H)2 shift of cytokines in peripheral blood of HIV-infected patients is detectable by reverse transcriptase polymerase chain reaction but not by enzyme-linked immunosorbent assay under nonstimulated conditions. J Acquir Immune Defic Syndr. 2000;23:287–94. doi: 10.1097/00126334-200004010-00001. [DOI] [PubMed] [Google Scholar]
- 38.Zhang M, Gong J, Iyer DV, Jones BE, Modlin RL, Barnes PF. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J Clin Invest. 1994;94:2435–42. doi: 10.1172/JCI117611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clerici M, Shearer GM. A TH1–>TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–11. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 40.Geldmacher C, Ngwenyama N, Schuetz A, et al. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J Exp Med. 2010;207:2869–81. doi: 10.1084/jem.20100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casazza JP, Brenchley JM, Hill BJ, et al. Autocrine production of beta-chemokines protects CMV-specific CD4 T cells from HIV infection. PLoS Pathog. 2009;5:e1000646. doi: 10.1371/journal.ppat.1000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jarvis JN, Meintjes G, Rebe K, et al. Adjunctive interferon-gamma immunotherapy for the treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial. AIDS. 2012;26:1105–13. doi: 10.1097/QAD.0b013e3283536a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wozniak KL, Levitz SM. Isolation and purification of antigenic components of Cryptococcus. Methods Mol Biol. 2009;470:71–83. doi: 10.1007/978-1-59745-204-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79:167–74. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brenchley JM, Karandikar NJ, Betts MR, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–20. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 47.Metcalfe JZ, Everett CK, Steingart KR, et al. Interferon-gamma release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and meta-analysis. J Infect Dis. 2011;204(Suppl 4):S1120–9. doi: 10.1093/infdis/jir410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levitz SM, Specht CA. The molecular basis for the immunogenicity of Cryptococcus neoformans mannoproteins. FEMS Yeast Res. 2006;6:513–24. doi: 10.1111/j.1567-1364.2006.00071.x. [DOI] [PubMed] [Google Scholar]
- 49.Levitz SM, North EA. Lymphoproliferation and cytokine profiles in human peripheral blood mononuclear cells stimulated by Cryptococcus neoformans. J Med Vet Mycol. 1997;35:229–36. doi: 10.1080/02681219780001201. [DOI] [PubMed] [Google Scholar]
- 50.Yauch LE, Lam JS, Levitz SM. Direct inhibition of T-cell responses by the Cryptococcus capsular polysaccharide glucuronoxylomannan. PLoS Pathog. 2006;2:e120. doi: 10.1371/journal.ppat.0020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.