Abstract
Staphylococcus aureus and group A Streptococcus pyogenes (GAS) express superantigen (SAg) exotoxin proteins capable of inducing lethal shock. To induce toxicity, SAgs must bind not only to the major histocompatibility complex II molecule of antigen-presenting cells and the variable β chain of the T-cell receptor but also to the dimer interface of the T-cell costimulatory receptor CD28. Here, we show that the CD28-mimetic peptide AB103 (originally designated “p2TA”) protects mice from lethal challenge with streptococcal exotoxin A, as well as from lethal GAS bacterial infection in a murine model of necrotizing soft-tissue infection. Administration of a single dose of AB103 increased survival when given up to 5 hours after infection, reduced inflammatory cytokine expression and bacterial burden at the site of infection, and improved muscle inflammation in a dose-dependent manner, without compromising cellular and humoral immunity. Thus, AB103 merits further investigation as a potential therapeutic in SAg-mediated necrotizing soft-tissue infection.
Keywords: superantigen, CD28, group A S. pyogenes, peptide antagonist, necrotizing soft tissue infection
Localized infections by gram-positive bacteria, including group A Streptococcus pyogenes (GAS) and Staphylococcus aureus, are often complicated by manifestations of systemic toxicity, including fever and hypotension, which may progress to sepsis and lethal septic/toxic shock [1, 2]. These bacteria can secrete exotoxins known as superantigens (SAgs), including staphylococcal enterotoxins SEA–SEE, toxic shock syndrome toxin 1, streptococcal pyrogenic exotoxins SPEA and SPEC, and streptococcal mitogenic exotoxin Z, that trigger an excessive cellular immune response [3–7].
SAgs bind to the outer face of major histocompatibility complex class II (MHC-II) molecules on antigen-presenting cells and to the variable β chain of the T-cell receptor, regions not involved in ordinary antigen recognition. Thus, in contrast to conventional antigen presentation, which stimulates approximately 0.02% of T cells, SAgs bypass the need for processing by antigen-presenting cells [8–10] and can activate 20%–40% of T cells, resulting in massive induction of proinflammatory T-helper 1 (Th1)–type cytokines, including interleukin 2, interferon γ, and tumor necrosis factor α [8, 9, 11], that induce lethal shock.
Therapies aimed at attenuating SAg-mediated cytokine overproduction downstream from T-cell activation (eg, with anti–tumor necrosis factor α monoclonal antibodies) proved unsuccessful [12]. However, the interaction of SAgs with cellular receptors at the onset of the toxicity cascade was targeted as a potential therapeutic approach. Although streptococcal and staphylococcal SAgs show only limited sequence homology, they harbor a domain distal to MHC-II/T-cell receptor binding sites that is highly conserved in sequence and structure [13]. SAgs use this conserved domain to bind directly to the costimulatory molecule CD28 on T cells, and this interaction is indispensable both for the induction of Th1-type cytokines by divergent SAgs and for their lethality [14]. Notably, the binding site for SAgs within CD28 is the homodimer interface [14]. To induce Th1-type cytokines, SAgs must bind directly into the CD28 dimer interface, defining this interface as a therapeutic target [14]. Short peptide mimetics of the CD28 dimer interface are capable of competing with CD28 holoreceptor in binding to SAgs, thereby preventing engagement of SAgs with CD28 and harmful induction of inflammatory cytokines in T cells; by inhibiting signaling through CD28, these peptides potently protect mice from the lethal effects of SAgs [14].
AB103 (originally designated “p2TA” [14]) is a CD28 dimer interface mimetic peptide that is active as a SAg antagonist and effectively protects mice from lethal SAg challenge, even when it is present at a level equimolar to that of SAg [14]. AB103 inhibits induction of genes encoding Th1-type cytokines (interleukin 2, interferon γ, and tumor necrosis factor α) by diverse SAgs (SEB, SEA, and TSST-1) in human peripheral blood mononuclear cells (PBMCs) [14]. On the basis of these findings, we hypothesized that the peptide might protect mice from infection with GAS.
Necrotizing soft-tissue infection (NSTI) is an acute, rapidly progressive cellulitis involving both superficial fascia and subcutaneous fat, characterized by pain at the infected site and systemic toxicity, including multiorgan injury [15, 16]. A major pathogen causing this infection is S. pyogenes. Since NSTI responds poorly to antibiotics, aggressive surgical intervention to remove necrotic tissue is mandatory. Nevertheless, mortality reaches 20% [15]. While streptococcal pyrogenic exotoxins may account for some of the pathophysiology of GAS, it is unclear whether they have an important role in systemic GAS toxicity or whether interventions targeting exotoxins may alter the course of disease. Currently, there are no available drugs approved for this indication, creating a significant unmet medical need for effective therapy. Here, we examined the ability of AB103, a peptide shown to block the interaction of SAg exotoxins with CD28, to prevent lethal GAS infection in a murine model of NSTI.
METHODS
SAgs were from Toxin Technology (Sarasota, FL). d-galactosamine was from Sigma-Aldrich.
Peptide
AB103 was GMP-grade peptide p2TA having the sequence SPMLVAYD, with d-alanine added to both termini to enhance protease resistance [14]. Once dissolved in phosphate-buffered saline (PBS) at 1 mg/mL, AB103 was used immediately at desired dilutions.
Bacteria
S. pyogenes strain 8198 (scarlet fever serotype M1T1, kindly provided by Dr Jonathan Cohen [Hammersmith Hospital, London, United Kingdom]) produces SPEA and SPEB and carries genes for SPEG, SPEJ, and SMEZ [3]; it was cultured in Todd-Hewitt broth (Becton Dickinson) at 37°C under aerobic conditions.
Animals
Pathogen-free 6–8-week-old female BALB/c mice were from Charles River Laboratories (Wilmington, MA). Animal studies were approved by the University of Maryland Institutional Animal Care and Use Committee.
Bacterial Counts in Organs
Thigh muscle tissue, spleen, and liver were harvested from euthanized infected mice, weighed, and placed in sterile PBS. Tissue homogenates, serially diluted in PBS, were plated on 5% sheep blood agar plates, and the number of colony-forming units (CFU) per milligram was determined.
Antibodies Against Virulence Factors
Serum was separated from cardiac blood of AB103-treated mice that survived GAS challenge. SPEA, SPEB, or SPEC, dissolved at 10 µg/mL in carbonate-bicarbonate buffer (pH 9.6), was used to coat 96-well enzyme-linked immunosorbent assay microtiter plates. Nonspecific binding was blocked with 50% fetal calf serum (FCS) in PBS. Plates were washed with 0.05% Tween20 (Fisher Scientific, Pittsburg, MA) in 0.5% FCS. Serum, diluted 1:100 in 1% FCS, was applied to the wells. Alkaline phosphatase–conjugated sheep anti-mouse immunoglobulin G antibody or goat anti-mouse immunoglobulin M antibody (Sigma), diluted 1:10 000 in 1% FCS, was applied before addition of p-nitrophenyl phosphate and determination of A405.
Cytokine Analysis
Mouse cytokine levels were assayed in serum, using a 9-multiplex immunoassay, with standard curves (Quansys Biosciences, Logan, UT).
Hematoxylin and Eosin Staining
Muscle samples were sectioned, embedded, and fixed at 5 μm; placed in 10 mM citrate buffer (pH 6.0); and heated for 10 minutes before hematoxylin and eosin staining.
Serum Chemistry
Creatinine, blood urea nitrogen, alanine transaminase, aspartate transaminase, alkaline phosphatase, and bilirubin levels were quantitated in sera collected 5 days after infection.
Effect of AB103 on Cell-Mediated Immune Response
Groups of 5 BALB/c mice were immunized with a single dose of 1 × 107 CFU of LVSΔguaB, a live attenuated Francisella tularensis vaccine (provided by Dr Eileen Barry), and challenged intraperitoneally on day 28 with 1 × 105 CFU of the live vaccine strain. Mice received 5 or 0.5 mg/kg AB103 intravenously 30 minutes before vaccination or neither peptide nor vaccine. Survival was determined on day 12.
Allogeneic Mixed Lymphocyte Reaction
Monocytes from healthy human PBMCs were cultured for 3 days in complete Roswell Park Memorial Institute (RPMI) medium supplemented with 50 ng/mL granulocyte-macrophage colony-stimulating factor and 25 ng/mL interleukin 4 (R&D Systems) to generate immature monocyte-derived dendritic cells. These immature cells were harvested, washed twice in complete RPMI medium, and plated in triplicate wells of U-bottomed culture plates at 2 × 104, 2 × 103, and 2 × 102 cells/well. Allogeneic responder PBMCs were added at 2 × 105 cells per 200-μL well.
Statistical Analysis
Values are expressed as means ± standard error of the mean. Differences between groups were analyzed using the Student t test. Differences are considered statistically significant at a P value of < .05.
RESULTS
Protection of Mice From Streptococcal Toxic Shock
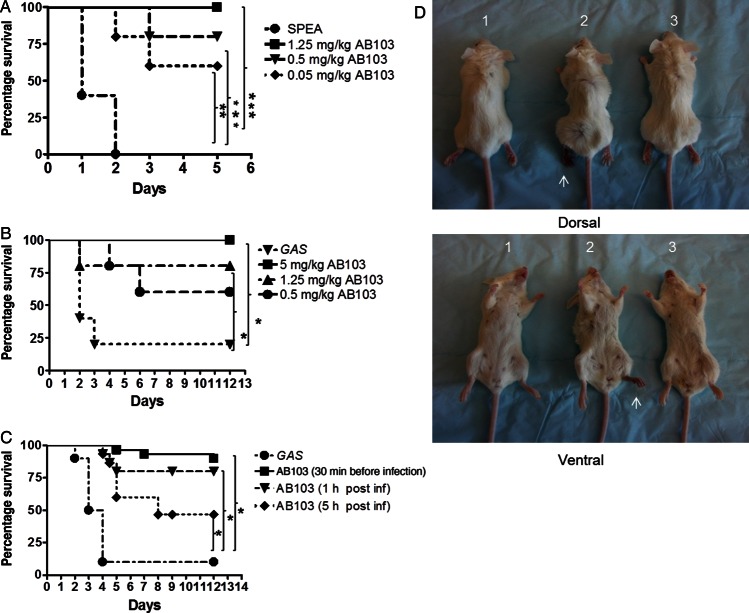
We evaluated the ability of AB103, a CD28 mimetic peptide, to protect mice from toxic shock induced by a lethal dose of SPEA. Since mice are naturally resistant to superantigen challenge, they were sensitized by pretreatment with d-galactosamine [13]. Whereas SPEA was 100% lethal for untreated mice, survival among AB103-treated mice increased in a dose-dependent fashion (Figure 1A). Survival was 80% (P < .0034) and 60% (P < .0051) when AB103 was administered at 0.5 and 0.05 mg/kg, respectively. At 1.25 mg/kg AB103, all mice survived (P < .0023).
Figure 1.
AB103 protects mice from lethal SPEA intoxication and lethal infection with Streptococcus pyogenes (GAS). A, BALB/c mice were sensitized with 20 mg of D-galactosamine intraperitoneally and challenged 2 hours later with 0.75 mg/kg SPEA intraperitoneally. Varying doses of AB103 (0.05, 0.5, and 1.25 mg/kg) were administered intravenously 30 minutes before challenge, and survival was monitored. B BALB/c mice were infected intramuscularly in the left upper thigh with 1 × 107 colony-forming units of GAS (100 µL). AB103 (0.5, 1.25, and 5 mg/kg) was administered intravenously 30 minutes prior to infection, and survival was monitored. C, BALB/c mice were infected with 1 × 107 GAS intramuscularly 30 minutes after or 1 or 5 hours before AB103 treatment (5 mg/kg intravenously), and survival was monitored. D, Mice (1 and 2) were infected with GAS intramuscularly and monitored for the development of necrotizing fasciitis in the ipsilateral leg (1). Mice administered AB103 were protected from GAS-induced necrotizing fasciitis (2). In the absence of AB103, mice infected with GAS intramuscularly developed necrotizing fasciitis in the foot pad within 24 hours after infection (3). Mice injected with phosphate-buffered saline injected (ie, uninfected) served as controls. Arrows indicate clinical signs of necrotizing fasciitis. Each mouse is representative of 1 group (n = 5). *P < .05, **P < .01, ***P < .005.
AB103 Protects Mice From GAS Challenge
Although AB103 protected mice from SPEA, this model does not mimic live infection by SAg-producing bacteria, considering that mice are resistant to SAgs unless sensitized and that, in murine models, SAgs are typically delivered as a bolus as compared to their more gradual and sustained release from replicating bacteria. Therefore, we tested the ability of the peptide to protect mice from GAS thigh infection, a model of NSTI [17].
BALB/c mice were injected intramuscularly with a clinical isolate of GAS at 1 × 107 CFU, the lowest dose causing 100% mortality. Before GAS challenge, AB103 was administered intravenously in a single dose, without any antibiotic treatment. Untreated mice started to die as early as 2 days after challenge, and by day 12, <20% had survived. By contrast, >60% of mice treated with 0.5 mg/kg AB103 survived GAS challenge for ≥12 days after infection (P < .0612). Over 75% of mice treated with 1.25 mg/kg AB103 survived (P < .0334), and 100% treated with 5 mg/kg AB103 survived (P < .0123; Figure 1B). Unlike untreated mice, those that received AB103 showed minimal signs of clinical illness (eg, piloerection, lethargy, and huddling). We next evaluated the effect of the peptide on established acute bacterial infection, administering AB103 1 or 5 hours after infection. Of the untreated GAS-infected mice, 28 of 30 (93%) died within 4 days of challenge, whereas 95% that received AB103 30 minutes before GAS infection survived (P < .0001). When administered 1 hour after challenge, AB103 protected 25 of 30 mice (83%; P < .0003), and when administered as late as 5 hours after GAS challenge, AB103 protected 47% of infected mice (P < .0005; Figure 1C).
Thus, following GAS bacterial infection, there is window of at least 5 hours during which a single administration of AB103 is beneficial, even in the absence of antibiotic treatment. Importantly, the same dose of 5 mg/kg was efficacious whether given at the time of infection or, as a delayed treatment, 1 or 5 hours after infection. As expected, survival rates depended on the timing of treatment after infection.
Mice infected with GAS in the thigh of their left hind leg developed a substantial necrotic lesion by 24 hours after infection, which had spread to the footpad (Figure 1D). By contrast, mice treated with AB103 1 hour after infection showed no signs of inflammation or necrosis, and their footpad appeared normal, similar to that of control mice injected with saline instead of GAS (Figure 1D). Moreover, in AB103-treated mice, the infected leg did not show any progression of signs of inflammation, even at 48 or 72 hours.
Hence, AB103 ameliorates disease symptoms both systemically and locally at the site of infection, culminating in increased survival. AB103 not only blocks toxic shock induced by challenge with a single lethal dose of SAg [14] (Figure 1A) but also protects mice from live, replicating S. pyogenes that produce a variety of SAgs (Figure 1B and 1C).
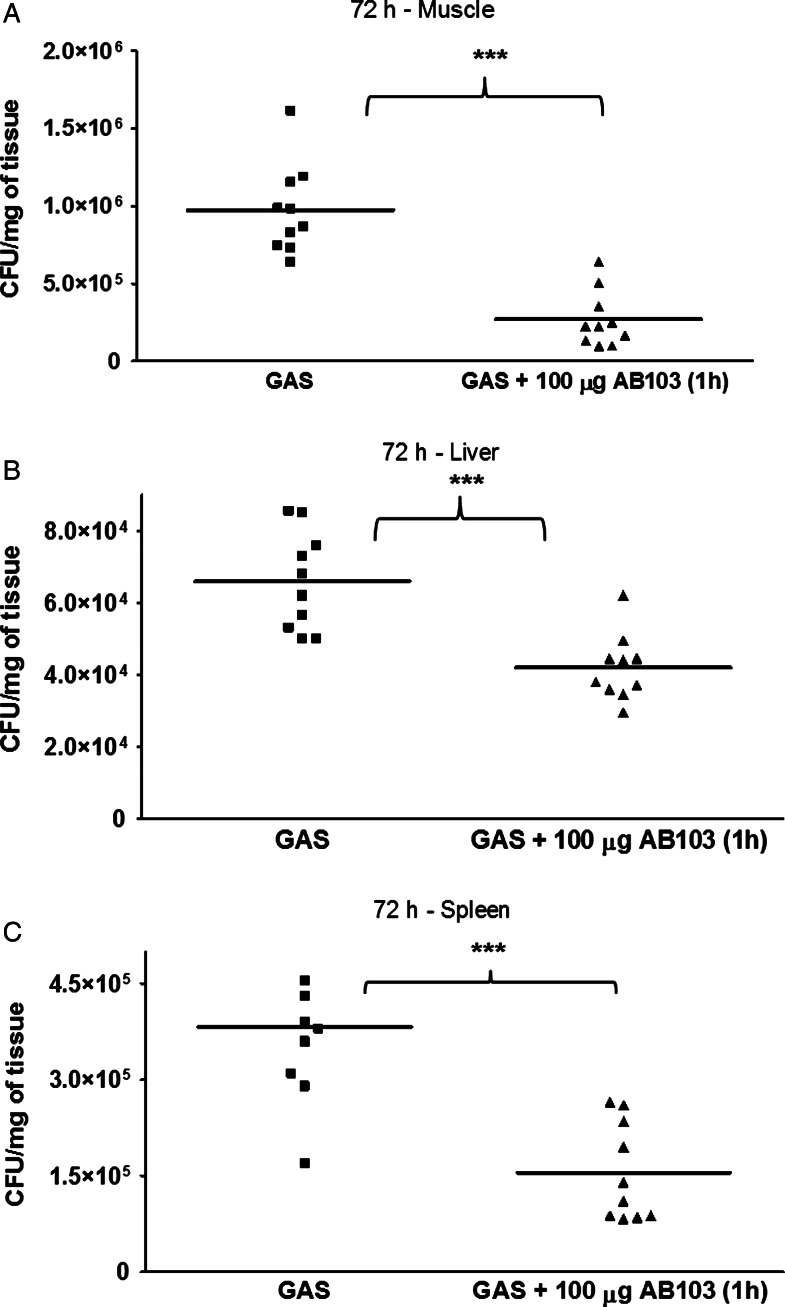
AB103 Attenuates the Bacterial Burden at the Site of Infection
A significant difference (P < .0001) in bacterial count in muscle tissue was observed between untreated and AB103-treated mice by 72 hours after infection (Figure 2A). All untreated animals died within 4 days of infection. Notably, in AB103-treated animals, clearance of GAS was complete only by day 60 after infection (data not shown).
Figure 2.
Bacterial burden of Streptococcus pyogenes (GAS) at 72 hours in muscle, liver, and spleen of AB103 treated and untreated mice. A, Mice treated with AB103 (5 mg/kg intravenously) had a lower bacterial burden in muscle tissue as compared to infected, untreated control mice. B and C, Although there was less dissemination of GAS to the liver and spleen, a significant reduction in bacterial load in each organ was observed in AB103-treated mice as compared to infected, untreated controls. Colony-forming unit (CFU) data points represent pooling of data from each of 2 experiments of 5 mice per group. ***P < .005.
There was no bacterial dissemination to the lungs. However, a low level of bacteria was detected in liver (P < .0001) and spleen (P < .0001), with a difference between treated and untreated groups (Figure 2B). AB103 did not kill GAS when added directly to the GAS culture. We next preincubated mouse peritoneal macrophages in vitro with different concentrations of AB103 and then added GAS to the cultures. We did not observe any difference in killing between macrophages that were exposed to AB103 and those that were not (data not shown). Thus, the peptide mimetic had neither a direct antibacterial effect nor a stimulatory effect on host phagocytic activity. These results suggest that, during GAS infection, fatal outcome may result predominantly from bacterial virulence factors and systemic release of cytokines, which is sensitive to inhibition by AB103 [14].
AB103 Attenuates Toxemia in Mice
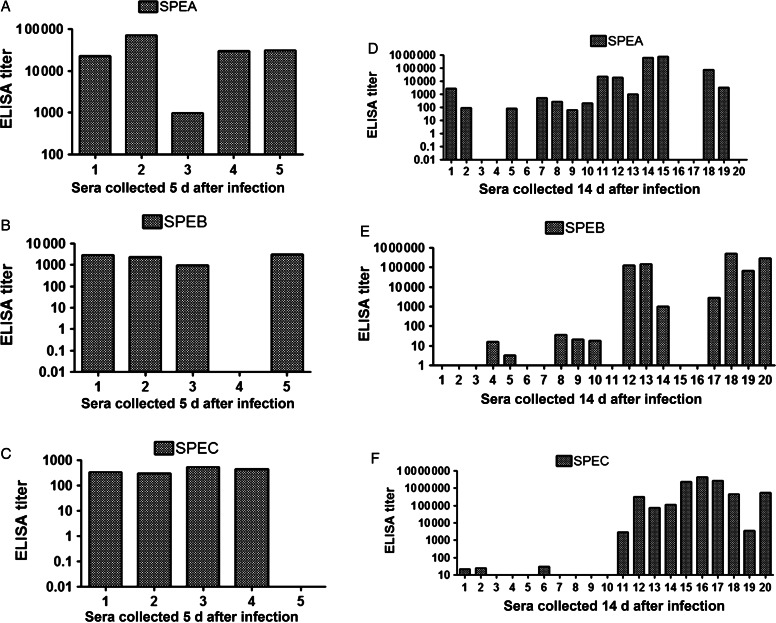
None of the mice had detectable antibodies against streptococcal pyrogenic exotoxins SPEA and SPEC or against the cysteine protease SPEB before infection (data not shown). Antibodies against all 3 virulence factors were observed 5 days after infection (Figure 3A–C), when untreated mice were typically moribund, and 14 days after infection, when most mice (17/20) showed antibodies against at least 1–2 of the SAgs (Figure 3D–F), and titers were higher. Conceivably, the 3 mice that failed to develop antibodies against assayed GAS toxins developed antibodies to other streptococcal SAgs, such as SMEZ or SEJ, that were not assayed. None of the mice tested had detectable antibodies before GAS infection (data not shown). Cumulatively, our data indicate that AB103 protects mice from GAS challenge by attenuating toxemia rather than bacteremia. Notably, treatment with AB103 did not compromise the ability of the challenged mice to raise antibodies against SAgs, indicating that it leaves the ability to develop a protective antibody response intact.
Figure 3.
Serum antibody titers against SPEA, SPEB, and SPEC. Mice were pretreated with AB103 (5 mg/kg intravenously) 30 minutes before intramuscular infection with Streptococcus pyogenes (GAS), and sera were harvested 5 days (A–C; n = 5) and 14 days (D–F; n = 20) later for analysis of immunoglobulin G levels against SPEA, SPEB, and SPEC. None of the mice tested had detectable antibodies before GAS infection (data not shown). None of the infected, untreated mice survived 5 days. Abbreviation: ELISA, enzyme-linked immunosorbent assay.
AB103 Attenuates Proinflammatory Cytokine Production in Mice Infected With S. pyogenes
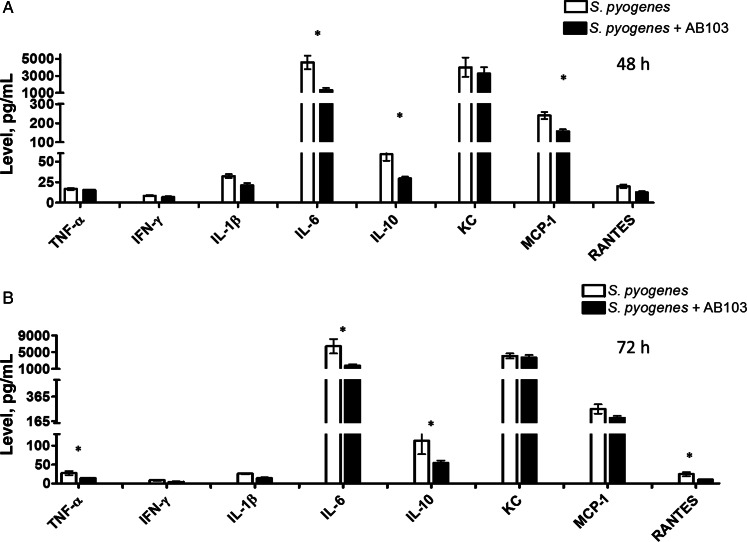
Since AB103 inhibits proinflammatory cytokine production triggered in PBMC by SAgs [14], we investigated whether the peptide can interfere in vivo with cytokine production downstream of CD28 signaling caused by infection with SAg-producing GAS.
Mice were infected with GAS and received AB103 or PBS (control) 1 hour after infection. Serum harvested at 12, 24, 48, and 72 hours after infection was assayed for cytokines and chemokines. Serum cytokines levels for uninfected and untreated mice were below the limit of detection. Compared with levels of interferon γ, interleukin 1β, and interleukin 6 in untreated mice, levels in peptide-treated mice declined significantly at all 4 time points, which is consistent with the data in human PBMC [14]. Cytokine/chemokine levels at 48 and 72 hours are shown in Figure 4. Concomitant survival analysis showed that only 5 of 10 untreated mice were alive at 48 hours and only 2 of 10 were alive at 72 hours, whereas 10 of 10 AB103-treated mice had survived GAS challenge by 72 hours.
Figure 4.
Serum cytokine profile in mice that were infected with Streptococcus pyogenes (GAS) and then received AB103. BALB/c mice (n = 10/group) were infected intramuscularly with GAS and treated with either AB103 (5 mg/kg intravenously) or phosphate-buffered saline (PBS) 1 hour after infection. Serum was obtained 48 hours (A) and 72 hours (B) after infection, and serum cytokine levels were measured. Cytokine levels obtained from mice that were neither infected nor treated with AB103 were below the limits of detection (data not shown). Data are means ± standard error of the mean. *P < .05, for comparison of cytokine levels between AB103- and PBS-treated mice. Abbreviations: IFN-γ, interferon γ; IL-1β, interleukin 1β; IL-6, interleukin 6; IL-10, interleukin 10; MCP-1, monocyte chemotactic protein 1; TNF-α, tumor necrosis factor α.
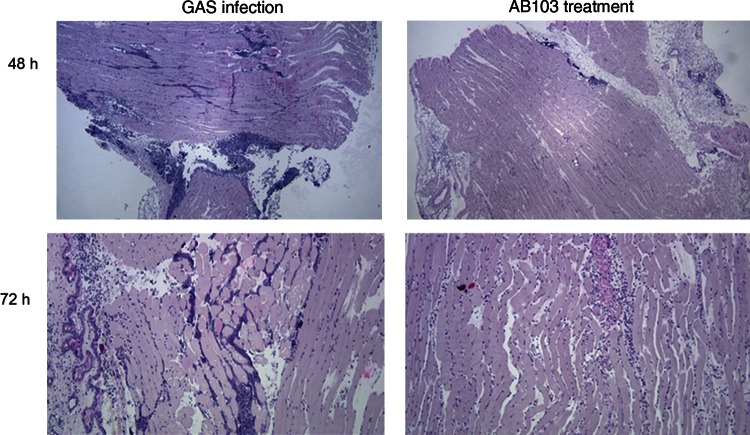
AB103 Effectively Attenuates Myositis in Muscle Tissue
In untreated mice, we observed the onset of NSTI as early as 24 hours after infection. By 48 hours after infection, muscle sections showed a severe acute inflammatory infiltrate composed mainly of neutrophils, primarily in the fascia and among the muscle fibers, that was milder in AB103-treated mice (Figure 5)
Figure 5.
AB103-treated mice exhibit fewer inflammatory cells than infected, untreated mice. Upper panels, Muscle section of a mouse 48 hours after Streptococcus pyogenes (GAS) infection. GAS-infected mice developed severe, acute inflammatory infiltrates primarily in the fascia (upper left panel). This was significantly reduced in mice treated with AB103 (5 mg/kg) and among muscle fibers (upper right panel). Lower panels, Muscle section of mice 72 hours after GAS infection. Apart from inflammatory infiltrates, necrosis of muscle cells was observed in GAS-infected, untreated mice (lower left panel). Necrosis was strongly attenuated in AB103-treated mice (lower right panel).
In sections of muscle taken from untreated controls at 72 hours, severe necrosis of muscle cells was apparent, infiltrated primarily by neutrophils with scattered lymphocytes, whereas in AB103-treated mice the necrosis was significantly milder (Figure 5). No bacteria were seen.
In untreated mice, severe infiltration of inflammatory cells to the site of infection correlated with significantly increased chemokine levels, whereas following AB103 treatment, chemokine levels declined.
No differences were observed in tissue pathology between treated and untreated groups in remote organs such as liver and kidney. This is further supported by no observable differences or abnormalities in kidney and liver function between the 2 groups, as judged on the basis of creatinine, alanine transaminase, aspartate transaminase, alkaline phosphatase, and bilirubin levels in serum from these mice (Supplementary Table 1).
AB103 Does Not Impede Induction of a T-Cell–Mediated Immune Response
We next tested the effect of AB103 on the development of the cell-mediated immune response induced by vaccination. As a model, we used mice infected with F. tularensis that were also vaccinated with LVSΔguaB, a live vaccine strain of the pathogen prior to infection. In the group that received LVSΔguaB 28 days before infection, 5 of 5 mice were protected from F. tularensis challenge, whereas 0 of 5 unvaccinated mice survived. Infected mice started to die as early as 5 days after infection, and all died by day 7. By contrast, in the group treated with AB103 before vaccination and infection, all mice (5/5) survived challenge with F. tularensis (Table 1). Thus, AB103 does not impede the action of a vaccine that induces a cell-mediated immune response response.
Table 1.
AB103 Does Not Impede the Cell-Mediated Immune Response
| Experimental Group | AB103 Dose,a mg/kg | LVSΔguaB Dose,b CFU | F. tularensis Inoculum, CFU | Survival Ratio | Time(s) to Death, d |
|---|---|---|---|---|---|
| 1 (5 mice) | None | None | 1 × 105 | 0/5 | 5, 5, 6, 7, and 7 |
| 2 (5 mice) | None | 1 × 107 | 1 × 105 | 5/5 | NA |
| 3 (5 mice) | 0.5 | 1 × 107 | 1 × 105 | 5/5 | NA |
| 4 (5 mice) | 5 | 1 × 107 | 1 × 105 | 5/5 | NA |
Abbreviations: CFU, colony forming units; F. tularensis, Francisella tularensis; NA, not applicable.
a Administered 30 min before LVSΔguaB vaccination.
b Administered 28 d before F. tularensis infection.
AB103 Does Not Impede Induction of Antigen-Presenting Cell–Mediated Costimulation of T-Cell Proliferation
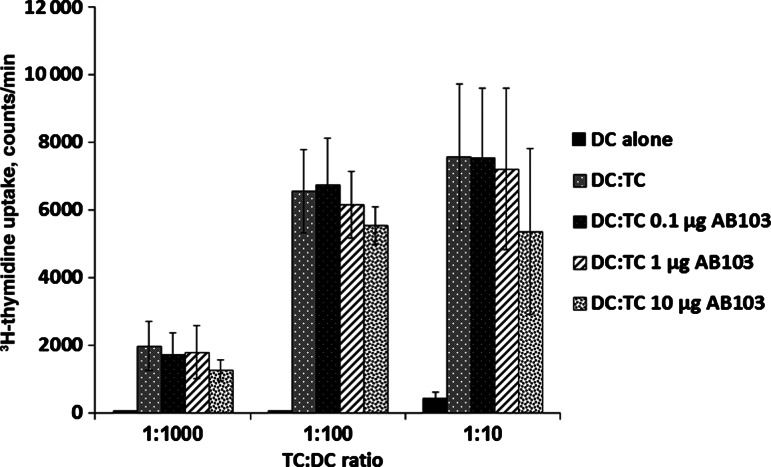
CD28 signaling results in several responses, including cell proliferation. Because AB103 effectively promoted survival of mice following both SAg and GAS lethal challenge (Figure 1), which involves attenuation of the inflammatory cytokine response [14] (Figure 4), we examined whether the peptide affects cell proliferation. To this end, we used a mixed lymphocyte reaction model and measured T-cell proliferation in the presence and absence of AB103. Varying doses of AB103 were used, and no inhibitory effect of the peptide on the mixed lymphocyte reaction was observed (Figure 6). These results support the conclusion that AB103 does not interfere with cell-mediated cross-activation of T cells by antigen-presenting cells that results in proliferation.
Figure 6.
AB103 does not interfere with mixed lymphocyte reactions. Dendritic cells (DCs) from 3 different individuals were cultured for 3 days with allogeneic peripheral blood mononuclear cells from 3 additional donors in the presence of 0.1, 1, or 10 µg/mL of AB103. Immature monocyte-derived dendritic cells alone were included as controls. Cells were pulsed with 1.0 μCi 3H-thymidine for 16 hours, and the level of incorporated label was determined. Data are means ± SD from 3 independent experiments. NS, no significant difference between DC:TC and increasing doses of AB103.
DISCUSSION
These results show that a single dose of AB103, a peptide mimetic of the dimer interface of the costimulatory receptor CD28 [14], protects mice from lethal NSTI. Treatment with AB103 attenuates signaling through the SAg receptor, CD28, a step essential for SAg lethality [14]. Administration of AB103 conferred substantial survival benefit, up to 100%, in a dose-responsive manner, depending on the interval between infection and treatment. Remarkably, upon treatment of established infection with a single dose of AB103 1 and 5 hours after bacterial administration, long-lasting protection was obtained, reaching 85% and 50% survival, respectively, compared with 0% survival among untreated controls. The peptide was protective not only following challenge with a streptococcal SAg, SPEA, after bolus administration but also after the more sustained release of multiple SAgs by replicating GAS bacteria that increasingly overwhelm the host, a model for clinical infection. As is true for most animal models, this NSTI model reflects an accelerated time course relative to the clinical illness in humans, which usually presents after the process is well underway. Furthermore, this murine model requires significantly higher bacterial inocula, and unlike the case with mice, supportive care is provided to infected patients. Surviving animals demonstrated no signs of local infection, as judged by lack of inflammation and necrosis, minimal systemic clinical symptoms, and minimized tissue damage. In mice that were followed for up to 60 days after infection, the number of GAS bacteria at the initial site of infection decreased progressively in AB103-treated mice, until total resolution was observed.
The protective effect of AB103 may be attributed, at least in part, to its ability to neutralize the biologic activity of SAgs, thereby enabling the host to develop an adaptive immune response to the GAS infection. Whereas the staphylococcal and streptococcal SAg families share little overall homology [13], Arad et al identified a 12-amino acid SAg domain distal to the binding sites for the T-cell receptor variable β chain and MHC-II molecules showing high structural conservation and showed that SAgs use this conserved domain to bind directly to CD28 [14]. A peptide mimetic of this conserved domain in SAgs protected mice from lethal challenge by SAgs, including SPEA [13]. Protected mice promptly developed protective antibodies against divergent SAgs [13, 18]. Thus, the peptide protected mice from subsequent challenge with lethal doses of other SAgs, and sera obtained from protected mice conferred passive protection against lethal SAg challenge in SAg- and peptide antagonist–naive mice [13]. These studies show that while AB103 protects from the lethal effect of SAg challenge, it enables the development of a protective humoral immune response, validating that this response remains intact. Because little systemic dissemination of bacteria was detected in untreated mice and surviving mice developed antibodies against bacterial toxins, we posit that, in this model, the major factor associated with mortality is toxemia rather than bacteremia.
The improved outcome also was associated with reduced systemic levels of cytokines and chemokines and reduced infiltration of inflammatory cells (primarily neutrophils) into the infected site. Rather than targeting a specific cytokine or chemokine, AB103 interferes with the cellular response elicited through CD28 that controls proinflammatory cytokine production in T cells, with a strong reduction, yet not full abrogation, of proinflammatory cytokines [14]. By preserving at least part of the proinflammatory cytokine response, treatment with AB103 would not be expected to render the host susceptible to microbial superinfection, as was observed in earlier clinical trials of sepsis therapies that abrogated responses by tumor necrosis factor α and interleukin 1β [18, 19], cytokines essential for host defense against lethal infection with gram-negative bacteria [19].
Indeed, AB103 did not impair the host adaptive immune response or the humoral and long-term activities of the immune system, as judged by (1) the lack of interference with vaccine-induced protection against lethal infection with the intracellular pathogen F. tularensis, (2) unimpaired development of a mixed lymphocyte reaction, (3) development of antibodies against bacterial toxins, and (iv) the lack of interference with the Th2 response, exemplified in human PBMCs by its lack of effect on the induction of interleukin 4 and interleukin 10 [14].
SAgs have been detected after immunostaining tissue biopsy specimens from patients with GAS toxic shock syndrome [20, 21] The plasma from patients with severe invasive GAS infection who received intravenous immunoglobulin neutralized SAg activity in vitro [22]. By targeting an essential step in the initiation of SAg-mediated signaling [14], AB103 inhibits the host-mediated response to multiple SAgs [14] and is not pathogen specific, and it may therefore be used to treat infections caused by other SAg-producing bacteria, such as S. aureus.
Previous attempts to treat lethal SAg intoxication with specific interventions targeting the proinflammatory cytokine response failed [23], likely because SAgs induce an excessive cytokine storm that cannot be controlled by neutralizing antibodies. Studies of drugs to mitigate systemic toxemia associated with streptococcal necrotizing skin infections, such as use of human intravenous immunoglobulin with and without clindamycin to inhibit protein synthesis, were inconclusive [1]. Other attempts focused on intervening at more proximal events, targeting SAg interactions with host receptors [23–25]. These studies did not examine efficacy for infections with SAg-producing bacteria, nor efficacy against multiple SAgs.
Our results support the conclusion that CD28 mimetic peptide AB103 may be an effective host-oriented therapeutic drug candidate for NSTI, particularly since no adequate therapy for NSTI is available. Therapy with this peptide is unlikely to increase susceptibility to infection. As neither surgical debridement nor antibacterial therapy directly address the immunological pathogenesis of NSTI, reducing the host inflammatory response could yield important clinical benefits in both morbidity and mortality.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant 2U54AI057168-06) and Atox Bio.
Potential conflicts of interest. A. S. and R. S. are employees of, R. K. is chief scientific officer of, and A. C. has received funding from Atox Bio. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Johansson L, Thulin P, Low DE, Norrby-Teglund A. Getting under the skin: the immunopathogenesis of Streptococcus pyogenes deep tissue infections. Clin Infect Dis. 2010;51:58–65. doi: 10.1086/653116. [DOI] [PubMed] [Google Scholar]
- 2.Brosnahan AJ, Schlievert PM. Gram-positive bacterial superantigen outside-in signaling causes toxic shock syndrome. FEBS J. 2011;278:4649–67. doi: 10.1111/j.1742-4658.2011.08151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sriskandan S, Moyes D, Buttery LK, et al. Streptococcal pyrogenic exotoxin A release, distribution, and role in a murine model of fasciitis and multiorgan failure due to Streptococcus pyogenes. J Infect Dis. 1996;173:1399–407. doi: 10.1093/infdis/173.6.1399. [DOI] [PubMed] [Google Scholar]
- 4.Unnikrishnan M, Altmann DM, Proft T, et al. The bacterial superantigen streptococcal mitogenic exotoxin Z is the major immunoactive agent of Streptococcus pyogenes. J Immunol. 2002;169:2561–9. doi: 10.4049/jimmunol.169.5.2561. [DOI] [PubMed] [Google Scholar]
- 5.Lynskey NN, Lawrenson RA, Sriskandan S. New understandings in Streptococcus pyogenes. Curr Opin Infect Dis. 2011;24:196–202. doi: 10.1097/QCO.0b013e3283458f7e. [DOI] [PubMed] [Google Scholar]
- 6.Unnikrishnan M, Cohen J, Sriskandan S. Complementation of a speA negative Streptococcus pyogenes with speA: effects on virulence and production of streptococcal pyrogenic exotoxin A. Microb Pathog. 2001;31:109–14. doi: 10.1006/mpat.2001.0453. [DOI] [PubMed] [Google Scholar]
- 7.Llewelyn M, Cohen J. Superantigens: microbial agents that corrupt immunity. Lancet Infect Dis. 2002;2:156–62. doi: 10.1016/s1473-3099(02)00222-0. [DOI] [PubMed] [Google Scholar]
- 8.Marrack P, Blackman M, Kushnir E, Kappler J. The toxicity of staphylococcal enterotoxin B in mice is mediated by T cells. J Exp Med. 1990;171:455–64. doi: 10.1084/jem.171.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miethke T, Wahl C, Heeg K, Echtenacher B, Krammer PH, Wagner H. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J Exp Med. 1992;175:91–8. doi: 10.1084/jem.175.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leder L, Llera A, Lavoie PM, et al. A mutational analysis of the binding of staphylococcal enterotoxins B and C3 to the T cell receptor beta chain and major histocompatibility complex class II. J Exp Med. 1998;187:823–33. doi: 10.1084/jem.187.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hackett SP, Stevens DL. Superantigens associated with staphylococcal and streptococcal toxic shock syndrome are potent inducers of tumor necrosis factor-beta synthesis. J Infect Dis. 1993;168:232–5. doi: 10.1093/infdis/168.1.232. [DOI] [PubMed] [Google Scholar]
- 12.Baracco GJ, Bisno AL. Therapeutic approaches to streptococcal toxic shock syndrome. Curr Infect Dis Rep. 1999;1:230–7. doi: 10.1007/s11908-999-0024-4. [DOI] [PubMed] [Google Scholar]
- 13.Arad G, Levy R, Hillman D, Kaempfer R. Superantigen antagonist protects against lethal shock and defines a new domain for T-cell activation. Nat Med. 2000;6:414–21. doi: 10.1038/74672. [DOI] [PubMed] [Google Scholar]
- 14.Arad G, Levy R, Nasie I, et al. Binding of superantigen toxins into the CD28 homodimer interface is essential for induction of cytokine genes that mediate lethal shock. PLoS Biol. 2011;9:e1001149. doi: 10.1371/journal.pbio.1001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olsen RJ, Musser JM. Molecular pathogenesis of necrotizing fasciitis. Annu Rev Pathol. 2010;5:1–31. doi: 10.1146/annurev-pathol-121808-102135. [DOI] [PubMed] [Google Scholar]
- 16.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 17.Stevens DL, Gibbons AE, Bergstrom R, Winn V. The Eagle effect revisited: efficacy of clindamycin, erythromycin, and penicillin in the treatment of streptococcal myositis. J Infect Dis. 1988;158:23–8. doi: 10.1093/infdis/158.1.23. [DOI] [PubMed] [Google Scholar]
- 18.Arad G, Hillman D, Levy R, Kaempfer R. Broad-spectrum immunity against superantigens is elicited in mice protected from lethal shock by a superantigen antagonist peptide. Immunol Lett. 2004;91:141–5. doi: 10.1016/j.imlet.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Cross AS, Sadoff JC, Kelly N, Bernton E, Gemski P. Pretreatment with recombinant murine tumor necrosis factor alpha/cachectin and murine interleukin 1 alpha protects mice from lethal bacterial infection. J Exp Med. 1989;169:2021–7. doi: 10.1084/jem.169.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norrby-Teglund A, Muller MP, McGeer A, et al. Successful management of severe group A streptococcal soft tissue infections using an aggressive medical regimen including intravenous polyspecific immunoglobulin together with a conservative surgical approach. Scand J Infect Dis. 2005;37:166–72. doi: 10.1080/00365540410020866. [DOI] [PubMed] [Google Scholar]
- 21.Sriskandan S, Moyes D, Cohen J. Detection of circulating bacterial superantigen and lymphotoxin-alpha in patients with streptococcal toxic-shock syndrome. Lancet. 1996;348:1315–6. doi: 10.1016/s0140-6736(05)65800-x. [DOI] [PubMed] [Google Scholar]
- 22.Norrby-Teglund A, Low DE, McGeer A, Kotb M. Superantigenic activity produced by group A streptococcal isolates is neutralized by plasma from IVIG-treated streptococcal toxic shock syndrome patients. Adv Exp Med Biol. 1997;418:563–6. doi: 10.1007/978-1-4899-1825-3_130. [DOI] [PubMed] [Google Scholar]
- 23.Buonpane RA, Churchill HR, Moza B, et al. Neutralization of staphylococcal enterotoxin B by soluble, high-affinity receptor antagonists. Nat Med. 2007;13:725–9. doi: 10.1038/nm1584. [DOI] [PubMed] [Google Scholar]
- 24.Kissner TL, Moisan L, Mann E, et al. A small molecule that mimics the BB-loop in the Toll interleukin-1 (IL-1) receptor domain of MyD88 attenuates staphylococcal enterotoxin B-induced pro-inflammatory cytokine production and toxicity in mice. J Biol Chem. 2011;286:31385–96. doi: 10.1074/jbc.M110.204982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang N, Mattis DM, Sundberg EJ, Schlievert PM, Kranz DM. A single, engineered protein therapeutic agent neutralizes exotoxins from both Staphylococcus aureus and Streptococcus pyogenes. Clin Vaccine Immunol. 2010;17:1781–9. doi: 10.1128/CVI.00277-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.