Abstract
Background. Modified vaccinia Ankara (MVA-BN, IMVAMUNE) is emerging as a primary immunogen and as a delivery system to treat or prevent a wide range of diseases. Defining the safety and immunogenicity of MVA-BN in key populations is therefore important.
Methods. We performed a dose-escalation study of MVA-BN administered subcutaneously in 2 doses, one on day 0 and another on day 28. Twenty-four hematopoietic stem cell transplant recipients were enrolled sequentially into the study, and vaccine or placebo was administered under a randomized, double-blind allocation. Ten subjects received vaccine containing 107 median tissue culture infective doses (TCID50) of MVA-BN, 10 subjects received vaccine containing 108 TCID50 of MVA-BN, and 4 subjects received placebo.
Results. MVA-BN was generally well tolerated at both doses. No vaccine-related serious adverse events were identified. Transient local reactogenicity was more frequently seen at the higher dose. Neutralizing antibodies (NAb) to Vaccinia virus (VACV) were elicited by both doses of MVA-BN and were greater for the higher dose. Median peak anti-VACV NAb titers were 1:49 in the lower-dose group and 1:118 in the higher-dose group. T-cell immune responses to VACV were detected by an interferon γ enzyme-linked immunosorbent spot assay and were higher in the higher-dose group.
Conclusions. MVA-BN is safe, well tolerated, and immunogenic in HSCT recipients. These data support the use of 108 TCID50 of MVA-BN in this population.
Clinical Trials Registration. NCT00565929.
Keywords: safety, immunogenicity, dose, stem cell transplant, modified vaccinia ankara, clinical trial
Less than 200 years after the introduction of the smallpox vaccine, variola virus (VARV) was successfully eradicated by use of vaccines produced with vaccinia virus (VACV) [1]. However, along with the dramatic success of the vaccination program, frequent and sometimes severe adverse reactions to VACV vaccine were encountered, particularly in subjects with immunologic defects or dermatopathologic conditions. Since eradication, VACV vaccine has been reserved for highly selected individuals at risk for orthopoxvirus infections, but there are ongoing concerns over the potential use of VARV as a biological weapon. The development of safer, yet effective vaccines for future use against smallpox therefore remains of considerable interest.
Modified vaccinia Ankara (MVA), an attenuated strain of VACV [2, 3], is much less reactogenic than widely used vaccinia strains, such as Dryvax or Elstree [4–8]. MVA was administered to approximately 120 000 persons [9] but was never used in a VARV-endemic area, and its effectiveness at preventing clinical smallpox is unknown. MVA is severely host restricted, and it is either unable to replicate in mammalian cell lines or replicates at a very low level [3, 10, 11]. Approximately 15% of the MVA genome was deleted during in vitro passage, compared with the parental strain [10, 12], but the block in replication in nonpermissive mammalian cells occurs late in the viral life cycle. Thus, because MVA-infected cells express very high levels of virally encoded proteins [13–15], including those encoded by foreign transgenes, there is considerable interest in using MVA as a vector in vaccines to prevent human immunodeficiency virus (HIV) infection, malaria, and infectious diseases due to other pathogens [16–18]. There is also substantial interest in the use of MVA as a vector to deliver tumor-specific antigens to induce immune responses that may help control malignancies, and several of these therapeutic vaccines have advanced to clinical trials [18–20].
Because preliminary studies suggested MVA was safe in immunocompromised hosts [21–24], we hypothesized that MVA would be safe, well-tolerated, and immunogenic in an important, well-characterized immunocompromised population—recipients of a hematopoietic stem cell transplant (HSCT). Vaccination with either traditional calf lymph–derived VACV (such as Dryvax or Elstree) or modern tissue culture-grown strains (the recently approved ACAM2000) are contraindicated for HSCT recipients [25]. Thus, establishing safety and immunogenicity of MVA in this group has implications for pre-event smallpox vaccine contingency planning, as well as for potential therapeutic vaccines against malignancies. We therefore conducted a randomized, placebo-controlled, double-blind study of MVA-BN (IMVAMUNE; Bavarian Nordic A/S, Kvistgaard, Denmark) in 24 individuals who received a HSCT >2 years previously.
METHODS
Vaccine and Placebo
The MVA vaccine used in this study was MVA-BN (lot numbers 0111208, 0040707, 0040704, 0050808, and 0031105) formulated with 1.22 mg/mL Tris and 0.9% NaCl USP, with a titer of 108 median tissue culture infective doses (TCID50) in 0.5 mL. The vaccine was reconstituted with 0.9% NaCl and diluted to the appropriate dose. Sterile saline, 0.9% NaCl, was used as the placebo.
Study Design and Subjects
We performed a dose-escalation study of MVA-BN administered subcutaneously in 2 doses, one on day 0 and another on day 28. Twenty-four HSCT recipients were enrolled sequentially into the study, and vaccine or placebo was administered under a randomized, double-blind, allocation (Figure 1, Supplementary Table 1). Ten subjects received 107 TCID50 of MVA-BN, 10 subjects received 108 TCID50 of MVA-BN, and 4 subjects received placebo.
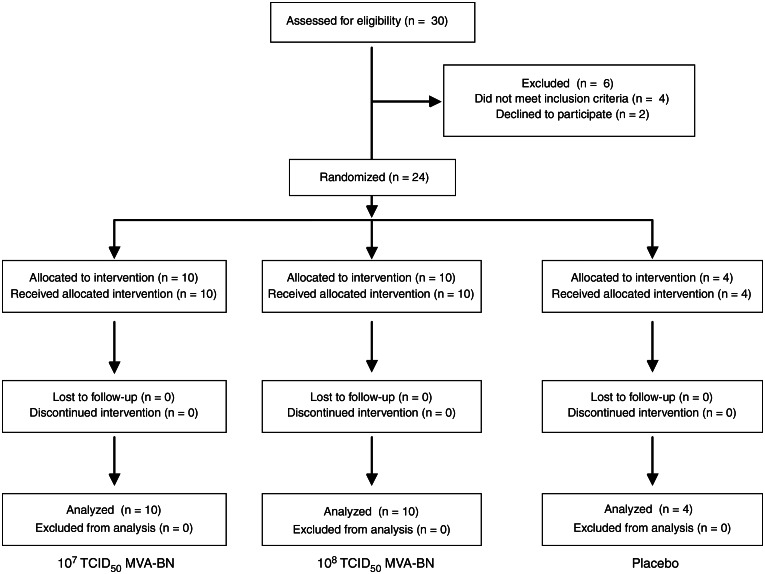
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) subject flow diagram demonstrating the number of patients recruited into the study, reasons for dropout, number of subjects randomized and vaccinated, and number of subjects analyzed. MVA-BN, modified vaccinia Ankara; TCID50, median tissue culture infective dose
Subjects were healthy men or women aged 18–60 years, had received an HSCT at least 2 years prior to enrollment, had no evidence of current graft-versus-host disease, and had taken no systemic immunosuppressant medications for at least 30 days prior to enrollment. The 2-year interval since transplantation was chosen because receipt of live vaccines is not recommended within 2 years after HSCT receipt [26, 27]. Current good health was verified by history, physical examination, and laboratory tests. Allogeneic and autologous HSCT recipients, VACV vaccine–naive individuals, and past recipients of VACV vaccine were eligible for the study. To mitigate the risk of cardiac events, which have been reported with vaccine containing replication-competent VACV [28–30], subjects were screened for cardiac risk factors and history of heart disease; we excluded potential subjects who had a risk of ≥20% for developing a myocardial infarction or coronary death within the next 10 years [31].
The study was approved by the local institutional review board and committee on microbiologic safety, and written informed consent was obtained from all subjects.
Safety and Reactogenicity Evaluation
To assess reactogenicity after each immunization, subjects maintained a diary to record daily body temperatures and reactions for 15 days or until symptoms resolved, if longer. Hematology and chemistry evaluations were performed at screening and on days 14, 28, 42, 56, and 84. Standardized cardiac questions were asked at every visit. Electrocardiography (ECG) and troponin levels were performed at screening and on days 14, 28, 42, 56, and 84. Nonserious adverse events were collected through day 56 after the last immunization, and serious adverse events were collected throughout the study period. Toxicity was graded according to standard Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases toxicity tables (available at: http://www.niaid.nih.gov/LabsAndResources/resources/DMIDClinRsrch/Documents/dmidadulttox.pdf).
Cell Lines and Viruses
HeLa, CV-1, and DF-1 cell lines and VACV strains (Western Reserve [VACV:WR], ACAM3000 MVA, and VACV and MVA recombinants containing a luciferase reporter gene [VACV:Luc and MVA:Luc, respectively]) are described in the Supplementary Material.
VACV and MVA Neutralization Assay
Neutralizing activity against VACV and MVA was measured on serum samples obtained on days 0, 14, 28, 42, 56, 84, and 180 following the first immunization, using a luciferase-based assay in HeLa or DF-1 cells as described elsewhere (Supplementary Material) [8, 32].
T-Cell Interferon γ (IFN-γ) Enzyme-Linked Immunosorbent Spot (ELISPOT) Assay
ELISPOT assays were performed on PBMCs obtained on days 0, 14, 28, 42, 56, 84, and 180 after immunization, essentially as described elsewhere (Supplementary Material) [8, 33].
Statistical Analysis
Kruskal-Wallis nonparametric 1-way analysis of variance was used to assess continuous baseline characteristics among the 3 groups. The Fisher exact test and the exact Wilcoxon rank-sum test were used to compare categorical and continuous outcomes between groups, respectively. For immunogenicity results, pairwise comparisons were performed between any 2 of the 3 groups. The cumulative probabilities of response were also analyzed at each time point after vaccination, using the Fisher exact test. All tests were 2-sided. All analyses are based on the intent-to-treat principle. No adjustments were made for multiple comparisons, and P values were considered significant at an α level of 0.05.
RESULTS
Subject Characteristics
Twenty-four subjects enrolled in the study from September 2008 through December 2011 (Figure 1). Fifteen participants (63%) were male, and all were non-Hispanic white individuals (Table 1). Subjects ranged in age from 21 to 60 years, with a median age of 50 years. Six subjects (25%) were born after 1972, did not have a smallpox vaccine scar, and were presumed to be VACV-vaccine naive; 17 of the 18 subjects (94%) born prior to 1972 had a vaccination scar. The interval between HSCT receipt and enrollment was longer for the low-dose group, but no other baseline characteristic differed among the groups. All subjects received both injections, and no subjects were lost to follow-up.
Table 1.
Baseline Characteristics of Subjects
| Characteristic | Placebo Group | 107 TCID50 Group | 108 TCID50 Group | Pa |
|---|---|---|---|---|
| Age | 36 (25–54) | 46.5 (25–59) | 52.5 (21–60) | .2191 |
| Male sex | 1 (25) | 6 (60) | 8 (80) | .1330 |
| White race | 4 (100) | 10 (100) | 10 (100) | >.999 |
| Allogeneic HSCT receipt | 1 (25) | 6 (60) | 3 (30) | .4642 |
| VACV vaccine naive | 2 (50) | 3 (30) | 1 (10) | .1631 |
| Time since HSCT receipt | 3 (3–5) | 4 (2–15) | 2 (2–3) | .0042 |
| Underlying disease | .867 | |||
| Leukemia | 1 (25) | 2 (20) | 2 (20) | |
| Lymphoma | 2 (25) | 5 (50) | 4 (40) | |
| Myeloma | 0 (0) | 1 (10) | 3 (30) | |
| Aplastic anemia | 1 (25) | 1 (25) | 0 (0) | |
| Myelodysplastic syndrome | 0 (0) | 0 (0) | 1 (10) | |
| Myelofibrosis | 0 (0) | 1 (10) | 0 (0) |
Data are no. (%) of subjects or median no. of years (interquartile range).
Abbreviations: HSCT, hematopoietic stem cell transplant; TCID50, median tissue culture infective dose; VACV, vaccinia virus.
a By the Fisher exact test, for categorical variables, or by Kruskal-Wallis 1-way analysis of variance, for continuous variables, among the 3 groups.
Safety and Reactogenicity
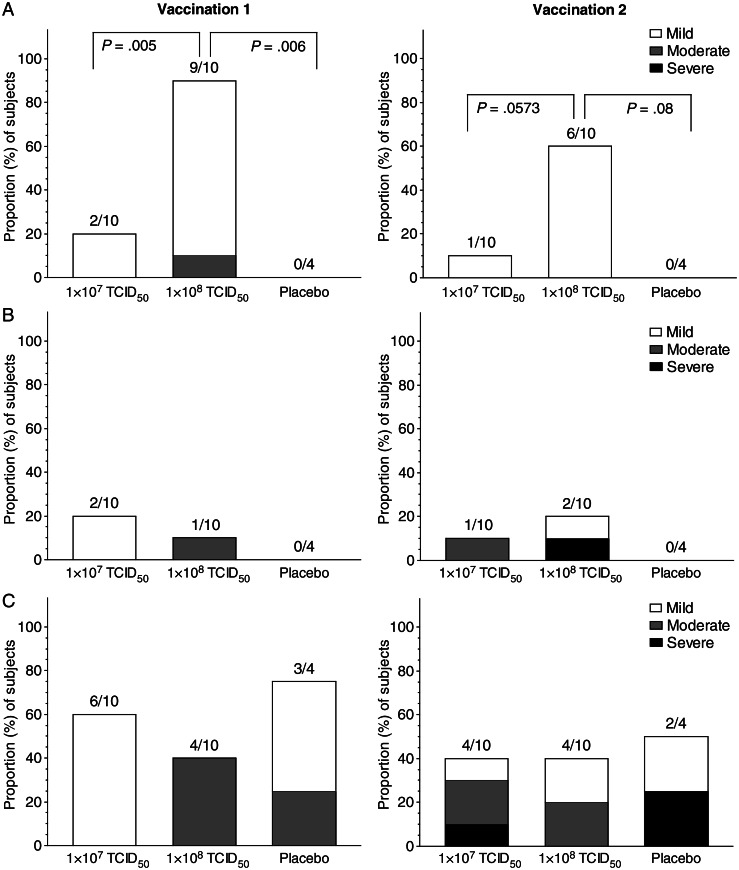
MVA immunization was well tolerated at both dose levels. Local reactogenicity was significantly more common in the 108 TCID50 group with both inoculations (Figure 2A) and consisted of pain, tenderness, or itchiness at the inoculation site or limitation of deltoid movement that generally resolved within 4–7 days, with either no treatment or with over-the-counter analgesics. Local erythema and induration was uncommon (Figure 2B), but 1 subject in the 108 TCID50 group, who had moderate erythema and induration after the first injection, had increased erythema and induration following the second inoculation, which resolved within 4 days.
Figure 2.
Proportion of vaccinees experiencing local reactogenicity (including pain or tenderness) (A), local erythema and induration (B), or systemic symptoms (C) after the first or second modified vaccinia Ankara vaccination, by dose group. Severity of symptoms were graded on the basis of Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases toxicity tables. TCID50, median tissue culture infective dose.
Systemic reactogenicity, consisting of low-grade fever (temperature <38°C), chills, malaise, myalgia, arthralgia, headache, or nausea occurred commonly and was generally graded as mild or moderate, with 1 subject each in the 107 TCID50 group and the placebo group noting more severe symptoms following the second inoculation (Figure 2C). All systemic reactogenic events were self-limited and resolved without sequelae. There were no significant differences in frequency or severity of systemic reactions between the higher and lower doses of MVA.
Adverse Events
A total of 69 nonserious adverse events were reported by 22 subjects: 9 of 10 (90%) in the 107 TCID50 group, 9 of 10 (90%) in the 108 TCID50 group, and 4 of 4 (100%) in the placebo group (P > .99 for all comparisons). The frequency of adverse events did not differ on the basis of the subjects’ history of prior smallpox vaccination: 6 of 6 VACV vaccine–naive subjects and 16 of 18 previously vaccinated subjects reported adverse events. Twenty-one of the adverse events were incidental infections, including urinary tract infections and upper respiratory tract infections, 10 were transient laboratory abnormalities, and 22 others were exacerbations of preexisting conditions, including hypertension, low back pain, and seasonal allergies. One upper respiratory tract infection was graded as severe, but the remaining 68 adverse events were graded as mild to moderate. All 69 adverse events were judged to be unrelated to vaccination.
Two serious adverse events occurred during the study. The first was a new diagnosis of prostate cancer and involved a subject in the 107 TCID50 group; the second was an episode of pneumonia, which occurred in a subject in the 108 TCID50 group and required hospitalization. Both serious adverse events were judged to be unrelated to vaccination.
Because of the reports of myopericarditis in recipients of live VACV vaccine, subjects were examined closely for possible cardiac side effects related to immunization. One subject in the 107 TCID50 group had asymptomatic dynamic ECG changes related to preexisting hypertension; a review of ECG findings prior to vaccination revealed identical dynamic ECG changes concurrent with hypertensive episodes. Another subject in the 107 TCID50 group had a transiently detectable troponin level that was not associated with any symptoms or ECG findings. A subject in the 108 TCID50 group had an increased QTc at baseline (474 ms), likely due to concomitant medications, that transiently increased (to 499 ms) following vaccination, was asymptomatic, and deemed not clinically significant. No subject had clinical symptoms or signs, ECG findings, or troponin levels suggestive of myopericarditis.
Neutralizing Antibody (NAb) Responses to MVA and VACV:WR
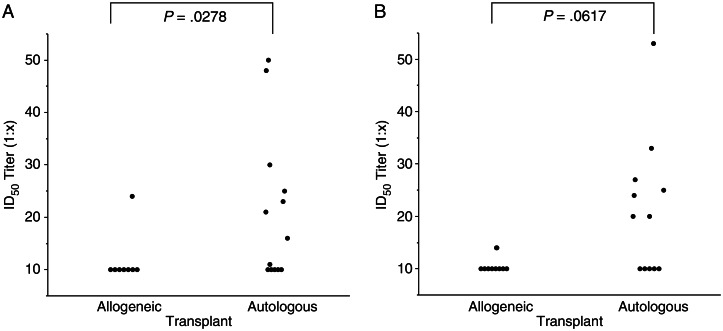
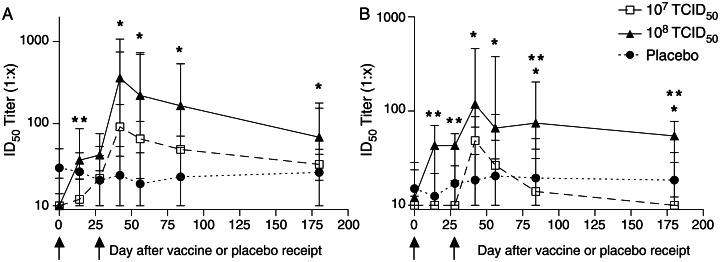
Because we did not exclude prior VACV vaccinees from the study, 7 of 24 subjects had MVA NAb titers of ≥1:20 at baseline, consistent with previous vaccination. Subjects who had received an autologous HSCT had higher anti-MVA NAb titers at enrollment (P = .0278), suggesting that residual immunity is more likely to be ablated by allogeneic HSCT receipt (Figure 3A). This finding also suggests that anti-VACV humoral responses are not transferred from the allogeneic HSCT donor. Baseline NAb against MVA were seen only in subjects born before 1972 (7/18 vs 0/6; P = .13) and did not differ between groups. Following vaccination, NAb responses to MVA were detected in 9 of 10 vaccine recipients (90%) in both the 107 TCID50 and 108 TCID50 groups (Figure 4A), although 2 subjects in the 107 TCID50 group had very low responses. The 90% response rates in the 2 MVA-BN groups, compared with the 0% response rate in the placebo group, are significantly different (P = .0014). Following primary immunization, elevated titers were observed on day 14 in the higher-dose group as compared to the lower-dose group (P = .004). These responses increased following the second immunization, and peak NAb titers typically occurred on day 42 (14 days after the second immunization; P = .01 for the 108 TCID50 group vs placebo). Median peak anti-MVA NAb titers were 1:92 in the lower-dose group and 1:361 in the higher-dose group. By day 180, titers in the 108 TCID50 group remained significantly increased as compared to titers in the placebo group (P = .01); whereas 2 of 9 responders in the 107 TCID50 group had seroreverted, none in the 108 TCID50 group had.
Figure 3.
Baseline neutralizing antibody responses against modified vaccinia Ankara (A) and vaccinia virus (B), stratified by the type of hematopoietic stem cell transplant (HSCT) the subjects had received. The limit of detection was a serum median infective dose (ID50) titer of 1:10.
Figure 4.
Neutralizing antibody responses elicited by modified vaccinia Ankara (MVA) prime/boost immunization. Serum samples were obtained at days 0, 14, 28, 42, 56, 84, and 180 following MVA immunization. Serial dilutions were tested for neutralizing activity against a modified vaccinia Ankara recombinant strain containing a luciferase reporter gene (A) or a vaccinia virus recombinant strain containing a luciferase reporter gene (B). Data are presented as median infective dose (ID50) titers with interquartile ranges for each dose group. The limit of detection was a serum ID50 titer of 1:10, and arrows indicate days of immunization. *P ≤ .01 for the 108 median tissue culture infective dose (TCID50) group vs the placebo group; **P ≤ .04 for the 108 TCID50 group vs the 107 TCID50 group.
The cross-reactivity of MVA-elicited NAb responses to VACV was also assessed. Baseline NAb titers to VACV of ≥1:20 were more frequently detected in autologous HSCT recipients than allogeneic HSCT recipients (7/14 vs 0/10; P = .0188), with a trend toward higher anti-VACV NAb titers in the autologous HSCT recipients (P = .0617; Figure 3B). As with MVA, baseline NAb against VACV were found only in subjects born before 1972 (7/18 vs 0/6; P = .13), but again, this finding did not differ by group. Overall, the kinetics of anti-VACV NAb responses were similar to the anti-MVA NAb responses, but the magnitude was diminished (Figure 4B). Increased NAb activity against VACV following MVA vaccination was seen in 6 of 10 subjects (60%) in the 107 TCID50 group and 8 of 10 subjects (80%) in the 108 TCID50 group, and this difference in response rates (vs 0/4 in the placebo group) is significant (P = .0331). After the first inoculation, titers on day 14 were significantly higher in the higher-dose group as compared to the lower-dose group (P = .009). By day 42, the 108 TCID50 group had NAb titers that were significantly increased as compared to those in the placebo group (P = .01) and the 107 TCID50 group (P = .01). Median peak anti-VACV NAb titers were 1:49 in the lower-dose group and 1:118 in the higher-dose group. The NAb titers in the higher-dose group remained higher than those in the placebo group on days 56 and 84 (P = .01 and P = .04, respectively) and were higher than those in the lower-dose group on days 84 and 180 (P = .01 and P = .03, respectively). Three of 6 responders in the 107 TCID50 group seroreverted by day 180, compared with 2 of 8 responders in the 108 TCID50 group.
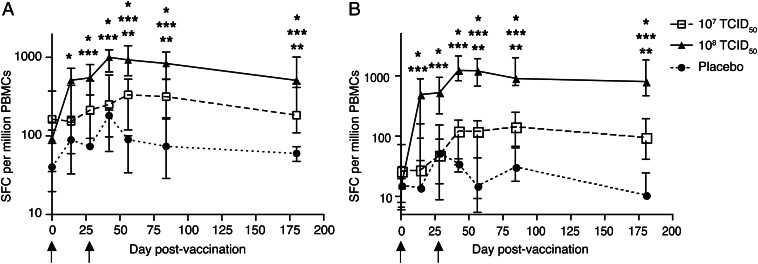
T-Cell Responses Determined by IFN-γ ELISPOT Assay
The magnitude and kinetics of orthopoxvirus-specific T-cell responses were assessed by an IFN-γ ELISPOT assay (Figure 5) and were similar whether MVA- or VACV-infected target cells were used to stimulate effector PBMCs. Baseline responses did not differ between the groups. At day 14, MVA-BN administration at 108 TCID50 elicited significantly higher responses, compared with both the lower-dose and the placebo groups (P < .004 for both comparisons), using VACV-infected target cells. Two weeks following the boost immunization (day 42), the 108 TCID50 group had significantly higher responses to both MVA and VACV stimulation than the placebo group (P = .002) and the 107 TCID50 group (P < .0001). From day 56 through day 180, the higher-dose group had significantly higher IFN-γ ELISPOT responses to both MVA and VACV stimulation than the lower-dose group and the placebo group (P ≤ .01 for all comparisons); the 107 TCID50 group had higher responses than the placebo group to MVA stimulation at the same time points (P ≤ .02) but only at days 84 and 180 for VACV stimulation (P ≤ .008). Interestingly, the lone subject in the 108 TCID50 group who did not mount a NAb response to MVA-BN vaccination (all titers were <1:10) exhibited a 350-fold increase in VACV-specific T-cell responses versus baseline, as measured by ELISPOT; this subject had received rituximab (anti-CD20 monoclonal antibody) therapy 64 days prior to enrollment.
Figure 5.
Cellular immune responses elicited by modified vaccinia Ankara (MVA) prime/boost immunizations. Peripheral blood mononuclear cells (PBMCs) were obtained on days 0, 14, 28, 42, 56, 84, and 180 following MVA vaccination and tested by the interferon γ enzyme-linked immunosorbent spot assay against autologous MVA-infected (A) and Western Reserve vaccinia virus strain–infected (B) target cells. Data are presented as the median number of spot-forming cells per 106 effector PBMCs with interquartile ranges for each dose group, following subtraction of responses to medium alone. *P ≤ .02 for the 108 median tissue culture infective dose (TCID50) group vs the placebo group; **P ≤ .02 for the 107 TCID50 group vs the placebo group; ***P ≤ .04 for the 108 TCID50 group vs the 107 TCID50 group.
Comparison of Immune Responses in Allogeneic and Autologous HSCT Recipients
Because roughly half our vaccinees (n = 9) were allogeneic HSCT recipients and half (n = 11) were autologous HSCT recipients, we determined whether humoral and cellular immune responses differed in the 2 populations. There were no differences in VACV-specific NAb response rates between allogeneic (7/9) and autologous (7/11) HSCT recipients when both MVA-BN dose groups were combined. When MVA-specific and VACV-specific NAb titers were compared between groups, the titers were lowest in the autologous HSCT recipients who were vaccinated with 107 TCID50 (Supplementary Figure 1A and 1B). While these differences are not significant, autologous HSCT recipients were more likely to have detectable NAb titers at baseline, suggesting that the higher dose may elicit immune responses despite remote vaccination. There was a more pronounced dose-response effect on cellular immune responses (Supplementary Figure 1C and 1D), but our small sample size precludes any firm conclusions.
Comparison of Immune Responses Between Previously Vaccinated Recipients and Smallpox Vaccine–Naive Subjects
Because a majority of our vaccinees (16/20) had been remotely vaccinated against smallpox, we determined whether anamnestic immune responses could be induced by MVA-BN despite HSCT receipt. There were no differences in VACV-specific NAb response rates between remotely vaccinated (11/16) and naive (3/4) HSCT recipients when both MVA-BN dose groups were combined. When MVA-specific and VACV-specific NAb titers were compared between groups, the titers were lowest in the remotely vaccinated HSCT recipients from the 107 TCID50 group (Supplementary Figure 2A and 2B). Interestingly, at day 14, the remotely vaccinated subjects in the 108 TCID50 group appeared to have a more rapid rise in virus-specific NAb titers, compared with other subjects, particularly against VACV (Supplementary Figure 2B). While these differences are not significant, our findings suggest that the higher dose of MVA-BN may elicit anamnestic immune responses in remotely vaccinated HSCT recipients. There was a more pronounced dose-response effect on cellular immune responses than for NAb (Supplementary Figure 2C and 2D), but these differences were not significant, likely because of our small sample size.
DISCUSSION
MVA-BN was safe and generally well tolerated at both doses, and self-limited local discomfort was the most frequent reactogenicity. More-pronounced local reactogenicity was more common in the higher-dose group. Self-limited systemic reactogenic events were encountered in about half of the vaccinees and did not differ among the groups. No serious adverse events were associated with vaccination, and no pattern of adverse events was detected. Phase I studies of other MVA candidate vaccines have also shown that they are well tolerated [4–8]. However, our study expands the safety profile of MVA-BN to include HSCT recipients, including allogeneic HSCT recipients. Because orthopoxvirus vectors have been proposed as therapeutic vaccines against cancer [18, 34] and HIV infection [23, 24], our data provide reassurance that the highly attenuated MVA vector is likely to be safe in immunosuppressed patient populations.
Similar to other findings of rapid induction of humoral immune responses with MVA [8, 35], we found that a single administration of 108 TCID50 of MVA-BN elicited detectable anti-MVA NAb titers in the majority of subjects by day 14. These titers substantially increased by 2 weeks after the second immunization, and NAb responses in the higher-dose group remained detectable through day 180. While antibody responses in the lower-dose group increased after the second immunization, peak anti-VACV NAb titers trended lower than those in the higher-dose group (P = .06) and did not differ significantly from placebo at any time point for either MVA or VACV. Overall, the anti-MVA and anti-VACV NAb titers in our HSCT recipients inoculated with MVA-BN were quite similar to those elicited in healthy volunteers by other strains of MVA [8, 35].
Dose-dependent induction of orthopoxvirus-specific antibody responses has been reported in prior studies with subcutaneous administration of MVA [4, 6, 8]. Since we did not exclude individuals with a history of smallpox vaccination, the lower NAb titers that we found in the 107 TCID50 group may be related to preexisting antibodies. Although our sample sizes were small, it appears that 108 TCID50 of MVA-BN may be able to elicit humoral immune responses in vaccinia-experienced as well as vaccinia-naive subjects. This observation has implications for the use of MVA as a cancer vaccine vector, because a substantial fraction of the target population received smallpox vaccine as children.
Only 1 subject in the higher-dose group failed to generate a NAb response to MVA vaccination (all NAb titers were <1:10). Interestingly, this subject exhibited a 350-fold increase in VACV-specific T-cell responses in response to vaccination, and their history is notable for receipt of rituximab therapy 2 months before enrollment. This suggests that B-cell cytotoxic therapy can have long-lasting effects on induction of humoral immune responses and expands the observation that patients receiving rituximab therapy respond very poorly to subunit influenza vaccination [36]. Only one other subject had received rituximab therapy, but their most recent dose was received >19 months prior to enrollment. No subjects had received other monoclonal antibody or biologic therapies.
While the correlates of protection against smallpox are not known, studies from the preeradication era suggested that an anti-VACV NAb titer of >1:32 was protective [37]. After the second inoculation in our present study, anti-VACV titers exceeded 1:32 in 6 of 10 subjects (60%) in the 107 group and 9 of 10 subjects (90%) in the 108 group. Because our method for assaying NAb titers differs from that of Mack and colleagues (reduction in luciferase expression vs plaque reduction), our results are not directly comparable but do provide additional evidence that MVA immunization elicits cross-reactive anti–orthopoxvirus NAb in a dose-dependent fashion.
T-cell responses were also observed, using an IFN-γ ELISPOT assay with VACV- and MVA-infected target cells, with a clear dose-response effect. A previous study did not find a dose-response relationship with respect to T-cell responses after immunization with MVA-BN [4]. Interestingly, the dose-response pattern we observed appeared similar in both autologous and allogeneic HSCT vaccinees, compared with NAb responses, in which the autologous HSCT recipients had somewhat lower titers with 107 TCID50 of MVA. A similar dose-response pattern was seen when we compared remotely vaccinated subjects with smallpox vaccine–naive subjects. This suggests that there may be differences in the long-term persistence of viral-specific cellular versus humoral immune responses from remote smallpox vaccination; alternatively, memory T cells may be more sensitive than memory B cells or plasma cells to cytotoxic therapies during HSCT procedures.
Despite our small sample size, we found that 108 TCID50 was highly immunogenic with respect to both humoral and cellular immune responses. As many MVA-vectored therapeutic cancer vaccines are intended to elicit tumor-specific cytotoxic T-cell responses [18], our findings clearly demonstrate that MVA can safely induce cellular immune responses in HSCT recipients.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the following people for their invaluable assistance in this study: Robert Johnson, Stephen P. Heyse, and Carol Ostrye at the DMID/NIAID; the Hematopoietic Stem Cell Transplant Team at Dana-Farber Cancer Institute; Lawrence Friedman; Thomas Talbot of Vanderbilt University; Eyal Attar of Massachusetts General Hospital; and Jon Gothing, Daniel Worrall, Francisco Marty, Nicolas Issa, Anju Nohria, and John Jarcho of Brigham and Women's Hospital.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases and the National Center for Advancing Translational Sciences (US Public Health Service grants U54 AI057159, U19 AI057330, UL1 RR025758, and K23 AI085181).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi I. Smallpox and its eradication. Geneva: World Health Organization; 1988. [Google Scholar]
- 2.Mayr A. Smallpox vaccination and bioterrorism with pox viruses. Comp Immunol Microbiol Infect Dis. 2003;26:423–30. doi: 10.1016/S0147-9571(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 3.Meyer H, Sutter G, Mayr A. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J Gen Virol. 1991;72:1031–8. doi: 10.1099/0022-1317-72-5-1031. [DOI] [PubMed] [Google Scholar]
- 4.Frey SE, Newman FK, Kennedy JS, et al. Clinical and immunologic responses to multiple doses of IMVAMUNE (Modified Vaccinia Ankara) followed by Dryvax challenge. Vaccine. 2007;25:8562–73. doi: 10.1016/j.vaccine.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parrino J, McCurdy LH, Larkin BD, et al. Safety, immunogenicity and efficacy of modified vaccinia Ankara (MVA) against Dryvax challenge in vaccinia-naïve and vaccinia-immune individuals. Vaccine. 2007;25:1513–25. doi: 10.1016/j.vaccine.2006.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vollmar J, Arndtz N, Eckl KM, et al. Safety and immunogenicity of IMVAMUNE, a promising candidate as a third generation smallpox vaccine. Vaccine. 2006;24:2065–70. doi: 10.1016/j.vaccine.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 7.von Krempelhuber A, Vollmar J, Pokorny R, et al. A randomized, double-blind, dose-finding phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE. Vaccine. 2010;28:1209–16. doi: 10.1016/j.vaccine.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilck MB, Seaman MS, Baden LR, et al. Safety and immunogenicity of modified vaccinia Ankara (ACAM3000): effect of dose and route of administration. J Infect Dis. 2010;201:1361–70. doi: 10.1086/651561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayr A, Stickl H, Muller HK, Danner K, Singer H. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism [author's translation; in German] Zentralbl Bakteriol B. 1978;167:375–90. [PubMed] [Google Scholar]
- 10.Antoine G, Scheiflinger F, Dorner F, Falkner FG. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology. 1998;244:365–96. doi: 10.1006/viro.1998.9123. [DOI] [PubMed] [Google Scholar]
- 11.Drexler I, Heller K, Wahren B, Erfle V, Sutter G. Highly attenuated modified vaccinia virus Ankara replicates in baby hamster kidney cells, a potential host for virus propagation, but not in various human transformed and primary cells. J Gen Virol. 1998;79(Pt 2):347–52. doi: 10.1099/0022-1317-79-2-347. [DOI] [PubMed] [Google Scholar]
- 12.Meisinger-Henschel C, Schmidt M, Lukassen S, et al. Genomic sequence of chorioallantois vaccinia virus Ankara, the ancestor of modified vaccinia virus Ankara. J Gen Virol. 2007;88:3249–59. doi: 10.1099/vir.0.83156-0. [DOI] [PubMed] [Google Scholar]
- 13.Blanchard TJ, Alcami A, Andrea P, Smith GL. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J Gen Virol. 1998;79:1159–67. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]
- 14.Carroll MW, Moss B. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a nonhuman mammalian cell line. Virology. 1997;238:198–211. doi: 10.1006/viro.1997.8845. [DOI] [PubMed] [Google Scholar]
- 15.Sutter G, Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci U S A. 1992;89:10847–51. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perkus ME, Tartaglia J, Paoletti E. Poxvirus-based vaccine candidates for cancer, AIDS, and other infectious diseases. J Leukoc Biol. 1995;58:1–13. doi: 10.1002/jlb.58.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Stittelaar KJ, Osterhaus ADME. MVA: a cuckoo in the vaccine nest? Vaccine. 2001;19:v–vi. doi: 10.1016/s0264-410x(01)00171-2. [DOI] [PubMed] [Google Scholar]
- 18.Walsh SR, Dolin R. Vaccinia viruses: vaccines against smallpox and vectors against infectious diseases and tumors. Expert Rev Vaccines. 2011;10:1221–40. doi: 10.1586/erv.11.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acres B, Bonnefoy JY. Clinical development of MVA-based therapeutic cancer vaccines. Expert Rev Vaccines. 2008;7:889–93. doi: 10.1586/14760584.7.7.889. [DOI] [PubMed] [Google Scholar]
- 20.Tykodi SS, Thompson JA. Development of modified vaccinia Ankara-5T4 as specific immunotherapy for advanced human cancer. Expert Opin Biol Ther. 2008;8:1947–53. doi: 10.1517/14712590802567298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosma A, Nagaraj R, Staib C, et al. Evaluation of modified vaccinia virus Ankara as an alternative vaccine against smallpox in chronically HIV type 1-infected individuals undergoing HAART. AIDS Res Hum Retroviruses. 2007;23:782–93. doi: 10.1089/aid.2006.0226. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg RN, Overton ET, Haas DW, et al. Safety, immunogenicity, and surrogate markers of clinical efficacy for modified vaccinia Ankara as a smallpox vaccine in HIV-infected subjects. J Infect Dis. 2013;207:749–58. doi: 10.1093/infdis/jis753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenough TC, Cunningham CK, Muresan P, et al. Safety and immunogenicity of recombinant poxvirus HIV-1 vaccines in young adults on highly active antiretroviral therapy. Vaccine. 2008;26:6883–93. doi: 10.1016/j.vaccine.2008.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrer E, Bauerle M, Ferstl B, et al. Therapeutic vaccination of HIV-1-infected patients on HAART with a recombinant HIV-1 nef-expressing MVA: safety, immunogenicity and influence on viral load during treatment interruption. Antivir Ther. 2005;10:285–300. [PubMed] [Google Scholar]
- 25.Wharton M, Strikas RA, Harpaz R, et al. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC) MMWR Recomm Rep. 2003;52:1–16. [PubMed] [Google Scholar]
- 26.Kroger AT, Sumaya CV, Pickering LK, Atkinson WL. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011;60:1–64. [PubMed] [Google Scholar]
- 27.Wilck MB, Baden LR. Vaccination after stem cell transplant: a review of recent developments and implications for current practice. Curr Opin Infect Dis. 2008;21:399–408. doi: 10.1097/QCO.0b013e328307c7c5. [DOI] [PubMed] [Google Scholar]
- 28.Arness MK, Eckart RE, Love SS, et al. Myopericarditis following smallpox vaccination. Am J Epidemiol. 2004;160:642–51. doi: 10.1093/aje/kwh269. [DOI] [PubMed] [Google Scholar]
- 29.Morgan J, Roper MH, Sperling L, et al. Myocarditis, pericarditis, and dilated cardiomyopathy after smallpox vaccination among civilians in the United States, January-October 2003. Clin Infect Dis. 2008;46(Suppl 3):S242–50. doi: 10.1086/524747. [DOI] [PubMed] [Google Scholar]
- 30.Swerdlow DL, Roper MH, Morgan J, et al. Ischemic cardiac events during the Department of Health and Human Services Smallpox Vaccination Program, 2003. Clin Infect Dis. 2008;46(Suppl 3):S234–41. doi: 10.1086/524745. [DOI] [PubMed] [Google Scholar]
- 31.Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 32.Grandpre LE, Duke-Cohan JS, Ewald BA, et al. Immunogenicity of recombinant modified vaccinia Ankara following a single or multi-dose vaccine regimen in rhesus monkeys. Vaccine. 2009;27:1549–56. doi: 10.1016/j.vaccine.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh SR, Gillis J, Peters B, et al. Diverse recognition of conserved orthopoxvirus CD8+ T cell epitopes in vaccinated rhesus macaques. Vaccine. 2009;27:4990–5000. doi: 10.1016/j.vaccine.2009.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walsh SR, Seaman MS, Grandpre LE, et al. Impact of anti-orthopoxvirus neutralizing antibodies induced by a heterologous prime-boost HIV-1 vaccine on insert-specific immune responses. Vaccine. 2012;31:114–9. doi: 10.1016/j.vaccine.2012.10.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Issa NC, Marty FM, Gagne LS, et al. Seroprotective titres against 2009 H1N1 influenza A virus after vaccination in allogeneic hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2011;17:434–8. doi: 10.1016/j.bbmt.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mack TM, Noble J, Jr, Thomas DB. A prospective study of serum antibody and protection against smallpox. Am J Trop Med Hyg. 1972;21:214–8. doi: 10.4269/ajtmh.1972.21.214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.