Abstract
The immunopathogenesis of severe dengue is poorly understood, but there is concern that induction of cross-reactive nonneutralizing antibodies by infection or vaccination may increase the likelihood of severe disease during a subsequent infection. We generated a total of 63 new human monoclonal antibodies to compare the B-cell response of subjects who received the National Institutes of Health live attenuated dengue vaccine rDEN1Δ30 to that of subjects following symptomatic primary infection with DENV1. Both infection and vaccination induced serum neutralizing antibodies and DENV1-reactive peripheral blood B cells, but the magnitude of induction was lower in vaccinated individuals. Serotype cross-reactive weakly neutralizing antibodies dominated the response in both vaccinated and naturally infected subjects. Antigen specificities were very similar, with a slightly greater percentage of antibodies targeting E protein domain I/II than domain III. These data shed light on the similarity of human B-cell response to live attenuated DENV vaccine or natural infection.
Keywords: human, B cells, hybridomas, dengue virus, antibodies, antibody-dependent enhancement, neutralization
Dengue viruses (DENVs) have become the most common arboviral pathogen worldwide. There are no licensed dengue vaccines available. The 4 DENV serotypes, denoted DENV1–4, are all capable of causing symptomatic disease that ranges in severity from an undifferentiated febrile illness to life-threatening hemorrhagic fever and shock. The most recent World Health Organization estimates suggest that 50–100 million DENV infections occur each year, resulting in >20 000 deaths [1–3], most often in children. The risk of developing severe disease is lower during the first infection with DENV (primary infection) than during a second infection with a different serotype of DENV (secondary infection) [4]. The most widely accepted model of the pathogenesis of severe dengue proposes that an initial infection with DENV occurs and induces serotype–cross-reactive antibodies. This event is followed, in most cases years later, with a second infection by a different serotype, during which time preexisting cross-reactive antibodies form nonneutralized DENV-antibody complexes that allow the virus to enter Fc receptor–expressing cells more efficiently. This mechanism might result in increased viral replication, viremia, and subsequent release of cytokines and vasoactive mediators that increase vascular permeability. This process has been termed antibody-dependent enhancement (ADE) of infection and has been demonstrated using cells in culture, in animal models, and, to some extent, in humans.
DENV belongs to the Flaviviridae family and displays pseudo-icosahedral symmetry, with 180 copies of the envelope (E) glycoprotein and 180 copies of the premembrane/membrane (prM/M) protein in the lipid bilayer membrane. The E glycoprotein monomer possesses 3 principal domains, designated domain I (DI), DII, and DIII. Panels of human monoclonal antibodies (mAbs) recently generated by several groups have demonstrated that the human response targets both E and prM proteins and is made up largely of serotype–cross-reactive weakly neutralizing antibodies [5–9]. Only a small percentage of antibodies directed against surface-exposed epitopes are serotype-specific or neutralizing at a concentration of <0.5 µg/mL [5]. DENV immune serum depletion studies have shown that the antibodies responsible for serum neutralizing activity in humans following DENV infection do not target E protein DIII, as was previously thought, but target other areas, such as complex epitopes existing only on the assembled virion particle [6, 10]. Furthermore, the detailed mapping of a strongly neutralizing virion-only binding antibody, in complex with DENV1, was recently published using cryoelectron microscopy [11]. Considered together, these studies establish a conceptual foundation that we can use to evaluate the human antibody response to DENV vaccination.
For a dengue vaccine to be successful, there is consensus that it must protect against all 4 serotypes, thus almost certainly requiring a tetravalent vaccine formulation. This goal creates a unique challenge. If protection is not achieved for ≥1 DENV serotype in an individual, cross-reactive nonneutralizing antibodies directed at other serotypes could bind the infecting virus, potentially resulting in enhanced disease severity. With this in mind, several vaccine strategies are currently under development, including live attenuated virus vaccines, killed or inactivated virus preparations, viral vectored constructs, DNA plasmid vaccines, and protein subunit vaccines [12, 13]. A recent publication described data acquired from a phase IIb clinical trial conducted in Thailand of ChimeriVax (Sanofi Pasteur), a live attenuated tetravalent dengue-yellow fever 17D chimeric virus vaccine [14, 15]. The overall efficacy of the vaccine was determined to be only 30.2%, with no vaccine-induced ADE detected, although the follow-up duration was only 13 months.
An additional live attenuated vaccine candidate being tested in clinical trials uses the National Institutes of Health (NIH) rDENΔ30 platform. The rDEN4Δ30 virus was first engineered through deletion of a 30-nucleotide segment of the 3′ untranslated region [16] and was later found to be safe and immunogenic in 3 phase I clinical trials [17–19]. The Δ30 mutation then was introduced into the homologous region of DENV1 Western Pacific strain, and the resulting mutant virus was designated rDEN1Δ30. The live attenuated rDEN1Δ30 vaccine candidate subsequently was tested in rhesus monkeys [20] and then evaluated in a phase I clinical trial [21]. A single dose was well tolerated by vaccinees and elicited a >4-fold rise in serum neutralizing antibody titers. A second trial was performed to test whether the humoral immune response could be boosted by a second dose of rDEN1Δ30 [22]. The second dose did not induce a significant rise in serum neutralizing antibody titers, suggesting that the primary vaccination induced sterilizing humoral immunity for vaccine virus [22]. An investigational tetravalent dengue vaccine currently moving into phase II evaluation builds on these results, using rDEN1Δ30 as one of the components [23].
In the present study, we compared in detail the memory B-cell response in subjects who received the NIH live attenuated rDEN1Δ30 vaccine to that of subjects following natural DENV1 infection. We generated a total of 63 human hybridomas to DENV1 and characterized the function of the encoded DENV-reactive monoclonal antibodies in detail.
METHODS
Human Subjects and Peripheral Blood Cell Isolation
Serum and peripheral blood mononuclear cells (PBMCs) were obtained 28 days following a second vaccine dose (7 months after the first dose) from subjects who received the live attenuated rDEN1Δ30 vaccine; informed consent was obtained from all subjects [22]. We also identified a panel of subjects in North Carolina or California who had acquired DENV infection naturally by screening volunteers with suspected symptomatic exposure during past travel to dengue-endemic regions, with informed consent received from all subjects. Subjects were confirmed to have had primary DENV1 infection by testing their serum for the presence of antibodies that neutralized each of the DENV serotypes. PBMCs were isolated by density gradient separation on Ficoll. The cells were cryopreserved immediately and stored in liquid nitrogen until study. The protocol for recruiting and collecting blood samples from subjects was approved by the institutional review boards of the University of North Carolina at Chapel Hill and the La Jolla Institute for Allergy and Immunology.
Viruses
DENV1 WestPac-74, DENV2 S-16803, DENV3 CH-53489, and DENV4 TVP-360 virus strains, provided by Dr Robert Putnak (Walter Reed Army Institute of Research, Silver Spring, MD) were used in the present study for both binding enzyme-linked immunosorbent assay (ELISA) and neutralization assays. Virus-containing cell culture supernatant used in virus-capture ELISA was prepared in C6/36 mosquito cells grown in complete minimum essential medium (catalog no. 51985-034, Gibco).
Generation of Human Hybridomas
Previously cryopreserved samples were thawed rapidly in a 37°C water bath and washed prior to transformation with Epstein-Barr virus (EBV), CpG, and additional supplements as described previously [5]. Cultures were incubated at 37°C with 5% CO2 for 10 days prior to screening for DENV1-reactive cell lines with ELISA (the capture ELISA method is described below). The minimal frequency of DENV1-reactive B cells was estimated on the basis of the number of wells with DENV1-reactive supernatants as compared to the total number of lymphoblastoid cell line colonies in the transformation plates, as follows: [number of wells with DENV1-reactive supernatants]/[number of LCL colonies in the plate]. Cells from wells with supernatants reacting in the DENV1 capture ELISA were expanded prior to cytofusion with HMMA2.5 nonsecreting myeloma cells, as previously described [5]. Following cytofusion, hybridomas were selected for growth in HAT medium containing ouabain and were biologically cloned.
MAb Production and Purification
Wells containing hybridomas producing DENV1-reactive antibodies were cloned biologically by 3 rounds of limiting dilution plating or by use of a ClonePix device (Molecular Devices) in accordance with the manufacturer's recommendations. Once clonal, the cell lines were used to produce mAb immunoglobulin G (IgG) in cell supernatants, using serum-free medium, followed by protein G column purification.
Virus and Recombinant Protein ELISA
For virus-capture ELISA, purified mouse mAb 4G2 (1 mg/mL stock) prepared in carbonate binding buffer (dilution, 1:1000) was used to coat 384 well ELISA plates at 25 µL per well (Nunc 242 757) and incubated overnight at 4°C. After blocking for 1 hour, plates were washed 5 times with phosphate-buffered saline (PBS), and 50 µL/well of DENV-containing culture supernatant from infected C6/36 cell culture monolayers was added (using the 4 strains listed above). Plates then were washed 10 times with PBS, and 5 µL of purified human monoclonal antibody (1 µg/µL) was added into 25 µL/well of blocking solution. Plates were incubated at room temperature for 1 hour prior to washing 5 times with PBS. Secondary antibody (goat anti-human Fc; catalog no. W99008A, Meridian Life Science) was added at a dilution of 1:5000 to blocking solution, using 25 µL/well, and plates again were incubated at room temperature for 1 hour. After the plates were washed 5 times with PBS, phosphatase substrate solution (1 mg/mL phosphatase substrate in 1 M Tris aminomethane; catalog no. S0942, Sigma) was added at 25 µL/well, and plates were incubated at room temperature for 2 hours before reading the optical density at 405 nm on a Biotec plate reader.
For recombinant protein capture ELISA that used E protein or prM protein constructs, purified mouse anti-Strep-tag II mAb (catalog no. IBA 2-1517-001, StrepMAB-Immo; 1 mg/mL stock) prepared in carbonate binding buffer (dilution, 1:500) was used to coat 384-well ELISA plates at 25 µL/well (Nunc 242 757) and incubated at 4°C overnight. After plates were exposed to blocking solution for 1 hour, they were washed 5 times with PBS, and 50 µL of recombinant protein construct containing culture supernatant (cultured in insect cells) was added (production of recombinant proteins is described in Supplementary Materials and Methods). Plates then were washed 10 times with PBS, and 5 µL of purified human monoclonal antibody (1 µg/µL) was added into 25 µL/well of blocking solution. All other steps were performed as described above for the virus-capture ELISA.
Neutralization Assay
Serum neutralization assays were performed as described elsewhere, using a 50% plaque reduction neutralization test (PRNT50) [17]. Virus strains used in the assay were as follows: DENV1, strain WestPac-74; DENV2, strain New Guinea C; DENV3, strain Sleman; and DENV4, strain 814 669.
The neutralizing potency of monoclonal antibodies was measured using a flow cytometry–based neutralization assay with the U937 human monocytic cell line stably transfected with DC-SIGN, as previously described [24].
ADE Assays
The ability of antibodies to enhance DENV infection was measured using U937 cells at a single dilution (concentration, 1 µg/mL). Because these Fc receptor cells do not have DENV virus attachment factor, the cells are only susceptible to infection in the presence of DENV-reactive antibodies. The assay was performed as described in detail previously [5]. ADE activity was expressed as the percentage increase of infected cells in the DENV-reactive antibody–treated sample as compared to the sample treated with a control antibody.
Statistical Analysis
Prism, version 5.0 (GraphPad), was used for data analysis. For statistical comparisons between 2 groups of data, the Student t test was used. A P value of ≤ .05 was considered a statistically significant difference.
RESULTS
Serum Neutralization Titers and DENV1-Reactive B-Cell Frequencies Were Lower in Vaccinated Individuals
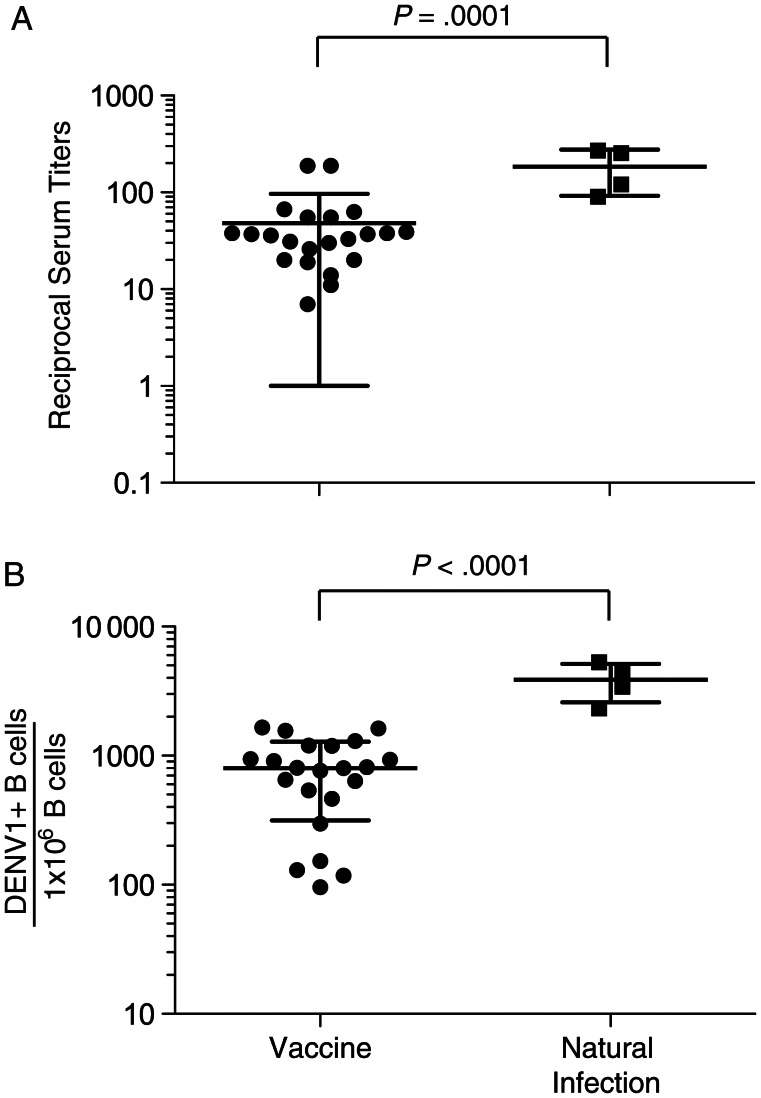
Twenty-two serum samples were obtained after vaccination with rDEN1Δ30 vaccine, and 4 serum samples were obtained after natural infection with DENV1. The times since vaccination or infection prior to serum collection are indicated in Table 1. To compare the serum neutralization potency of serum in vaccinated and naturally infected individuals, we performed a PRNT50. Mean reciprocal serum neutralizing titers (±SD) in vaccinated subjects were 48 ± 48, while those in individuals with natural infection were 184 ± 92. The difference in reciprocal serum titers was statistically significant (P=.0001; Figure 1A).
Table 1.
Clinical and Serologic Characteristics of and Hybridoma Yield From Recipients of Live Attenuated Dengue Vaccine and Travelers With Naturally Acquired Dengue Virus Serotype 1 (DENV1) Infection
| Subject | Infection Type | Time Since Vaccination or Infection | Reciprocal Serum Ab Neut50 to DENV1 | DENV1-Reactive EBV Wells/Wells Tested, No. | DENV1-Reactive B-Cell Frequency, ×10−4a | Hybridomas Obtained, No. |
|---|---|---|---|---|---|---|
| 31 | rDEN1Δ30 vaccination | 28 db | 14 | 1/384 | 1 | 0 |
| 32 | rDEN1Δ30 vaccination | 28 db | 31 | 5/384 | 7 | 1 |
| 33 | rDEN1Δ30 vaccination | 28 db | 55 | 6/384 | 5 | 2 |
| 35 | rDEN1Δ30 vaccination | 28 db | 37 | 13/384 | 8 | 3 |
| 36 | rDEN1Δ30 vaccination | 28 db | 67 | 4/384 | 3 | 0 |
| 38 | rDEN1Δ30 vaccination | 28 db | 19 | 5/384 | 9 | 1 |
| 39 | rDEN1Δ30 vaccination | 28 db | 189 | 5/384 | 5 | 2 |
| 40 | rDEN1Δ30 vaccination | 28 db | 38 | 14/384 | 9 | 2 |
| 41 | rDEN1Δ30 vaccination | 28 db | 7 | 1/384 | 2 | 0 |
| 43 | rDEN1Δ30 vaccination | 28 db | 63 | 5/384 | 8 | 0 |
| 44 | rDEN1Δ30 vaccination | 28 db | 55 | 6/384 | 12 | 1 |
| 45 | rDEN1Δ30 vaccination | 28 db | 36 | 17/384 | 9 | 3 |
| 46 | rDEN1Δ30 vaccination | 28 db | 33 | 1/384 | 1 | 0 |
| 47 | rDEN1Δ30 vaccination | 28 db | 38 | 12/384 | 6 | 3 |
| 49 | rDEN1Δ30 vaccination | 28 db | 37 | 7/384 | 2 | 2 |
| 50 | rDEN1Δ30 vaccination | 28 db | 20 | 4/384 | 1 | 0 |
| 52 | rDEN1Δ30 vaccination | 28 db | 189 | 9/384 | 16 | 0 |
| 53 | rDEN1Δ30 vaccination | 28 db | 39 | 10/384 | 15 | 2 |
| 55 | rDEN1Δ30 vaccination | 28 db | 30 | 5/384 | 8 | 1 |
| 56 | rDEN1Δ30 vaccination | 28 db | 26 | 1/384 | 1 | 0 |
| 57 | rDEN1Δ30 vaccination | 28 db | 11 | 11/384 | 6 | 2 |
| 58 | rDEN1Δ30 vaccination | 28 db | 20 | 6/384 | 13 | 1 |
| 006 | Primary DENV1 infection | Unknown | 121c | 17/384 | 23 | 2 |
| 106 | Primary DENV1 infection | 2 y | 90c | 78/768 | 44 | 29 |
| GL10 | Primary DENV1 infection | 7 y | 254c | 71/768 | 34 | 5 |
| GL24 | Primary DENV1 infection | 15 y | 271c | 159/1152 | 53 | 1 |
Abbreviations: Ab, antibody; EBV, Epstein-Barr virus; Neut50, concentration at which 50% of virus was neutralized.
a Data are estimated on the basis of the total number of EBV-transformed B-cell colony counts in the transformation plate.
b Since second vaccination.
c Titers to the heterologous DENV serotypes 2, 3, and 4, respectively, were as follows: for subject 006, 11, 5, and <5; for subject 106, 15, 13, and 11; for subject GL10, 16, 13, and 7; and for subject GL24, 55, 30, and 10.
Figure 1.
Dengue virus serotype 1 (DENV1) reciprocal serum neutralizing antibody titers and B cell frequencies following vaccination or natural infection. A, Fifty percent plaque reduction neutralization test assays were performed against DENV1 on 22 serum samples obtained after vaccination and 4 obtained after natural infection; results are shown as reciprocal serum titers. Statistical analysis was performed using a t test. B, Frequencies of circulating DENV–reactive B cells were estimated on the basis of supernatants from Epstein-Barr virus (EBV)–transformed peripheral blood mononuclear cell samples during clonal colony formation. Average DENV1-reactive B-cell frequencies were 800 and 3870 per million circulating EBV-transformable B cells for vaccinated and naturally infected subjects, respectively. Statistical analysis was performed using t test. The error bars represent SDs.
To assess the circulating DENV1-reactive B-cell frequencies, PBMCs from vaccinated or naturally infected individuals were transformed with EBV, as described in the Methods section. The minimal frequency of DENV1-reactive B cells was determined for 22 PBMC samples obtained after vaccination and for 4 PBMC samples obtained after natural infection (Table 1). Mean frequencies (±SD) in vaccinated subjects were 800 ± 485 DENV1-reactive B cells per million circulating EBV-transformable B cells. Naturally infected subjects had a mean frequency (±SD) of 3870 ± 1283 DENV1-reactive B cells per million circulating EBV-transformable B cells. The difference in frequencies was highly statistically significant (P < .0001; Figure 1B).
B Cells Induced by Vaccination or Natural Infection Displayed Similar Serotype–Cross-reactivity Profiles
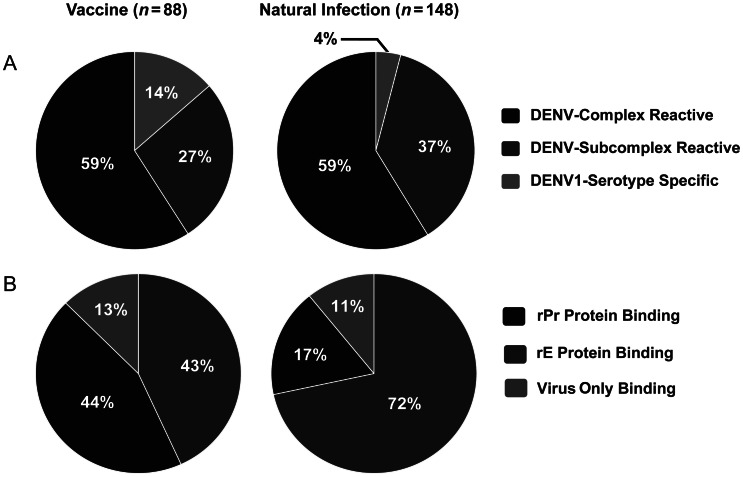
To compare the serotype–cross-reactivity profile of circulating B cells induced by vaccination or natural infection, DENV1-positive EBV-transformed B-cell culture supernatants were analyzed in a virion-capture ELISA. A total of 88 DENV1-positive B-cell culture supernatants obtained after vaccination and 148 obtained after natural infection were studied (Figure 2A). In both groups, 59% were found to be DENV-complex reactive (ie, they bound to all 4 DENV serotypes). DENV-subcomplex–reactive antibodies (ie, those binding to >1 but <4 DENV serotypes) made up the majority of the remaining supernatants tested, at 27% and 37%, in the vaccinated and naturally infected groups, respectively. Antigen-binding specificity also was tested, and results showed that E protein binding accounted for 43% and 72% of the antibodies obtained after vaccination and after natural infection, respectively (Figure 2B).
Figure 2.
Virus cross-reactivity and antigen-specificity profiles of Epstein-Barr virus (EBV)–transformed dengue virus (DENV)–reactive B-cell cultures following vaccination or natural infection. A, Eighty-eight DENV serotype 1 (DENV1)–positive B-cell culture supernatants obtained after vaccination (left) and 148 obtained after natural infection (right) underwent enzyme-linked immunosorbent assay for binding to heterologous DENV. DENV-complex reactive: binding to all 4 DENV serotypes; DENV-subcomplex reactive, binding to 2 or 3 DENV serotypes; DENV-serotype specific, binding only to DENV1. B, Eighty-eight DENV1-positive B-cell culture supernatants obtained after vaccination (left) and 148 obtained after natural infection underwent ELISA for binding to recombinant premembrane (rPr) or E (rE) protein. Virus-only binding, B-cell culture supernatants that bound to whole virus but did not show binding to either rPr or rE protein.
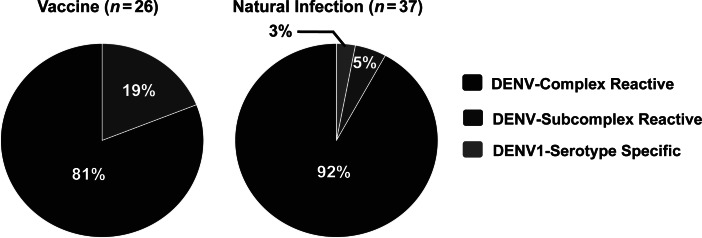
We generated human hybridomas from DENV1-positive B-cell cultures to study at a clonal level the antigen-reactivity profiles, neutralization potency, and antibody-dependent enhancement properties of antibodies produced following vaccination and natural infection (Table 1). A total of 63 hybridomas was generated, 26 from vaccinated subjects and 37 from naturally infected subjects (Table 2). The cross-reactivity profiles of mAbs were tested (Figure 3). Following vaccination, all of the mAbs obtained were found to be cross-reactive: 81% were DENV-complex reactive, while 19% were DENV-subcomplex reactive. Similar results were obtained following natural infection, where only 1 serotype-specific mAb was identified.
Table 2.
Genetic and Functional Characteristics of Human Monoclonal Antibodies (mAbs)
| Infection Type, Subject, mAb | Binding Resulta |
Neut50, µg/mL, by Serotype |
Fold-Enhancement of Infection at 1 µg/mL, by Serotype |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG Characteristica |
To Whole Virus at 1 µg/mL, by Serotype |
|||||||||||||||||
| Subclass | Light Chain | D1 | D2 | D3 | D4 | To rE | To rEDIII Fragment | To rEDI-II Fragment | To rPr | D1 | D2 | D3 | D4 | D1 | D2 | D3 | D4 | |
| rDENV1Δ30 vaccination | ||||||||||||||||||
| 32 | ||||||||||||||||||
| 1K12 | IgG1 | λ | + | + | + | + | + | + | – | – | >10 | >10 | >10 | >10 | 56 | 1 | 1 | 1 |
| 33 | ||||||||||||||||||
| 1C8 | IgG1 | κ | + | + | + | + | – | – | – | + | >10 | >10 | >10 | >10 | 13 | 31 | 130 | 15 |
| 1O21.2 | IgG1 | κ | + | + | + | + | + | + | – | – | >10 | >10 | >10 | >10 | 1 | 0 | 1 | 1 |
| 35 | ||||||||||||||||||
| 1D7.3 | IgG1 | λ | + | + | + | + | + | + | – | – | >10 | >10 | >10 | >10 | 27 | 9 | 20 | 1 |
| 1N23 | IgG3 | κ | + | + | + | – | + | + | – | – | >10 | >10 | >10 | >10 | 3 | 18 | 6 | 1 |
| 1C9 | IgG1 | λ | + | + | + | + | – | – | – | + | >10 | >10 | >10 | >10 | 15 | 40 | 54 | 13 |
| 38 | ||||||||||||||||||
| 1I23 | IgG1 | κ | + | + | + | – | + | + | – | – | >10 | >10 | >10 | >10 | 4 | 18 | 28 | 1 |
| 39 | ||||||||||||||||||
| 1O6 | IgG1 | κ | + | + | + | + | – | – | – | + | >10 | >10 | >10 | >10 | 119 | 67 | 54 | 15 |
| 1I15 | IgG1 | κ | + | + | + | + | – | – | – | + | >10 | >10 | >10 | >10 | 92 | 74 | 37 | 4 |
| 40 | ||||||||||||||||||
| 1C19.2 | IgG1 | κ | + | + | + | + | + | + | – | – | 0.22 | >10 | >10 | >10 | 5 | 1 | 1 | 1 |
| 1O10 | IgG1 | λ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 146 | 166 | 40 | 6 |
| 44 | ||||||||||||||||||
| 1J16.2 | IgG1 | κ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 97 | 88 | 26 | 3 |
| 45 | ||||||||||||||||||
| 1D7.2 | IgG1 | κ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 65 | 53 | 17 | 2 |
| 1L18 | IgG1 | λ | + | + | + | + | – | – | – | + | >10 | >10 | >10 | >10 | 81 | 60 | 30 | 6 |
| 1E8 | IgG1 | κ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 6 | 3 | 24 | 4 |
| 47 | ||||||||||||||||||
| 1C22 | IgG1 | λ | + | + | + | – | + | + | – | – | 2.5 | >10 | >10 | >10 | 3 | 11 | 10 | 2 |
| 1I14 | IgG1 | κ | + | + | + | + | – | – | – | + | >10 | >10 | >10 | >10 | 5 | 10 | 7 | 2 |
| 1E1 | IgG1 | κ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 8 | 8 | 26 | 4 |
| 49 | ||||||||||||||||||
| 1M12.2 | IgG1 | κ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 8 | 8 | 30 | 1 |
| 1H7 | IgG1 | κ | + | – | + | + | – | – | – | + | >10 | >10 | >10 | >10 | 13 | 1 | 13 | 5 |
| 53 | ||||||||||||||||||
| 1L13 | IgG1 | λ | + | + | + | + | – | – | – | + | >10 | >10 | 0.24 | >10 | 9 | 15 | 15 | 9 |
| 1I7 | IgG1 | λ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 8 | 28 | 10 | 2 |
| 55 | ||||||||||||||||||
| 1P11 | IgG3 | λ | + | + | + | + | – | – | – | + | >10 | >10 | >10 | >10 | 26 | 23 | 213 | 4 |
| 57 | ||||||||||||||||||
| 1I6 | IgG1 | λ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 11 | 11 | 56 | 5 |
| 1D3 | IgG1 | κ | + | + | + | – | – | – | – | + | >10 | >10 | >10 | >10 | 11 | 11 | 56 | 5 |
| 58 | ||||||||||||||||||
| 1M18 | IgG1 | λ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 8 | 7 | 35 | 1 |
| Primary DENV1 infection | ||||||||||||||||||
| 006 | ||||||||||||||||||
| 1B17.2 | IgG1 | κ | + | + | + | + | + | + | – | – | >10 | >10 | >10 | >10 | 3 | 3 | 4 | 3 |
| 1O12 | IgG1 | κ | + | + | + | + | + | + | – | – | >10 | >10 | >10 | >10 | 1 | 1 | 12 | 1 |
| 106 | ||||||||||||||||||
| 1E23 | IgG1 | λ | + | + | + | + | – | – | – | + | >10 | >10 | >10 | >10 | 8 | 6 | 55 | 3 |
| 2F13 | IgG1 | κ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 5 | 5 | 3 | 1 |
| 1L21 | IgG1 | κ | + | + | + | + | + | + | – | – | >10 | >10 | >10 | >10 | 5 | 7 | 1 | 1 |
| 1M6.2 | IgG1 | κ | + | + | + | + | + | – | + | – | >10 | 8.2 | >10 | >10 | 4 | 3 | 29 | 1 |
| 1H7.2 | IgG1 | λ | + | + | + | + | – | – | – | + | >10 | >10 | >10 | >10 | 11 | 9 | 30 | 4 |
| 2E14 | IgG1 | κ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 31 | 13 | 10 | 4 |
| 1K16.2 | IgG1 | λ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 8 | 35 | 19 | 3 |
| 1K4.2 | IgG1 | κ | + | + | + | + | – | – | – | + | >10 | >10 | >10 | >10 | 5 | 15 | 6 | 2 |
| 2H21 | IgG1 | λ | + | + | + | + | – | – | – | + | >10 | >10 | >10 | >10 | 21 | 4 | 18 | 4 |
| 2B17 | IgG1 | λ | + | + | + | + | – | – | – | + | >10 | >10 | >10 | >10 | 3 | 3 | 4 | 3 |
| 2G3 | IgG1 | λ | + | + | + | + | – | – | – | + | >10 | >10 | >10 | >10 | 22 | 4 | 47 | 8 |
| 2J9 | IgG1 | λ | + | + | + | + | – | – | – | + | >10 | >10 | >10 | >10 | 9 | 8 | 25 | 11 |
| 1G19 | IgG1 | κ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 4 | 10 | 4 | 2 |
| 1N21 | IgG1 | κ | + | + | + | + | + | + | – | – | >10 | >10 | >10 | >10 | 10 | 7 | 44 | 2 |
| 1I13.2 | IgG1 | λ | + | + | + | + | – | – | – | + | >10 | >10 | >10 | >10 | 8 | 15 | 16 | 8 |
| 1D8 | IgG1 | κ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 5 | 9 | 8 | 6 |
| 1A17 | IgG1 | κ | + | + | + | + | – | – | – | + | >10 | >10 | >10 | >10 | 118 | 60 | 47 | 4 |
| 1D6 | IgG1 | κ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 3 | 6 | 4 | 4 |
| 1N6 | IgG1 | κ | + | – | – | – | + | + | – | – | >10 | >10 | >10 | >10 | 5 | 0 | 1 | 1 |
| 2M23 | IgG1 | λ | + | + | + | + | + | + | – | – | >10 | >10 | >10 | >10 | 15 | 5 | 34 | 4 |
| 2I11 | IgG1 | λ | + | + | + | + | – | – | – | + | >10 | >10 | >10 | >10 | 24 | 6 | 26 | 5 |
| 2J21 | IgG1 | κ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 18 | 7 | 28 | 1 |
| 2I21 | IgG1 | λ | + | + | + | + | + | + | – | – | >10 | >10 | >10 | >10 | 14 | 7 | 4 | 1 |
| 2E19 | IgG1 | λ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 1 | 3 | 2 | 2 |
| 2I23 | IgG3 | λ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 5 | 1 | 1 | 1 |
| 2O19 | IgG1 | λ | + | + | + | + | + | + | – | – | >10 | >10 | >10 | >10 | 2 | 2 | 2 | 1 |
| 2J23 | IgG1 | κ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 25 | 6 | 7 | 3 |
| 2B20 | IgG1 | κ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 19 | 5 | 0 | 1 |
| 2D22.2 | IgG1 | λ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 10 | 6 | 1 | 2 |
| GL10 | ||||||||||||||||||
| 2F17.2 | IgG1 | λ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 2 | 3 | 2 | 1 |
| 1H16.2 | IgG1 | κ | + | + | + | – | – | – | – | + | >10 | >10 | >10 | >10 | 1 | 5 | 3 | 2 |
| 1M4 | IgG1 | κ | + | + | + | + | + | – | + | – | 0.56 | 0.27 | 5.4 | 2.3 | 6 | 1 | 56 | 1 |
| 1I3.2 | IgG1 | κ | + | + | + | + | + | – | + | – | >10 | >10 | >10 | >10 | 9 | 5 | 18 | 8 |
| 2H2 | IgG1 | κ | + | + | + | + | – | – | – | + | >10 | >10 | >10 | >10 | 6 | 9 | 15 | 1 |
| GL24 | ||||||||||||||||||
| 2G19 | IgG2 | λ | + | + | + | – | – | – | – | + | >10 | >10 | >10 | >10 | 14 | 13 | 53 | 7 |
Abbreviations: DENV1, dengue virus serotype 1; IgG, immunoglobulin G; Neut50, concentration at which 50% of virus was neutralized; rE, recombinant E protein; rEDI-II, recombinant E protein domain I-II; rEDIII, recombinant E protein domain III; rPr, recombinant premembrane protein.
a The Neut50, determined by enzyme-linked immunosorbent assay, is shown for each DENV serotype. A dash indicates a neut50 value of >10 µg/mL, neut50 values between 1.0–10.0 µg/mL are shown, and neut50 values <0.5 µg/mL are in bold.
b Values were determined by antibody-dependent enhancement assay. Values indicating >25-fold enhancement are in bold.
Figure 3.
Dengue virus (DENV) serotype cross-reactivity profiles of purified human monoclonal antibodies (mAbs) generated following vaccination or natural infection. Twenty-six purified human mAbs isolated after vaccination (left) or 37 mAbs recovered after natural infection (right) were tested by enzyme-linked immunosorbent assay for binding to heterologous DENV. DENV-complex reactive, binding to all 4 DENV serotypes; DENV-subcomplex reactive, binding to 2 or 3 DENV serotypes; DENV-serotype specific, binding only to DENV serotype 1.
B Cells Induced by Vaccination or Natural Infection Displayed Similar Antigen-Binding Profiles
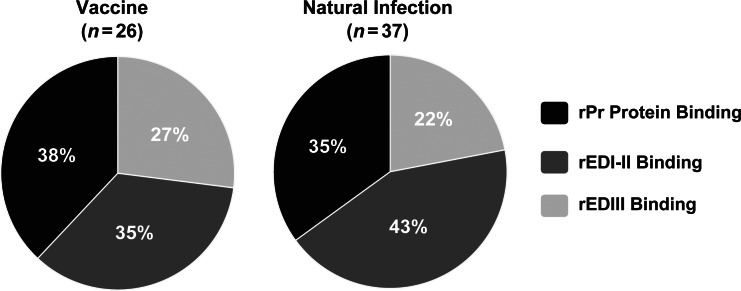
To understand the distribution of antigenic sites targeted by the immune response to DENV1 infection or vaccination, mAbs were tested for binding to recombinant E protein or prM protein constructs by ELISA (Figure 4). Approximately one-third of all the mAbs isolated were determined to bind to the pr portion of prM protein. The other two-thirds in each group (ie, 62% following vaccination and 65% following natural infection) were found to bind to E protein. Also, of the E protein–binding mAbs, both groups had a greater percentage directed toward domain I-II than to domain III. Thirty-five percent of the total mAbs isolated following vaccination and 43% isolated following natural infection bound to E protein domain I-II. In both groups, the antibody response appeared to be divided roughly equally into thirds among the prM protein, E protein domain I-II, and E protein domain III.
Figure 4.
Antigen specificity of purified human monoclonal antibodies (mAbs) generated following vaccination or natural infection. Twenty-six purified human mAbs isolated after vaccination (left) and 37 mAbs isolated after natural infection (right) were tested for binding to recombinant prM (rPr), E protein domain I-II (rEDI-II), or E protein domain III (rEDIII) constructs by enzyme-linked immunosorbent assay. Data are the percentage of total mAbs in each group.
Neutralization and Enhancement Characteristics of B Cells Induced by Vaccination or Natural Infection Were Similar
The neutralizing potency of each mAb was tested against representative viruses from each of the 4 DENV serotypes. The results are shown in Table 2. The vast majority of mAbs from both groups were poorly neutralizing, with a Neut50 of >10 µg/mL. One antibody from the vaccine group and 1 from the natural infection group showed moderate neutralizing capability (Neut50, 1–10 µg/mL). Three mAbs, 1C19.2 (EDIII binding) and 1L13 (prM binding) following vaccination and 1M4 (EDI-II binding) following natural infection, were strongly neutralizing, with a Neut50 of <1.0 µg/mL (Table 3).
Table 3.
Binding Patterns and Functional Activities of Dengue Virus–Reactive Human Monoclonal Antibodies (mAbs)
| Activity Pattern, Infection Type | mAbs, No. |
Functional Activity, No. |
||||||
|---|---|---|---|---|---|---|---|---|
| By Bound Protein, Domain, Virion |
||||||||
| Total | E | EDIII | EDI/II | pr | Virion Only | Neutralizinga | Enhancingb | |
| Cross-reactive | ||||||||
| Vaccination | 26 | 16 | 7 | 9 | 10 | 0 | 2 | 18 |
| Natural infection | 36 | 23 | 7 | 16 | 13 | 0 | 1 | 15 |
| Serotype specific | ||||||||
| Vaccination | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Natural infection | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: E, E protein; EDI-II, E protein domain I-II; EDIII, E protein domain III; pr, premembrane protein.
a Defined as an inhibitory effect at a concentration <0.5 µg/mL.
b Defined as a >25-fold increase in virus replication in vitro at a concentration of 1 µg/mL.
We also investigated the antibody-dependent infection-enhancing properties of each mAb against viruses from each of the 4 DENV serotypes (Table 2). At the concentration tested, most of the mAbs showed some degree of infection enhancement toward ≥1 of the DENV serotypes. The proportion of strongly enhancing mAbs (ie, those exhibiting >25-fold enhancement of infection) was 18 of 26 (69%) in the vaccine group and 15 of 37 (41%) in the natural infection group. In the vaccine group, 69% of E protein–binding mAbs (11/16) and 70% of prM protein–binding mAbs (7/10) possessed strong infection-enhancing capacity. In the natural infection group, 33% of E protein–binding mAbs (8/24) and 54% of prM protein–binding mAbs (7/13) enhanced infection in vitro by >25-fold (Table 2).
DISCUSSION
Isolation and characterization of a large panel of human mAbs from subjects who received the NIH live attenuated vaccine rDEN1Δ30 or subjects who were naturally infected with DENV1 revealed a high degree of similarity in the induced memory repertoire. Serum neutralization titers and DENV1-reactive B-cell frequencies were lower in vaccinated individuals. However, this observation should be interpreted with the understanding that we screened symptomatic travelers, using neutralization to identify natural primary DENV1 infections, and that live attenuated vaccination results in a clinically asymptomatic infection. Thereby only subjects with robust DENV1 neutralizing antibody titers were included in the DENV1 natural infection group. In most respects, however, the human B-cell response was similar following DENV1 vaccination or natural infection. Results showed that the repertoire in both groups was dominated by cross-reactive antibodies with low or no neutralizing potency and significant potential to enhance infectivity by Fc-mediated mechanisms. The frequency of strongly neutralizing antibodies was similar, albeit very low, making up only 5% of all antibodies tested. Antigen specificities of the response also were similar, with roughly two-thirds of antibodies targeting the E protein and one-third targeting the prM protein.
This study demonstrates for the first time that the high frequency of B cells encoding serotype–cross-reactive weakly neutralizing antibodies that has been described for natural DENV infection [5, 7–9] is also induced following live attenuated virus vaccination. These findings are in concordance with the concept that the DENV neutralizing activity in serum, which likely contributes to protection against reinfection with the inducing virus serotype, is determined principally by rare populations of B cells, not a collective effect of weakly neutralizing cross-reactive populations [6]. DENV1-reactive B-cell frequencies in vaccinated subjects were lower than those in naturally infected patients, a finding that also was reflected in the serum neutralization activities. It is possible that vaccination induces a lower frequency of DENV-reactive memory B cells and a lower level of secreted antibodies. Caveats about the interpretation of these data are that collection of samples from naturally infected donors occurred at significantly later time points than for vaccinees, and the DENV1 wild-type and vaccine strain antigens were likely not identical. Another limitation of this study is that the profile of B-cell clones described does not necessarily predict whether protection would be afforded by the vaccine, because vaccine efficacy can only be determined by large clinical trials.
Recently, we identified a subpopulation of potently neutralizing antibodies, termed virion-only binding antibodies (because they target epitopes present only on the virion particle), that appear to be the primary contributors to the neutralization activity in human immune serum. In the present study, we screened for virus-reactive antibodies, using purified whole virus preparations expected to contain both immature and mature virus particles and all of the virus structural proteins. This approach allowed for the unbiased interrogation of B-cell cultures and thus identified B cells secreting antibodies to diverse domains of the E and prM proteins. However, given the rarity of virion-only binding antibody-producing B cells in circulation following infection or vaccination, this comprehensive approach is not likely to isolate type-specific ultrapotent antibodies, which compose <1% of induced antibodies. In this study, as expected, virion-only binding antibodies were not isolated from vaccinees or naturally infected subjects with the panel of antibodies identified, since the panel contained 63 new human mAbs. Likely, it would be necessary to generate thousands of human mAbs to isolate a large panel of virion-only binding or potently neutralizing antibodies from DENV-immune subjects, since unbiased screening for mAbs reveals a low frequency of such clones. This finding is not likely a technical problem with our study; rather, the rarity of neutralizing clones appears to be the nature of the biology of the response.
The antigen specificity profiles of mAbs isolated from vaccinated and naturally infected individuals were remarkably similar, despite significant differences in the interval since exposure to antigen. Samples from vaccinated individuals were obtained 1 month following a second dose of vaccine, while samples from naturally infected individuals were obtained 2–15 years after recovery from infection. The similarity in this memory B-cell response may reflect durability and suggests that the response to vaccination is long-lived. We were unable to test how the memory response to vaccination might mature over time, because only 1 sample time point was used to generate mAbs from each subject.
In summary, the human B-cell response encoding DENV-reactive antibodies is similar between live attenuated vaccination and natural infection. Most of the E protein– and prM protein–specific antibodies in both groups exhibit ADE activity, whereas only rare clones possessed strong neutralizing activity. The studies suggest that live attenuated monovalent vaccination does not induce a dramatically aberrant B-cell response that is of concern, nor does it induce an unusually potent neutralizing response that fundamentally differs from that following natural infection. This is not entirely unexpected, since both groups experienced a DENV infection presenting wild-type structural proteins, albeit with differing clinical features. Ideally, one might like to manipulate the antigen exposure during vaccination, using antigen preparations designed to discourage the dominant recognition of cross-reactive nonneutralizing sites by the humoral immune response, while enhancing the induction of protective neutralizing antibodies directed to epitopes that are determinants of type-specific potent neutralizing responses. The immune profiling described here provides molecular information that could contribute to the rational design of dengue vaccines that enhance the induction of protective antibodies while reducing the potential for development of enhanced or severe disease.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Frances Smith-House, for excellent laboratory management support; and Dr Alessandro Sette, Dr Sujan Shresta, and the staff of the La Jolla Institute for Allergy and Immunology, for providing cells from 2 DENV-immune donors.
Financial Support. This work was supported by the NIH (grant U54 AI057157- the Southeastern Regional Center of Excellence for Emerging Infections and Biodefense; contract HHSN272200900042C to Alessandro Sette); grant K08 AI103038 to S. A. S.), and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, NIH.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10:100–3. doi: 10.1016/s0966-842x(01)02288-0. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Dengue and severe dengue. Fact sheet no. 117. 2012. November Available at: http://www.who.int/mediacentre/factsheets/fs117/en/ Accessed 7 December 2012. [Google Scholar]
- 3.Guzman MG, Halstead SB, Artsob H, et al. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8:S7–16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–80. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 5.Smith SA, Zhou Y, Olivarez NP, Broadwater AH, de Silva AM, Crowe JE., Jr Persistence of circulating memory B cell clones with potential for dengue virus disease enhancement for decades following infection. J Virol. 2012;86:2665–75. doi: 10.1128/JVI.06335-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Alwis R, Smith SA, Olivarez NP, et al. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc Natl Acad Sci U S A. 2012;109:7439–44. doi: 10.1073/pnas.1200566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Alwis R, Beltramello M, Messer WB, et al. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Negl Trop Dis. 2011;5:e1188. doi: 10.1371/journal.pntd.0001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dejnirattisai W, Jumnainsong A, Onsirisakul N, et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328:745–8. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beltramello M, Williams KL, Simmons CP, et al. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe. 2010;8:271–83. doi: 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wahala WM, Kraus AA, Haymore LB, Accavitti-Loper MA, de Silva AM. Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology. 2009;392:103–13. doi: 10.1016/j.virol.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teoh EP, Kukkaro P, Teo EW, et al. The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci Transl Med. 2012;4:139ra83. doi: 10.1126/scitranslmed.3003888. [DOI] [PubMed] [Google Scholar]
- 12.Durbin AP, Whitehead SS. Next-generation dengue vaccines: novel strategies currently under development. Viruses. 2011;3:1800–14. doi: 10.3390/v3101800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webster DP, Farrar J, Rowland-Jones S. Progress towards a dengue vaccine. Lancet Infect Dis. 2009;9:678–87. doi: 10.1016/S1473-3099(09)70254-3. [DOI] [PubMed] [Google Scholar]
- 14.Sabchareon A, Wallace D, Sirivichayakul C, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012;12:61428–7. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 15.Halstead SB. Dengue vaccine development: a 75% solution? Lancet. 2012;12:61510–4. doi: 10.1016/S0140-6736(12)61510-4. [DOI] [PubMed] [Google Scholar]
- 16.Men R, Bray M, Clark D, Chanock RM, Lai CJ. Dengue type 4 virus mutants containing deletions in the 3′ noncoding region of the RNA genome: analysis of growth restriction in cell culture and altered viremia pattern and immunogenicity in rhesus monkeys. J Virol. 1996;70:3930–7. doi: 10.1128/jvi.70.6.3930-3937.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durbin AP, Karron RA, Sun W, et al. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3′-untranslated region. Am J Trop Med Hyg. 2001;65:405–13. doi: 10.4269/ajtmh.2001.65.405. [DOI] [PubMed] [Google Scholar]
- 18.Durbin AP, Whitehead SS, McArthur J, et al. rDEN4delta30, a live attenuated dengue virus type 4 vaccine candidate, is safe, immunogenic, and highly infectious in healthy adult volunteers. J Infect Dis. 2005;191:710–8. doi: 10.1086/427780. [DOI] [PubMed] [Google Scholar]
- 19.Durbin AP, Kirkpatrick BD, Pierce KK, Schmidt AC, Whitehead SS. Development and clinical evaluation of multiple investigational monovalent DENV vaccines to identify components for inclusion in a live attenuated tetravalent DENV vaccine. Vaccine. 2011;29:7242–50. doi: 10.1016/j.vaccine.2011.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitehead SS, Falgout B, Hanley KA, Blaney Jr JE, Jr., Markoff L, Murphy BR. A live, attenuated dengue virus type 1 vaccine candidate with a 30-nucleotide deletion in the 3′ untranslated region is highly attenuated and immunogenic in monkeys. J Virol. 2003;77:1653–7. doi: 10.1128/JVI.77.2.1653-1657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durbin AP, McArthur J, Marron JA, et al. The live attenuated dengue serotype 1 vaccine rDEN1Delta30 is safe and highly immunogenic in healthy adult volunteers. Hum Vaccin. 2006;2:167–73. doi: 10.4161/hv.2.4.2944. [DOI] [PubMed] [Google Scholar]
- 22.Durbin AP, Whitehead SS, Shaffer D, et al. A single dose of the DENV-1 candidate vaccine rDEN1Delta30 is strongly immunogenic and induces resistance to a second dose in a randomized trial. PLoS Negl Trop Dis. 2011;5:e1267. doi: 10.1371/journal.pntd.0001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durbin AP, Kirkpatrick BD, Pierce KK, et al. A single dose of any of four different live attenuated tetravalent dengue vaccines is safe and highly immunogenic in flavivirus-naïve adults: a randomized, double blind clinical trial. J Infect Dis. 2012;207:957–65. doi: 10.1093/infdis/jis936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraus AA, Messer W, Haymore LB, de Silva AM. Comparison of plaque- and flow cytometry-based methods for measuring dengue virus neutralization. J Clin Microbiol. 2007;45:3777–80. doi: 10.1128/JCM.00827-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.