Abstract
The gingival epithelium plays a key role in protecting the supporting structures of the teeth from bacteria and their products. In ex vivo experiments, we recently showed that the cytolethal distending toxin (Cdt) from the periodontal pathogen Aggregatibacter actinomycetemcomitans causes extensive damage to gingival tissue. Morphological changes included detachment of the keratinized outer layer, distention of spinous and basal cells in the oral epithelium, disruption of rete pegs, and apparent dissolution of cell junctions. Adherens junctions (zonula adherens) are essential for maintaining barrier function and integrity of gingival epithelium. Therefore, immunohistochemical and RT-PCR analyses of human gingival explants (HGX) and human gingival epithelial cells (HGEC) were utilized for a closer examination of the effects of the Cdt on E-cadherin, the key membrane component of adherens junctions. Although there was some variability among tissue donors, exposure of gingival tissue or isolated epithelial cells to the toxin generally resulted in a pronounced increase in the expression and cytosolic distribution of E-cadherin, accompanied by an increase in levels of the intracellular scaffolding proteins β-catenin and β-actin. These results indicate that the Cdt induced substantial remodeling of adherens junctions, with a potential impact on the barrier function of gingival epithelium.
Abbreviations: cytolethal distending toxin (Cdt), 4′,6-diamidino-2-phenylindole (DAPI), human gingival epithelial cells (HGEC), human gingival explants (HGX), human gingival fibroblasts (HGF), transepithelial resistance (TER).
Keywords: adherens junctions, Aggregatibacter actinomycetemcomitans, cytolethal distending toxin, E-cadherin, gingival epithelial cells, gingival explants
Introduction
Plaque-induced periodontal disease (PIPD) is initiated by a persistent mixed-bacterial infection and sustained by interactions between the microbial antagonists and host immune system (Armitage, 2004; Darveau, 2010). The gingival epithelium is an early line of defense against numerous biofilm-forming micro-organisms, creating a barrier that minimizes bacterial invasion of the underlying tissue (Gibbons, 1989). Bacterial products, along with factors released from recruited inflammatory cells, induce degradation of connective tissue and bone, altering the structural support of the teeth. Therefore, breakdown of the epithelial barrier early in the development of PIPD has significant consequences for periodontal health.
Contact between epithelial cells is stabilized by multi-protein cell junction complexes composed of transmembrane, cytosolic, and cytoskeletal proteins. These are symmetrical structures formed between cells that are critical for maintaining the physical and functional integrity of tissues. Adherens junctions (zonula adherens) are a ubiquitous form of cell junction that interacts with a core structure of intracellular scaffolding and signaling molecules (Miyaguchi, 2000; Meng and Takeichi, 2009; Kowalczyk and Nanes, 2012). Desmosomes (macula adherens) are composed of closely cis-packed transmembrane glycoproteins (desmogleins) that are abundant in epidermis and other stratified squamous epithelia (Franke, 2009). Tight junctions (zonula occludens) are composed of transmembrane proteins arranged head-to-head and include the claudins, occludin, and cytoplasmic adaptor proteins such as ZO-1 (Shen, 2012). Gap junctions are formed by paracrystalline connexin channels (Hirokawa and Heuser, 1981; Franke, 2009).
Aggregatibacter actinomycetemcomitans is a key periodontal pathogen thought to play a significant role in the pathogenesis of localized aggressive periodontitis (LAP; Henderson et al., 2002). A secreted heterotrimeric cytolethal distending toxin (Cdt) is among its putative virulence factors. The results of in vitro (Lepine et al., 1998; Kang et al., 2005) and ex vivo (Damek-Poprawa et al., 2011) studies have provided evidence that the toxin selectively affects the integrity and physiology of cells from human gingival tissue. Disorganization of cell-cell contact and consequent breakdown of tissue architecture are often the result of pathophysiological conditions (Franke, 2009). Therefore, our hypothesis is that Cdt-induced changes within the gingival epithelium are secondary to effects on cell-cell junctions. The goal of this study was to evaluate whether the Cdt alters the organization of adherens junction components in situ in a gingival explant model and in monolayers of isolated human gingival epithelial cells (HGEC).
Materials & Methods
Human Gingival Explants and Epithelial Cells
Gingival tissue (n = 19) was obtained from periodontally healthy and diseased (n = 2) adults, as described previously (Damek-Poprawa et al., 2011), during routine procedures performed at the University of Pennsylvania School of Dental Medicine in accordance with an Institutional Review Board-approved protocol. All patients provided informed consent. Excised tissue was immediately processed as described (Damek-Poprawa et al., 2011). HGEC were isolated from periodontally healthy tissue and cultured as described.
Cdt Purification and Assembly
Recombinant Cdt subunits were isolated by affinity chromatography as described previously (Cao et al., 2005). Heterotrimers were reconstituted, in a refolding buffer, with equimolar amounts of wild-type subunits (Mao and DiRienzo, 2002) or CdtBH160A substituted for CdtB (DiRienzo et al., 2009). The TD50 for HGEC, based on cell-cycle arrest, is 1.1 μg/mL.
Transepithelial Resistance (TER) Measurements
HGEC were seeded at a density of 5 x 104, in serum-free keratinocyte medium, on 12 mm 0.4-μm-pore-size Transwell® inserts (Corning, Lowell, MA, USA) and incubated for 3 days to allow the cells to reach confluence. TER measurements were taken by means of an EVOM2 Epithelial Voltohmmeter (World Precision Instruments, Inc., Sarasota, FL, USA) (Sabath and Denker, 2006). Madin-Darby Canine Kidney (MDCK) cells (CCL-34; ATCC, Manassas, VA, USA) were cultured on the filters in Eagle’s Minimum Essential Medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin mix (Gibco®|Life Technologies, Grand Island, NY, USA).
Tissue Processing
Untreated and Cdt-treated HGX were fixed in 4% buffered formalin for paraffin sectioning at the University of Pennsylvania School of Dental Medicine Tissue Processing Facility. Sections were stained with hematoxylin and eosin and examined by light microscopy. Additional sections were processed for immunohistochemistry as described in the Fig. legend for each experiment. Stained slides were preserved with ProLong Gold anti-fade reagent containing DAPI (Life Technologies) and viewed with a Nikon Eclipse 80i fluorescence microscope. Digital images were recorded with Spot Advanced 4.6 software (Diagnostic Instruments, Inc., Sterling Heights, MI, USA).
Real-time (RT)-PCR
HGX were homogenized in TRIzol reagent (Life Technologies). Total RNA was isolated according to the manufacturer’s protocol, and a 2-μg quantity was converted into cDNA by means of the First-Strand cDNA Synthesis Kit with NotI-(dT)18 primers (GE Healthcare, Piscataway, NJ, USA). RT-PCR reactions were performed in an ABI 7300 Real-Time PCR System with Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). Forward/reverse primers (5′ to 3′) [GAAGG TGACAGAGCCTCTGGA/GACCCCCTCCACAAATTGC (CDH1, E-cadherin); GATACCCAGCGCCGTACGT/GACCC CCTCCACAAATTGC (CTNNB1, β-catenin); GCATGGAGT CCTGTGGCAT/CTAGAAGCATTTGCGGTGGAC (ACTB, β-Actin)] were designed with Primer Express software (Applied Biosystems). TATA binding protein (TBP) mRNA expression level was used as a normalizing control. Results were expressed as relative fold change by the ΔΔCt method as described previously (Damek-Poprawa et al., 2011).
Results
HGEC Do Not Form Tight Junctions in vitro
TER was used to measure tight junctions in cultures of HGEC. MDCK cells, a commonly used positive control for tight junctions (Dukes et al., 2011), had resistance values as high as 2,000 Ω x cm2 (Fig. 1). In contrast, TER for HGEC averaged 100 Ω x cm2. Claudin 1, a component of tight junction filaments (Furuse et al., 2002), was poorly detected in both HGEC and HGX (data not shown). Therefore, the presence of tight junctions was not clearly evident. When the MDCK cells with established tight junctions were exposed to 10 μg/mL of Cdt, a slight decrease in the TER was observed within minutes. The resistance decreased by approximately 80% by 72 hrs post-treatment. Untreated MDCK cells maintained a high TER for up to 100 hrs after reaching confluence.
Figure 1.
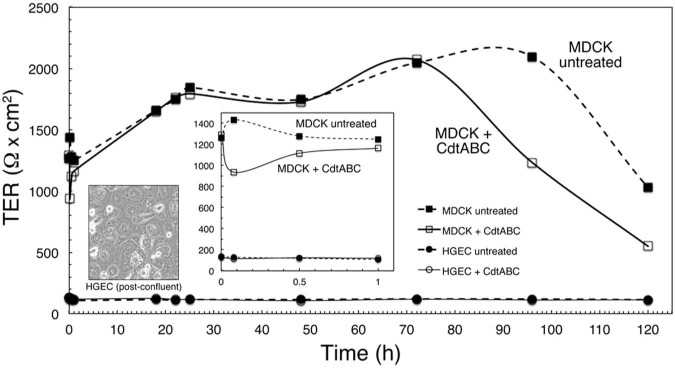
Transepithelial resistance (TER) of HGEC. HGEC cultured on polycarbonate filters were untreated and exposed to CdtABC for 18 hrs. No increase in the TER of either culture was detected over a 120-hour period. Cultures of untreated and CdtABC-treated MDCK cells reached a TER of approximately 1,800 Ω x cm2 by 30 hrs of incubation. This experiment was performed twice.
Effect of Cdt on Cellular Levels and Distribution of E-cadherin
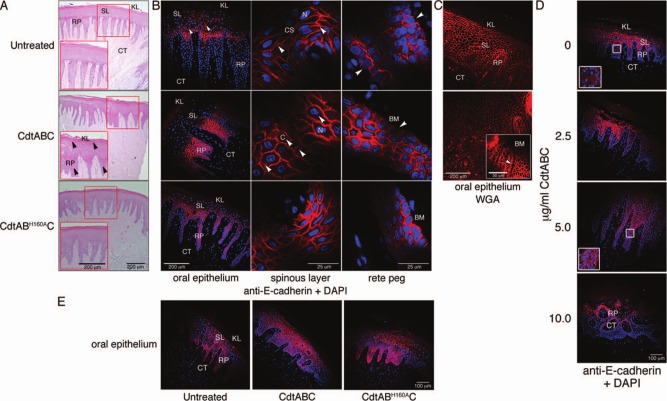
HGX from healthy patients were either left untreated or exposed to 10 µg/mL of wild-type CdtABC or CdtABH160AC for 18 hrs. Hematoxylin and eosin staining of untreated HGX confirmed that the tissue was stable for the duration of the experiment. Rete pegs remained intact, cells in the spinous layer were densely packed, and the keratinized layer was undamaged (Fig. 2A). Upon exposure to CdtABC, there was extensive separation of the keratinized surface layer at the junction of the oral epithelium, as well as extensive disruption of the spinous epithelial layer and rete pegs. Structural integrity of HGX incubated with CdtABH160AC appeared similar to that of the untreated tissue.
Figure 2.
Cellular distribution of E-cadherin in untreated and Cdt-treated HGX. (A) Hematoxylin and eosin-stained sections of HGX that were untreated or treated with either 10 µg/mL CdtABC or CdtABH160AC for 18 hrs. Arrows show the location of tissue damage. (B) Staining untreated and Cdt-treated (10 µg/mL for 18 hrs) HGX for the presence of E-cadherin. Arrows show the location of E-cadherin on the epithelial cell surface (CS), in the cytosol (C), and lack of staining at the junction of basal epithelial cells and the basement membrane (BM). (C) Untreated and Cdt-treated HGX labeled with 1:1,000 dilution of wheat germ agglutinin Alexa Fluor 555 conjugate (Life Technologies). Inset shows rete pegs (arrow) from CdtABC-treated HGX at a higher magnification. (D) Dose response of CdtABC-treated HGX stained for the presence of E-cadherin after 18 hrs of exposure. Insets show enlargement of outlined areas. The orientation of the tissue section in the bottom panel is showing a cross-section of the rete pegs. (E) E-cadherin distribution in the spinous layer of Cdt-treated gingival tissue. HGX sections shown in B, D, and E were labeled with a 1:100 dilution of rabbit polyclonal anti-E-cadherin IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and 5 µg/mL of conjugated goat anti-rabbit IgG Alexa Fluor 594 (red fluorescence; Life Technologies) and DAPI (blue fluorescence). KL, keratinized layer; SL, spinous layer; RP, rete peg; CT, connective tissue. Tissue from the same donor is shown in A through C, while those in D and E are from two distinct donors. Experiments were performed a minimum of 3 times with tissue from different patients.
E-cadherin localized predominantly at the plasma membrane, outlining the epithelial cell-cell contact zone, in untreated HGX (Fig. 2B, top row). Exposure to CdtABC resulted in a pronounced increase in fluorescence intensity, thus representing an increase in the relative amount of E-cadherin. The protein was detected throughout the cytosol, indicating extensive intracellular redistribution (Fig. 2B, middle row). Lack of E-cadherin staining in untreated HGX suggested the absence of adherens junctions at the border of the epithelial cells and basement membrane. The presence of intact epithelial cell membranes adjacent to the basement membrane in all samples was confirmed by staining with wheat germ agglutinin (Fig. 2C). E-cadherin was detected circumferentially around cells adjacent to the basement membrane in HGX treated with CdtABC. Not only did this observation confirm that Cdt induces redistribution of E-cadherin within the epithelial cells, but it also suggests that the toxin causes detachment of basal cells from the basement membrane. Exposure of HGX to CdtABH160AC affected the level of E-cadherin but not to the extent of that observed in HGX incubated with wild-type toxin (Fig. 2B, bottom row). The Cdt-induced change in distribution of E-cadherin was evident when the HGX were treated with between 2.5 and 5.0 μg/mL of the toxin (Fig. 2D).
Comparison of tissue from 14 different individuals revealed 2 distinct E-cadherin labeling patterns (9 spinous layer and 5 rete pegs), suggesting that distribution within the gingival epithelium may be patient-specific. As exemplified in Figs. 2B and 2D, the strongest reaction with the anti-E-cadherin antibody in untreated HGX was observed in the spinous layers. Upon exposure to increasing concentrations of CdtABC (Fig. 2D), the intensity of the signal within the basal epithelial cells increased. However, in HGX from other individuals, the staining pattern was reversed such that more intense labeling was apparent in the spinous cell layer after exposure of the HGX to the Cdt (Fig. 2E).
Effect of Cdt on Intracellular Scaffolding Proteins
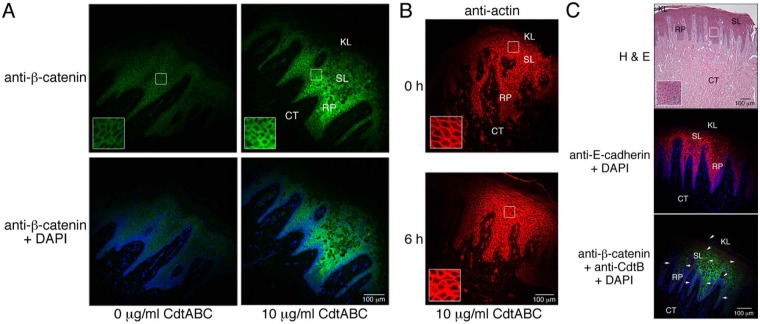
Since adherens junction structure relies on dynamic interactions of E-cadherin with catenin complexes and the actin cytoskeleton (Drees et al., 2005; Yamada et al., 2005), we also examined the localization and relative levels of β-catenin and β-actin in the gingival epithelium of untreated and Cdt-treated HGX. Both proteins were detected in all tissue sections tested. Following 18 hrs of incubation with 10 µg/mL of CdtABC, the β-catenin concentration in the spinous layer was elevated relative to that in untreated HGX (Fig. 3A). Under similar treatment, there was a marked increase in actin concentration as early as 6 hrs after the addition of the Cdt (Fig. 3B).
Figure 3.
Localization of cell junction and structural proteins in untreated and CdtABC-treated HGX and gingival tissue from an individual with clinical signs of plaque-induced periodontal disease (PIPD).. (A) Sections from a HGX obtained from a healthy individual were treated with 0 or 10 µg/mL of CdtABC for 18 hrs and then were labeled with a 1:100 dilution of mouse monoclonal anti-β-catenin IgG (Santa Cruz Biotechnology) followed by 5 µg/mL of conjugated goat anti-mouse Alexa Fluor 488 (green fluorescence; Life Technologies). Sections were co-stained with DAPI. Outlined areas are enlarged in the insets. (B) Sections from HGX obtained from a healthy individual were treated with 10 µg/mL of CdtABC for 0 and 6 hrs and were labeled with a 1:100 dilution of rabbit polyclonal anti-actin IgG (Santa Cruz Biotechnology) followed by 5 µg/mL of conjugated goat anti-rabbit IgG Alexa Fluor 594 (Life Technologies). (C) Untreated HGX from an individual with clinical signs of disease. Sections were stained with hematoxylin and eosin and were co-labeled with anti-E-cadherin IgG, anti-β-catenin IgG, anti-CdtB IgG (red fluorescence in bottom panel), and DAPI. Antibody concentrations were the same as those used in Figs. 2 and 3A. The presence of CdtB (arrows) was detected with rabbit polyclonal anti-CdtB IgG (1:50,000 dilution) and 5 µg/mL of conjugated goat anti-mouse Alexa Fluor 488 as described previously (Damek-Poprawa et al., 2011). The explants shown in A and B are from two separate healthy donors. Experiments were performed on healthy and diseased tissue a minimum of 3 and 2 times, respectively, with tissue from different patients.
Hematoxylin and eosin-stained sections of untreated HGX from patients with clinical signs of periodontal disease showed extensive tissue damage marked by detachment of the keratinized epithelium and disruption of the spinous layer and rete pegs (Fig. 3C). Relatively high levels of E-cadherin and β-catenin were present in the spinous layer. Even though the tissue examined was from a diseased donor, it was surprising to detect anti-CdtB-positive deposits throughout the oral epithelium (red fluorescence in bottom panel).
Quantitative Evaluation of Junction Protein Gene Expression in HGX and HGEC
In response to the toxin, a statistically significant increase in E-cadherin mRNA expression level was detected in at least 50% of HGX and 66% of HGEC cultures from independent individuals (Fig. 4). Twenty-five percent of HGX and 50% of HGEC cultures exhibited a statistically significant increase in β-catenin expression. Actin expression was increased in 63% of HGX and 83% of HGEC cultures.
Figure 4.
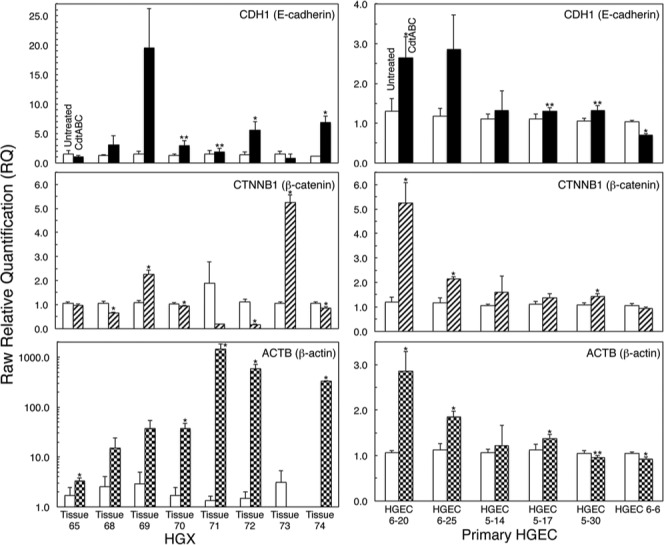
Quantitative expression of genetic markers for adherens junction and intracellular scaffolding proteins. RT-PCR of human genes for E-cadherin (CDH1), β-catenin (CTNNB1), and β-actin (ACTB) in untreated and CdtABC-treated HGX (eight individuals) and primary HGEC (six individuals). Untreated, white bars; CdtABC-treated (10 μg/mL), black and patterned bars. Note that the data are plotted with difference scales on the Y-axes. Mean values ± SD are shown (n = 3 in each group). Asterisks mark statistically significant differences (two-sample unequal variance test) between the relative expression levels of mRNA from untreated and CdtABC-treated HGX or HGEC (*p ≤ .05, **p ≤ .1). Experiments were performed a minimum of 3 times.
Discussion
Recently, we showed that the Cdt from A. actinomycetemcomitans significantly altered the structure of the epithelial compartment of HGX (Damek-Poprawa et al., 2011). In this study, we assessed the effects of the Cdt on adherens junctions, complexes that maintain tissue integrity under physiological conditions.
Our attempts to identify tight junctions, utilizing TER and immunolabeling for claudin-1, in post-confluent HGEC cultures were unsuccessful. The occurrence of tight junctions in human gingival epithelium had been suggested previously but not unequivocally demonstrated (Thilander and Bloom, 1968; Barnett and Szabo, 1973). Meyle and co-workers (1999) provided microscopic evidence of tight junctions in human gingival biopsies. However, they also indicated that there were areas in the tissue where tight junctions were not detected. The increase in TER of cultured human gingival keratinocytes was approximately 10 times lower than that of MDCK cells and was highly variable. The TER of gingival epithelial cell multilayers was only 150 Ω x cm2 and not altered by exposure of the cells to either Porphyromonas gingivalis or A. actinomycetemcomitans for up to 24 hrs (Dickinson et al., 2011). Based on these findings, and on data obtained ex vivo, it is possible that tight junctions may not be critical to maintaining the integrity of gingival epithelium.
Adherens junctions, composed of dynamic cadherin-catenin-actin complexes, are the most well-studied cell-cell adhesion complex in epithelial cells (Wheelock and Knudsen, 1991; Cavey and Lecuit, 2009; Meng and Takeichi, 2009; Brieher and Yap, 2012). We evaluated the distribution of E-cadherin and β-catenin in HGX. Relatively high concentrations of both proteins were detected in juxtaposition with the membrane of oral epithelial cells in untreated HGX. Interestingly, the lack of E-cadherin at the border between epithelial cells and basement membrane indicated the absence of adherens junctions in this region. This is most likely due to the fact that these cells attach to basement membrane via hemidesmosomes. These results supported previous observations that normal oral epithelium showed strong pericellular staining of E-cadherin in the basal and suprabasal cell layers but not on the basal aspect of keratinocytes adjacent to the basement membrane (Downer and Speight, 1993). Ye et al. (2000) also found marked expression of E-cadherin in the basal cell layer of healthy gingiva. With these findings at hand, we were confident that the HGX model would allow us to evaluate the effect of the Cdt on adherens junctions.
Our results indicated that the Cdt induces significant changes in both the distribution and expression of the component proteins of adherens junctions. Exposure of the HGX to wild-type Cdt resulted in a subtle but clearly detectable alteration in the cytosolic distribution of E-cadherin. Based on RT-PCR analysis, there was also an increase in expression of genes encoding E-cadherin, β-catenin, and β-actin in both HGEC and HGX from a majority of the donors examined. These effects may be associated with the cellular distension caused by the toxin. The variability that was detected in the response to the Cdt could be due to a multitude of environmental and/or donor-specific factors. Perturbations in the regulation of cell-cell junction stability and dynamics affect the barrier properties of epithelial layers and can have detrimental effects on tissue remodeling throughout development (Cavey and Lecuit, 2009). Therefore, barrier function is most likely a consequence of the stability of adherens junctions.
Considering the effect of Cdt on epithelial barrier function, we propose a model to explain these unique clinical findings. The clinical presentation of patients with LAP is typified by rapidly progressing bone loss despite a relative paucity of calculus and gingival inflammation. Secreted Cdt disrupts the gingival epithelium particularly, but not exclusively, by altering cell-cell contact. The bacterium and/or secreted products gain entry into the underlying connective tissue. Leukotoxin, another A. actinomycetemcomitans cytotoxin, induces cell death in a variety of inflammatory cells, thereby down-modulating the extent of clinically detectable gingival inflammation. The localized immunosuppression that results allows for additional influx of Gram-negative bacteria inhabiting the periodontal pocket. Lipopolysaccharide, a potent inducer of osteoclastogenesis, produced by the mixed infection causes the extensive bone loss seen in affected sites. The central premise of this model is that A. actinomycetemcomitans plays an early role in the pathogenesis of the disease via the elaboration of virulence factors that abrogate the host response. It is quite feasible that other organisms play a more critical role in latter events that directly lead to clinical manifestations of the disease.
Acknowledgments
A portion of this work was performed by R.G. as partial fulfillment of a Master’s Degree in Oral Biology. We thank Alla Volgina for able technical assistance.
Footnotes
The research was supported by NIH Grant R01-DE012593 (J.M.D.) from the National Institute of Dental and Craniofacial Research.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Armitage GC. (2004). Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000 34:9-21. [DOI] [PubMed] [Google Scholar]
- Barnett ML, Szabo G. (1973). Gap junctions in human gingival keratinized epithelium. J Periodontal Res 8:117-126. [DOI] [PubMed] [Google Scholar]
- Brieher WM, Yap AS. (2013). Cadherin junctions and their cytoskeleton(s). Curr Opin Cell Biol 25:39-46. [DOI] [PubMed] [Google Scholar]
- Cao L, Volgina A, Huang CM, Korostoff J, DiRienzo JM. (2005). Characterization of point mutations in the cdtA gene of the cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Mol Microbiol 58:1303-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavey M, Lecuit T. (2009). Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harb Perspect Biol 1:a002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damek-Poprawa M, Haris M, Volgina A, Korostoff J, DiRienzo JM. (2011). Cytolethal distending toxin damages the oral epithelium of gingival explants. J Dent Res 90:874-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau RP. (2010). Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol 8:481-490. [DOI] [PubMed] [Google Scholar]
- Dickinson BC, Moffatt CE, Hagerty D, Whitmore SE, Brown TA, Graves DT, et al. (2011). Interaction of oral bacteria with gingival epithelial cell multilayers. Mol Oral Microbiol 26:210-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo JM, Cao L, Volgina A, Bandelac G, Korostoff J. (2009). Functional and structural characterization of chimeras of a bacterial genotoxin and human type I DNAse. FEMS Microbiol Lett 291:222-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer CS, Speight PM. (1993). E-cadherin expression in normal, hyperplastic and malignant oral epithelium. Eur J Cancer B Oral Oncol 29(B):303-305. [DOI] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. (2005). Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 123:903-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukes JD, Whitley P, Chalmers AD. (2011). The MDCK variety pack: choosing the right strain. BMC Cell Biol 12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke WW. (2009). Discovering the molecular components of intercellular junctions – a historical view. Cold Spring Harb Perspect Biol 1:a003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, et al. (2002). Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol 156:1099-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons RJ. (1989). Bacterial adhesion to oral tissues: a model for infectious diseases. J Dent Res 68:750-760. [DOI] [PubMed] [Google Scholar]
- Henderson B, Wilson M, Sharp L, Ward JM. (2002). Actinobacillus actinomycetemcomitans. J Med Microbiol 51:1013-1020. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Heuser JE. (1981). Quick-freeze, deep-etch visualization of the cytoskeleton beneath surface differentiations of intestinal epithelial cells. J Cell Biol 91(2 Pt 1):399-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P, Korostoff J, Volgina A, Grzesik W, DiRienzo JM. (2005). Differential effect of the cytolethal distending toxin of Actinobacillus actinomycetemcomitans on co-cultures of human oral cells. J Med Microbiol 54(Pt 8):785-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk AP, Nanes BA. (2012). Adherens junction turnover: regulating adhesion through cadherin endocytosis, degradation, and recycling. Subcell Biochem 60:197-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepine G, Caudry S, DiRienzo JM, Ellen RP. (1998). Epithelial cell invasion by Actinobacillus actinomycetemcomitans strains from restriction fragment-length polymorphism groups associated with juvenile periodontitis or carrier status. Oral Microbiol Immunol 13:341-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, DiRienzo JM. (2002). Functional studies of the recombinant subunits of a cytolethal distending holotoxin. Cell Microbiol 4:245-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W, Takeichi M. (2009). Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol 1:a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyle J, Gultig K, Rascher G, Wolburg H. (1999). Transepithelial electrical resistance and tight junctions of human gingival keratinocytes. J Periodontal Res 34:214-222. [DOI] [PubMed] [Google Scholar]
- Miyaguchi K. (2000). Ultrastructure of the zonula adherens revealed by rapid-freeze deep-etching. J Struct Biol 132:169-178. [DOI] [PubMed] [Google Scholar]
- Sabath E, Denker BM. (2006). Cell-cell interactions in the kidney: inducible expression of mutant G protein alpha-subunits in Madin-Darby canine kidney cells for studies of epithelial cell junction structure and function. Methods Mol Biol 341:61-72. [DOI] [PubMed] [Google Scholar]
- Shen L. (2012). Tight junctions on the move: molecular mechanisms for epithelial barrier regulation. Ann NY Acad Sci 1258:9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilander H, Bloom GD. (1968). Cell contacts in oral epithelia. J Periodontal Res 3:96-110. [DOI] [PubMed] [Google Scholar]
- Wheelock MJ, Knudsen KA. (1991). Cadherins and associated proteins. In Vivo 5:505-513. [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. (2005). Deconstructing the cadherin-catenin-actin complex. Cell 123:889-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Chapple CC, Kumar RK, Hunter N. (2000). Expression patterns of E-cadherin, involucrin, and connexin gap junction proteins in the lining epithelia of inflamed gingiva. J Pathol 192:58-66. [DOI] [PubMed] [Google Scholar]