Abstract
Gdown1 is a substoichiometric RNA polymerase II subunit that modulates effects of both Mediator and elongation factors in vitro and is broadly distributed across the human genome. Here we assemble the existing information on Gdown1, provide additional bioinformatics analyses, and propose a working model for Gdown1 function.
Keywords: DSIF, Gdown1, Mediator, NELF, P-TEFb, POLR2M, RNA polymerase II
Regulating the level of expression of all genes at any given moment is at the heart of specifying the identity and function of every cell in multicellular organisms. Although DNA and chromatin modifications, as well as the initiation stage of transcription are important, controlling the elongation phase of transcription by RNA Polymerase II (RNAP II) has surfaced as a critical, regulated step.1,2 Hundreds of differentially expressed transcription factors are involved in the regulatory process, but detailed mechanisms describing exactly what these factors do are still lacking. Information presented here supports the hypothesis that a functional interaction between Mediator and Gdown1 is used to bridge the regulatory factors with the transcription complex to control the elongation phase of transcription.
RNAP II elongation control is characterized by the default action of negative elongation factors that halt RNAP II in promoter proximal positions and the regulated release of this block by the positive transcription elongation factor, P-TEFb.1 The DRB sensitivity inducing factor, DSIF,3 works with the negative elongation factor, NELF,4 to slow the rate of elongation5,6 and NELF is found over promoter proximal paused (poised) polymerases that are prevalent across metazoan genomes.7,8 Which of these poised polymerases enter productive elongation is dictated by selective P-TEFb function.1,2 After the transition into productive elongation, RNAP II moves at about 4000 bases per minute until it passes the region encoding the polyadenylation signal, at which point it slows again and ultimately terminates.9
In 2006, Averell Gnatt’s lab discovered that a little less than half of mammalian RNAP II has a 13th subunit called Gdown110 that is encoded by the GRINL1A gene,11 recently renamed POLR2M. At that time, the Roeder lab showed that Gdown1 was required for Mediator to function as a co-activator in vitro.10 Six years later, two papers provided many more details of the function of Gdown1.12,13 We will summarize the current state of understanding of Gdown1 function and provide a revealing comparison of the genome wide distributions of Gdown1 and Mediator. This information links the role of Mediator in regulating transcription14,15 with that of Gdown1 during transcription elongation.
Biochemical functions of Gdown1
Perhaps as expected for a tightly associated subunit of RNAP II, Gdown1 has a wide variety of effects on transcription by RNAP II and on the factors that influence RNAP II. First, a surprising finding was made using a defined in vitro transcription system comprising TFIIA, TFIIB, TFIID, TFIIE, TFIIF and TFIIH, along with PC4. Most of the activator-dependent stimulation in runoff required a Mediator fraction only if the reactions were driven by RNAP II containing Gdown1, RNAP II(G).10 In a more minimal in vitro system, in which transcription was reconstituted on supercoiled templates with TBP, TFIIB, TFIIF and RNAP II, Gdown1 had a strong negative effect attributed to inhibition of binding of TFIIF to RNAP II by Gdown1, presumably at the level of initiation.13 Inhibition of TFIIF binding was confirmed by immunoprecipitation experiments and a competition between TFIIF and Gdown1 was demonstrated in that at 0.3 M KCl a 10-fold molar excess of TFIIF could partially remove Gdown1 from RNAP II.13 Far Western assays demonstrated that Gdown1 could interact with the largest and 5th largest subunits of RNAP II. Interestingly, western blotting using immobilized templates suggested that RNAP II(G) could enter a pre-initiation complex, but required an activator and Mediator.13
Gdown1 dramatically affects the elongation phase of transcription. It bound to elongation complexes formed on immobilized templates and the association became completely resistant to extensive washing at low salt or with 1.6 M KCl.12 This latter property suggests a hydrophobic interaction with the polymerase. Because Gdown1 is easily made as a soluble recombinant protein in E. coli, the hydrophobic faces needed for binding to RNAP II likely interact with each other in such a way as to block formation of large aggregates and this suggests that Gdown1 undergoes a large conformational change upon binding to RNAP II. As was found for free RNAP II(G), Gdown1 also blocked the association of TFIIF to RNAP II in elongation complexes and eliminated the 20-fold stimulation of elongation by TFIIF.12 It modulated the individual and combined effects of DSIF and NELF, but did not influence either the capping enzyme or TFIIS, both of which extensively interact with RNAP II.12
One of the most profound effects of Gdown1 is that it completely blocks the termination activity of TTF2.12 This property prolongs the lifetime of promoter proximal paused RNAP II(G),12 but this results in a potential dilemma. During mitosis TTF2 is responsible for termination of all polymerases as the chromosomes condense.16 There must be a mechanism to reverse the influence of Gdown1 during mitosis and this would have to be globally applied during this tight window of time. It is possible that the proposed Mediator driven rearrangement of Gdown1 during initiation that lets TFIIF12 function could also occur during mitosis to allow TTF2 to function.
Bioinformatics analyses of Gdown1 distribution
The distribution of RNAP II and Gdown1 across the human genome was determined using ChIP-Seq.12,13 Although the conclusions in the two recent papers concerning the generality of Gdown1 function were described as a controversy in the Preview on the two papers,17 our analysis of the ChIP-Seq data sets from both studies indicates that there is no discrepancy. We took the mapped reads from the two published studies and analyzed them in an identical manner by generating files that can be viewed on the UCSC genome browser (GEO accession number GSE35886). Extensive comparison of the distribution of Gdown1 in IMR90 and HeLa cells was performed and a remarkably similar pattern across the genome was found. In both cell types, Gdown1 maps to almost all positions of RNAP II, indicating a general function for the factor in both cell types. This conclusion was stated in the study on HeLa cells12 but, because of an errant input data set (see our comment associated with Jishage et. al13 on the Molecular Cell website), the global distribution of Gdown1 was not described for IMR90 cells.13 In both cell types it is clear that the ratio of RNAP II to Gdown1 is not uniform at all sites of occupancy. This was quantified in HeLa cells and RNAP II in promoter proximal paused positions of genes experiencing more productive elongation had relatively less Gdown1 than RNAP II in the same position in poorly expressed genes.12
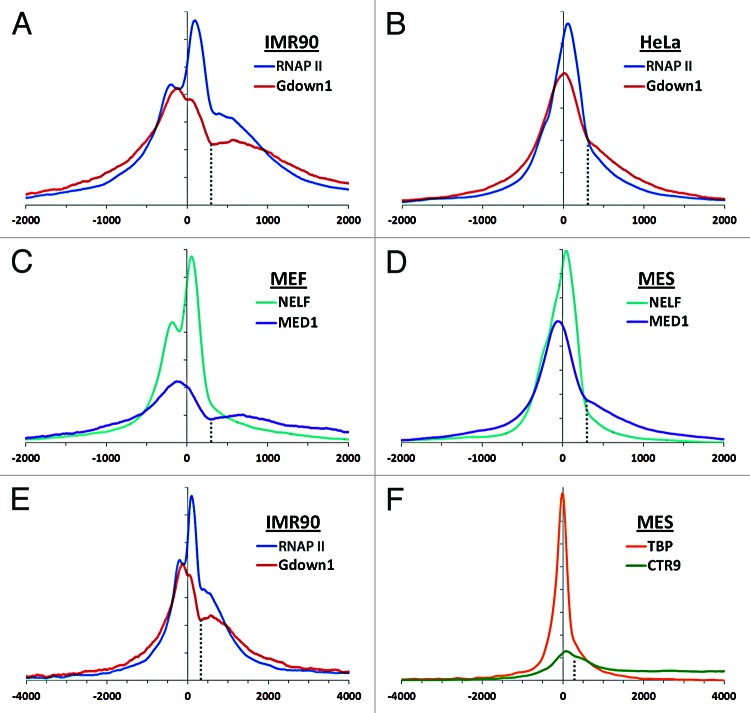
Using the newly derived IMR90 and HeLa data sets, average signals were calculated as described12 around the transcription start site (TSS) for all genes with a TSS more than 1000 bp from another. In both cell types the major peaks for RNAP II were downstream of the TSS and a second peak or shoulder from divergent transcription18 was present upstream (Fig. 1A and B). For both cell types Gdown1 peaked closer to the TSS than RNAP II. A second, more prominent transition in the profiles of RNAP II and Gdown1 is seen at +300 in both cell types (Fig. 1A and B, dotted line) and the region from +300 to about +1000 has a relatively high occupancy compared with further downstream. Because of the functional connection between Gdown1 and Mediator, ChIP-Seq data sets for the MED1 Mediator subunit19 as well as a subunit of NELF8,20 were analyzed. These data sets were derived from mouse cells, but the nature of the analyses allows comparison across species. Data sets from mouse embryonic fibroblasts MEFs or mouse embryonic stem (MES) cells, which closely match the growth rate of IMR90 and HeLa cells, respectively, demonstrate that NELF is found primarily over promoter proximal paused polymerases and that Mediator peaks upstream of the TSS (Fig. 1C and D). Significantly, the +300 transition was seen for Mediator in both cell types. In fact, the patterns of Gdown1 and Mediator were remarkably similar to each other in both cell types. This suggests that Gdown1 and Mediator are in close proximity on the average gene and suggests that the region downstream of +300 is important for Mediator/Gdown1 function.
Figure 1. Metagene analyses. The relative distribution of the indicated proteins around the average transcription start site (TSS) for a custom set of genes in which genes with TSSs within 1000 bp of another were excluded (20,799 mouse genes or 20,286 human genes). The profiles were generated for regions -10 kb to + 10 kb, backgrounds were subtracted, and the curves were normalized as described earlier.12 Relative amplitudes within a given curve or between curves can be compared but absolute amplitudes between curves should not be compared. Cell types: human cervical cancer cells (HeLa), human fibroblasts (IMR90), mouse embryonic stem cells (MES), mouse embryonic fibroblasts (MEF). Data sets analyzed: human (GSE35886),12,13 MEF (GSE24113, GSE22562),19,20 MES (GSE20530, GSE22562, GSE22303).8
Biochemical experiments demonstrated that Gdown1 could enter productive elongation in HeLa cells and Gdown1 was found downstream of the paused polymerase and in regions downstream of 3′ ends of genes.12 The relative distributions of Gdown1 and RNAP II in the interval from -4 kb to +4 kb indicate that this is also the case in IMR90 cells (Fig. 1E). A clue to the functional significance of the +300 transition is seen when comparing the distribution of the initiation factor TBP with that of the PAF1 complex subunit CTR9. TBP peaks slightly upstream of the TSS, as expected, and rapidly declines on both sides of the peak, while CTR9 is mostly far downstream and relatively absent from the region of promoter proximal pausing (Fig. 1F). CTR9 is a prime candidate for a factor that becomes associated with productive elongation complexes and it travels with the polymerase and is involved in co-transcriptional RNA processing events.8 The data are consistent with the transition of RNAP II(G) into productive elongation occurring at and slightly downstream of +300.
Model for Gdown1 function
Together, these findings suggest that Gdown1 plays a major role in regulating each step of transcription by altering the interactions with initiation, elongation and termination factors. The simplest model shown in Figure 2 illustrates parallel pathways for RNAP II vs. RNAP II(G) that have significant differences. In the absence of Gdown1, RNAP II interacts with TFIIF in the pre-initiation complex, initiates, and then comes under the control of DSIF and NELF leading to a transiently paused polymerase. RNAP II(G) can also enter a pre-initiation complex, but cannot initiate without Mediator. This was shown directly in vitro13 and was inferred from the effect of flavopiridol on the genome-wide distribution of Gdown1 in HeLa cells, which moved downstream over the promoter proximally paused polymerase when P-TEFb was inhibited.12 Mediator may “remodel” Gdown1 such that TFIIF can now function leading to initiation. Alternatively, initiation of RNAP II(G) might occur without TFIIF function, similar to what was recently described by the Luse lab.21 Either way, this leads to promoter proximally paused RNAP II(G) under the influence of negative elongation factors including the Gdown1 negative accessory factor, GNAF. Because Gdown1 blocks TTF2 function, these polymerases are stably bound compared with paused RNAP II. NELF could be a component of the paused RNAP II(G) complex found close to the promoter, but the RNAP II(G) found in the +300 to +1000 region has significantly less NELF (Fig. 1C and D). This region may represent an intermediate in the transition of RNAP II(G) into productive elongation. It is not clear if DSIF is associated with paused RNAP II(G), and the lack of DSIF is supported by bioinformatics analyses demonstrating that DSIF is substoichiometric with RNAP II in this region. Regardless of the presence of Gdown1, P-TEFb can cause the transition into productive elongation. However, on genes that do not experience much productive elongation, RNAP II(G) builds up in promoter proximal positions due to termination of transiently paused RNAP II and replacement with stably paused RNAP II(G).12 Examination of ChIP-Seq data provides thousands of examples of polymerases with relatively high levels of Gdown1. These types of polymerases may be holding regions of the genome in a special chromatin state22 that might be utilized to enhance or silence gene expression nearby or to insulate promoters.23
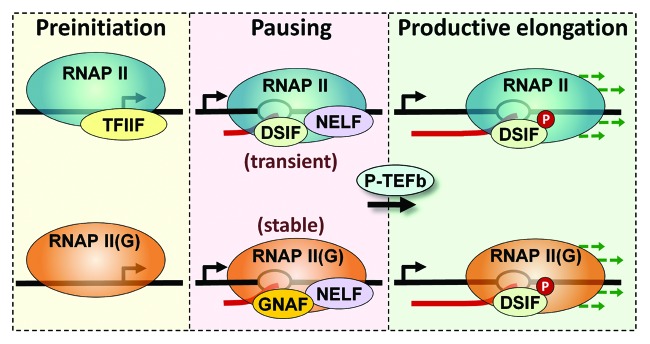
Figure 2. Model for Gdown1 function. The diagram illustrates the transcription cycles performed by RNAP II (blue) and RNAP II(G) (orange). As described in the text, both forms of polymerase can initiate and ultimately enter productive elongation. RNAP II(G) requires Mediator (not shown) for initiation. A major difference between the two cycles is that promoter proximal paused RNAP II is transient while paused RNAP II(G) is stable due to the ability of Gdown1 to block the termination activity of TTF2.
In conclusion, Gdown1 seems to play a significant role in all phases of RNAP II transcription. Although Mediator was originally thought of as a factor that affected initiation, it is becoming clear that it also affects elongation.15,24-27 The connections made between Mediator and Gdown1 suggest that Gdown1 may play a role in the intermolecular signaling between remote activators and the transcription complex and that the function of these activators may be to encourage P-TEFb function on RNAP II(G). In the future, it is important to determine if Mediator only works on RNAP II(G) or if it has an effect on all polymerases in vivo.
Acknowledgments
This work was funded by National Institutes of Health grant GM35500 to DHP. We thank Miles Pufall and the reviewers for helpful comments during the preparation of the final manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/transcription/article/20600
References
- 1.Zhou Q, Li T, Price DH. RNA Polymerase II Elongation Control. Annu Rev Biochem. 2012 doi: 10.1146/annurev-biochem-052610-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nechaev S, Adelman K. Pol II waiting in the starting gates: Regulating the transition from transcription initiation into productive elongation. Biochim Biophys Acta. 2011;1809:34–45. doi: 10.1016/j.bbagrm.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wada T, Takagi T, Yamaguchi Y, Ferdous A, Imai T, Hirose S, et al. DSIF, a novel transcription elongation factor that regulates RNA polymerase II processivity, is composed of human Spt4 and Spt5 homologs. Genes Dev. 1998;12:343–56. doi: 10.1101/gad.12.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaguchi Y, Takagi T, Wada T, Yano K, Furuya A, Sugimoto S, et al. NELF, a multisubunit complex containing RD, cooperates with DSIF to repress RNA polymerase II elongation. Cell. 1999;97:41–51. doi: 10.1016/S0092-8674(00)80713-8. [DOI] [PubMed] [Google Scholar]
- 5.Renner DB, Yamaguchi Y, Wada T, Handa H, Price DH. A highly purified RNA polymerase II elongation control system. J Biol Chem. 2001;276:42601–9. doi: 10.1074/jbc.M104967200. [DOI] [PubMed] [Google Scholar]
- 6.Cheng B, Price DH. Properties of RNA polymerase II elongation complexes before and after the P-TEFb-mediated transition into productive elongation. J Biol Chem. 2007;282:21901–12. doi: 10.1074/jbc.M702936200. [DOI] [PubMed] [Google Scholar]
- 7.Gilchrist DA, Nechaev S, Lee C, Ghosh SK, Collins JB, Li L, et al. NELF-mediated stalling of Pol II can enhance gene expression by blocking promoter-proximal nucleosome assembly. Genes Dev. 2008;22:1921–33. doi: 10.1101/gad.1643208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, et al. c-Myc regulates transcriptional pause release. Cell. 2010;141:432–45. doi: 10.1016/j.cell.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh J, Padgett RA. Rates of in situ transcription and splicing in large human genes. Nat Struct Mol Biol. 2009;16:1128–33. doi: 10.1038/nsmb.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu X, Malik S, Negroiu CC, Hubbard K, Velalar CN, Hampton B, et al. A Mediator-responsive form of metazoan RNA polymerase II. Proc Natl Acad Sci U S A. 2006;103:9506–11. doi: 10.1073/pnas.0603702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roginski RS, Mohan Raj BK, Birditt B, Rowen L. The human GRINL1A gene defines a complex transcription unit, an unusual form of gene organization in eukaryotes. Genomics. 2004;84:265–76. doi: 10.1016/j.ygeno.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Cheng B, Li T, Rahl PB, Adamson TE, Loudas NB, Guo J, et al. Functional association of Gdown1 with RNA polymerase II poised on human genes. Mol Cell. 2012;45:38–50. doi: 10.1016/j.molcel.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jishage M, Malik S, Wagner U, Uberheide B, Ishihama Y, Hu X, et al. Transcriptional regulation by Pol II(G) involving mediator and competitive interactions of Gdown1 and TFIIF with Pol II. Mol Cell. 2012;45:51–63. doi: 10.1016/j.molcel.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taatjes DJ. The human Mediator complex: a versatile, genome-wide regulator of transcription. Trends Biochem Sci. 2010;35:315–22. doi: 10.1016/j.tibs.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conaway RC, Conaway JW. Function and regulation of the Mediator complex. Curr Opin Genet Dev. 2011;21:225–30. doi: 10.1016/j.gde.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Y, Liu M, Spencer CA, Price DH. Involvement of transcription termination factor 2 in mitotic repression of transcription elongation. Mol Cell. 2004;14:375–85. doi: 10.1016/S1097-2765(04)00234-5. [DOI] [PubMed] [Google Scholar]
- 17.Espinosa JM. Get back TFIIF, don’t let me Gdown1. Mol Cell. 2012;45:3–5. doi: 10.1016/j.molcel.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seila AC, Core LJ, Lis JT, Sharp PA. Divergent transcription: a new feature of active promoters. Cell Cycle. 2009;8:2557–64. doi: 10.4161/cc.8.16.9305. [DOI] [PubMed] [Google Scholar]
- 19.Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–5. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun J, Pan H, Lei C, Yuan B, Nair SJ, April C, et al. Genetic and genomic analyses of RNA polymerase II-pausing factor in regulation of mammalian transcription and cell growth. J Biol Chem. 2011;286:36248–57. doi: 10.1074/jbc.M111.269167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Čabart P, Újvári A, Pal M, Luse DS. Transcription factor TFIIF is not required for initiation by RNA polymerase II, but it is essential to stabilize transcription factor TFIIB in early elongation complexes. Proc Natl Acad Sci U S A. 2011;108:15786–91. doi: 10.1073/pnas.1104591108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilchrist DA, Adelman K. Coupling polymerase pausing and chromatin landscapes for precise regulation of transcription. Biochim Biophys Acta. 2012 doi: 10.1016/j.bbagrm.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chopra VS, Cande J, Hong JW, Levine M. Stalled Hox promoters as chromosomal boundaries. Genes Dev. 2009;23:1505–9. doi: 10.1101/gad.1807309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang G, Balamotis MA, Stevens JL, Yamaguchi Y, Handa H, Berk AJ. Mediator requirement for both recruitment and postrecruitment steps in transcription initiation. Mol Cell. 2005;17:683–94. doi: 10.1016/j.molcel.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Donner AJ, Ebmeier CC, Taatjes DJ, Espinosa JM. CDK8 is a positive regulator of transcriptional elongation within the serum response network. Nat Struct Mol Biol. 2010;17:194–201. doi: 10.1038/nsmb.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer KD, Lin SC, Bernecky C, Gao Y, Taatjes DJ. p53 activates transcription by directing structural shifts in Mediator. Nat Struct Mol Biol. 2010;17:753–60. doi: 10.1038/nsmb.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi H, Parmely TJ, Sato S, Tomomori-Sato C, Banks CA, Kong SE, et al. Human mediator subunit MED26 functions as a docking site for transcription elongation factors. Cell. 2011;146:92–104. doi: 10.1016/j.cell.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]