Abstract
Genome-wide chromatin profiling efforts have shown that enhancers are often located at large distances from gene promoters within the noncoding genome. Whereas enhancers can stimulate transcription initiation by communicating with promoters via chromatin looping mechanisms, we propose that enhancers may also stimulate transcription elongation by physical interactions with intronic elements. We review here recent findings derived from the study of the hematopoietic system.
Keywords: enhancer, Myb, chromatin looping, chromosome conformation capture, long-range interactions, transcription elongation, transcription regulatory networks
Introduction
The development of multicellular organisms relies upon the capacity of stem and progenitor cells to respond to their microenvironment and differentiate upon exposure to specific stimuli. This multi-step process involves complex epigenetic changes within regulatory transcriptional networks which contribute to the timely activation and repression of key developmental genes. Transcription of mammalian genes relies on the presence of a variety of cis-DNA regulatory sequences such as promoters and enhancers. Whereas gene promoters can be relatively easy to identify (i.e., at the 5′ end of transcriptional units), locating and characterizing enhancers is far more complicated. Transgenic experiments performed over the last few decades have taught us important lessons about transcriptional enhancers and genomic organization. Attempts to express transgenes in animals, under the control of endogenous promoter sequences, often resulted in weak expression, altered tissue specificity, and frequent transcriptional silencing after stable integration of the transgene into the genome. Efficient transgene expression (maintaining developmental transcription dynamics and expression levels) required the use of large genomic DNA sequences. Besides promoter elements, these large DNA fragments contained introns and sequences surrounding the genes. It was deduced that natural sequences surrounding the genes contain tissue-specific transcriptional enhancers. These findings suggest that promoters work in combination with additional regulatory sequences that may be remote from transcription start sites (TSS). Therefore the de novo identification of transcriptional enhancers is difficult since they show a great variety in sequence and localization with respect to their target genes.
The recent advances in high throughput sequencing technologies, such as ChIP Sequencing (ChIP-Seq), have allowed chromatin structure and transcription factor occupancy to be analyzed on a genome-wide scale. Enhancers have recently been defined as discrete genomic sites harboring a local combination of open chromatin structure (hypersensitivity to DNase I), specific covalent histone modifications like mono- and di-methylation of histone 3 lysine 4 (H3K4me1, H3K4me2), acetylation of H3K27, low levels of H3K4me3, and RNA polymerase (pol) II and transcription factor (TF) occupancy.1-3 Based on this epigenetic definition thousands of potential enhancers were localized genome-wide, of which some have been functionally validated in vivo.4 However, this definition of enhancers may not be sufficient to predict enhancer function in a spatio-temporal fashion during development. Furthermore, with the recent identification of 67 novel types of histone modifications,5 the typical enhancer signature is likely to evolve and reveal a high degree of complexity and diversity. Presently, the best way to identify these critical regulatory elements is by combining histone modification profiling with the binding of general and tissue-specific TFs.
The genome is big: why not just use all this space?
ChIP-Seq-based studies have determined the genome-wide binding sites of TFs with unprecedented ease and speed. Unexpectedly a highly complex picture of transcriptional regulatory networks (TRNs) has been revealed. Text-book examples of TF binding at promoters or near genes are in fact exceptional cases, with several studies showing that critical tissue-specific TFs bind at large distances from genes TSS either in intergenic regions or within introns. For example, the binding profiles of the essential hematopoietic factors GATA1, TAL1, LDB1 and RUNX1 show distal occupancy (intronic/intergenic) in up to 90% of the cases6–7. Binding sites are often localized several dozen to hundreds of kb from the nearest TSS, indicative of long-range transcriptional regulation. In addition, it appears that developmentally regulated genes may harbor multiple binding sites for the same TF complexes, raising the possibility that TF-bound regulatory elements may act in a cooperative and/or specialized fashion during development,6-9 underscoring the complexity of TRNs. Similar findings were reported for non-hematopoietic tissues,10 indicating that transcriptional regulatory elements are generally spread within the non-coding fraction of mammalian genomes. Importantly, enhancer location is clearly not restricted to the immediate vicinity of their cognate target genes as they may be found upstream, downstream, or within genes. Long-range transcriptional regulation by distal enhancers hence emerges as an important mechanism driving proper spatio-temporal regulation of gene expression during development.
In agreement with this observation, examination of genome-wide association studies revealed that mutations in non protein-coding genomic regions contribute to disease traits in up to 40% of the cases.11 For example, single nucleotide polymorphisms (SNPs) affecting the severity of the erythroid disorders β-thalassemia and sickle cell anemia were found within the HBS1L-MYB and BCL11A loci.12 The causative SNPs fall into intergenic and intronic regions, respectively, up to 80 kb away from the gene promoters. An intronic SNP within the HERC2 gene has recently been linked to the regulation of the downstream OCA2 gene which is involved in human pigmentation.13 One of the most extreme examples is the location of an enhancer 1 Mb upstream from the Sonic Hedgehog SHH gene, within an intron of the unrelated LMBR1 gene.14 SNPs were found in this region in humans and shown to affect spatio-temporal SHH expression resulting in the congenital abnormality preaxial polydactyly, one of the most frequently observed hand malformations.15 These intriguing findings reveal the incredible functional complexity of the noncoding genome, with intergenic, intronic, and even gene desert areas16,17 having the potential to play critical roles in gene regulatory networks both in development and disease. This raises the question of how mammalian genome organization relates to transcriptional regulation, and how this organization dynamically changes during cellular differentiation to allow distal enhancers to regulate transcription over large distances in vivo.
Long-range transcription regulation by chromatin looping
New insights derived from ChIP-Seq analyses have provided a very detailed view of the regulatory potential of the genome although it is restrained to a linear perspective. Functional genomics studies are now facing the challenge of linking distal enhancers to their cognate genes, functionally dissecting enhancer-gene relationships, and understanding the impact of noncoding sequence variations in disease. The current dominant model for long-range transcriptional regulation proposes that distal enhancers are brought into physical proximity to their target genes in the three-dimensional nuclear space by chromatin looping mechanisms. The analysis of such spatial organization has been made possible thanks to the development of Chromosome Conformation Capture (3C) technology18 and its high throughput derivatives (4C, 5C, 3C-Seq, HiC).19 3C allows measuring the interaction frequency between two distal DNA elements and thereby provides information about local genomic topology and chromatin looping. 3C was originally used to study chromosome conformation in yeast18 and the regulation of the β-globin gene cluster by distal regulatory elements during erythroid development.20 We recently developed 3C-Seq technology which couples chromosome conformation capture to high throughput sequencing.6,9 3C-Seq measures interaction frequencies between a viewpoint (a DNA fragment of choice, e.g., gene promoter) and (distal) regulatory elements on a genome-wide scale. We recently used 3C-Seq for the unbiased analysis of the spatial organization of the Myb proto-oncogene locus in erythroid cells.9 Myb is a critical hematopoietic regulator required for the proliferation and expansion of all blood progenitors, and is dramatically downregulated during terminal differentiation. Failure to silence Myb expression is linked to impaired differentiation and may play a key role in leukemogenesis.21 We showed that Myb transcription is regulated by an array of distal intergenic enhancers localizing up to 109kb upstream of the gene, which are occupied by the essential hematopoietic TFs GATA1, TAL1, and LDB1. 3C-Seq profiling revealed that the enhancers loop to the Myb gene when it is transcriptionally active, forming an active chromatin hub resembling the one detected on the β-globin locus. Importantly, the spatial organization of the locus is highly dynamic. During terminal differentiation the active chromatin hub is destabilized and the enhancers no longer loop to the Myb gene, a feature correlating with a loss of TF occupancy at the distal sites, and a loss of transcriptional activity of the locus (Fig. 1).9 This and earlier studies suggest that dynamic chromatin looping and changes in spatial organization represent important features within gene regulatory networks.9,16,20,22-24

Figure 1. Transcription factor occupancy and three-dimensional structure of the Myb locus. (A) ChIP-Seq profiles of CTCF (red), LDB1 (blue) and KLF1 (gray) at the Myb-Hbs1l intergenic region. A schematic of the area including all TF-binding sites and their distance relative to Myb TSS is shown. (B) Spatial organization of the Myb locus in erythroid cells. On top, a linear schematic of the locus is shown (as in A), with the looping events toward the promoter summarized by the gray arrow. Below, the actual model of the three-dimensional conformation of the locus in vivo is shown, for both erythroid progenitors (expressing Myb) and differentiated erythroid cells (silencing Myb expression).
Chromatin loop formation and maintenance
The mechanisms by which chromatin loops are specifically established or maintained remain unclear. Furthermore, whether chromatin looping is a cause or a consequence of gene activity remains unknown. However, it is clear that chromatin loops depend on the local binding of structural and transcriptional regulatory factors. Structural proteins such as CTCF and Cohesin have been shown to participate in three-dimensional genomic interactions.25-27 For instance, both CTCF and Cohesin were shown to be crucial for imprinting at the H19/IGF2 locus28; a locus subjected to long-range regulation by differential looping. It is worth noting that CTCF is also well known for its enhancer-blocking function, and as such can limit the range of activity of nearby enhancers.25,29 Within the immunoglobulin к light chain locus (Igк), conditional inactivation of the Ctcf gene in pre-B cells results in increased usage of the proximal Vк-3 gene family, which is rarely used in normal B cells. Increased Vк-3 genes usage correlates with increased interaction between the Igк locus enhancers and the Vк-3 genes in the absence of CTCF, suggesting that CTCF drives the specificity of enhancer-genes contacts at the Igк locus.26 The absence of CTCF has been linked to disruption of loop formation at several other developmentally regulated loci. For instance, targeted disruption of a CTCF binding motif in the β-globin locus 3′HS1 element, abolishing CTCF binding, disrupts local loop formation.27 The Cohesin complex has also been linked to higher order chromatin structure formation and/or maintenance and it was shown that depletion of the Cohesin complex subunit Smc1 resulted in reduced enhancer-promoter loop formation at the Nanog locus in ES cells.30 In addition, TFs were also shown to play a role in long-range gene regulation, e.g., the hematopoietic TFs LDB1, GATA1, FOG1, KLF1 and BCL11A are required to maintain chromatin looping within the β-globin, Myb and other loci.9,22,31-34 Differential enhancer-gene looping correlating with gene expression was also observed at the Kit oncogene locus. Kit expression in hematopoietic progenitors is controlled by a distal enhancer -114kb upstream of the gene which is occupied by GATA2 TF complexes and loops to the kit gene when transcriptionally active. At the onset of terminal differentiation, the GATA2 complexes are replaced by GATA1-nucleated complexes, correlating with a spatial reorganization of the locus, a modification of enhancer-gene interactions, and a loss of Kit expression.22 These findings emphasize that complex interplay between regulatory factors binding to distal enhancers takes place during development, and suggest that the dynamic and timely establishment of higher order chromatin structures is involved in establishing and maintaining transcriptional regulatory networks.
Regulation of transcriptional elongation by distal enhancers
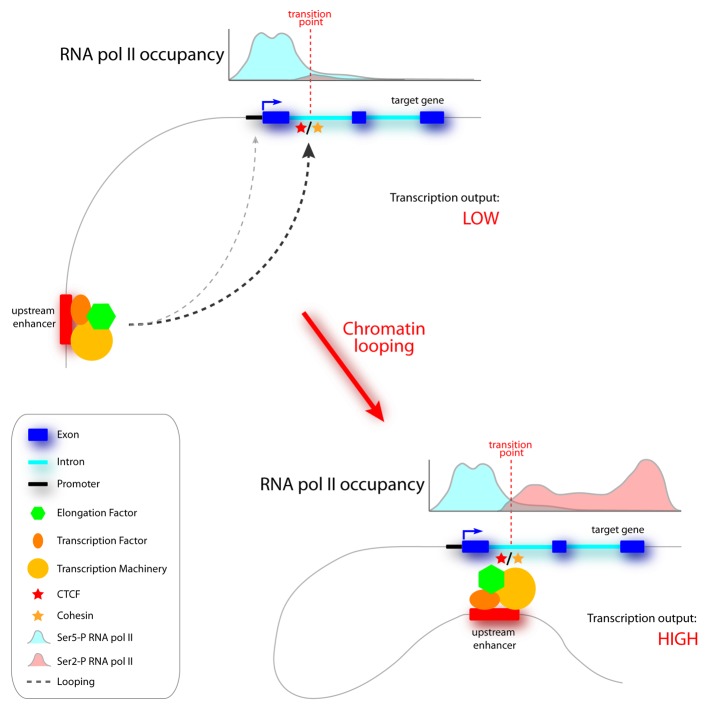
Despite detailed information from a number of model loci,9,20,23,25 higher order chromatin structure and local genomic reorganization upon signaling remain, for the majority of genes poorly understood or even completely uncharacterized. Importantly, the functional relationship between distal enhancer-gene interactions and transcriptional activity is still a matter of debate. The prevalent model is that distal enhancers loop to target gene promoters where they stimulate transcription by providing an increased local concentration of positive acting factors.25,30 However, this model does not apply to all cases. Sometimes distal enhancers show a preferential interaction with the transcribed part of their target genes (e.g., at intronic sites) rather than at promoter regions.9,22 These observations raise questions regarding the functionality of such enhancer-gene contacts. We recently showed that the -81kb Myb enhancer preferentially associates with the first intron of the gene.. This region is strongly occupied by CTCF and was previously shown to harbor an ‘attenuator’ site regulating transcription elongation.21 Accordingly, we demonstrated that this region represents the site where RNA pol II switches from the initiating to the elongating form, as characterized by phosphorylation of serine (Ser) residues 5 and 2. The appearance of transcription elongation-associated chromatin marks (e.g., H3K36me3) also occurs just downstream of the intronic CTCF site.9 However, both this site and the Myb promoter harbor only minor quantities of the positive elongation factor CDK9, a kinase involved in the phosphorylation of RNA pol II Ser2, which regulates transcription elongation. Instead, strong enrichments of CDK9 and an additional positive elongation factor, Tif1γ, were found at the upstream regulatory sites, including the -81kb enhancer. We proposed a model where RNA pol II stalls at Myb intron 1, close to the CTCF site, and requires stimulatory activity from the distal enhancers to bypass the attenuator element. Interestingly, when erythroid cells were treated with the CDK9 kinase inhibitor DRB, and transcription elongation was inhibited, distal enhancers still looped to Myb intron 1. This suggests that the loops became non-functional due to their inability to provide kinase activity. Intriguingly, our unpublished observations suggest that this mechanism also operates on other developmentally regulated genes in erythroid cells (van den Heuvel, Kolovos et al. unpublished). Furthermore, previous experiments have shown that the β-globin LCR controls high level globin transcription primarily through a stimulatory effect on transcription elongation.35 Similar to the Myb upstream regulatory elements, the LCR was highly enriched for positive elongation factors, while proximal promoter sequences showed less binding of these factors.36 Together, these data suggest that the function of at least a subclass of distal enhancers may be to provide direct local stimulation of transcription elongation (Fig. 2). In support of this view, a recent genome-wide histone modification profiling study, performed in differentiating erythroid cells, suggested that the regulation of transcription elongation plays a key role in gene induction and repression processes during cellular differentiation.37 Future investigations will reveal whether direct transcription elongation stimulation by enhancers is a general mechanism.
Figure 2. Speculative model of enhancer-mediated long-range stimulation of transcription elongation. The upper half shows a model gene with an upstream enhancer occupied by transcription factors, elongations factors and the transcription machinery. In the absence of chromatin looping, expression of the gene is kept low due to inefficient transcriptional elongation. Enhancer looping toward the gene results in the stimulation of elongation by increased RNA pol II Ser 2 phosphorylation and high level gene expression. Structural factors involved in chromatin looping (i.e., CTCF and/or Cohesin, depicted by star symbols) possibly contribute to establishing local enhancer-gene communication.
Important technical challenges and remaining questions
Since 3C-based technologies only provide topological information, their functional relevance should be interpreted with caution and needs to be supported by additional experiments. These experiments typically aim at correlating gene expression and TF occupancy with chromatin looping dynamics but assessing the functionality of a looping event remains a difficult task. One way to address this question is to generate mutant alleles and conditional enhancer deletions to address their roles in vivo, and to selectively disrupt specific loop formation.8,27 In the case of genes controlled by multiple regulatory elements (e.g., Myb), this will show whether transcriptional activity directly depends on all the active regulatory elements or whether there are specific elements and/or subsets driving stage-specific high level expression. Despite the availability of high throughput recombineering technologies, such approaches remain laborious and time consuming.
It remains a challenge to obtain a broader picture of genomic architecture with sufficient resolution to visualize individual enhancer-gene contacts, and explore the correlation with gene transcription. Several new technological developments have provided new possibilities with approaches such as HiC allowing the capture of the genomic “loop-ome” or ChIA-PET which allows the detection of genome-wide loop formation nucleated by specific transcription factors (for review see ref. 18). Defining the nuclear architecture, its dependence on regulatory factors and its impact on gene expression remains an important challenge in the field of functional genomics. However, it is likely to highlight key features which will grant us with a superior understanding of the regulatory role of the noncoding genome. One of the major challenges in this field may be to decipher the mechanism by which long-range interactions can switch a stalled to an elongating form of polymerase.
Concluding remarks
The current genome-wide characterizations of enhancers provide a picture of increasing complexity and diversity in both enhancer structure and function.25,38 We expect that different classes of enhancers will fulfill specific functions, such as facilitating transcriptional pause release or enhancing transcription elongation. The presence of multiple enhancers at single gene loci suggests that subsets of functionally specialized enhancers may provide a means to precisely drive transcription during specific developmental windows within specific lineages. Analyzing transcriptional regulation in both time and space emphasized the highly dynamic nature of the genome, which has recently been compared with a “regulatory jungle”17, bearing “regulatory archipelagos.”16 The rules governing the genomic regulatory landscape in its incredible complexity are only now just being discovered. Understanding the interplay between distal enhancers, their target genes, and their individual roles within complex genetic loci will remain a major task both in basic and disease-driven research.
Acknowledgments
We are thankful to the members of the FG laboratory for helpful comments and discussions. ES is supported by grants from the Netherlands Genomics Initiative (Horizon breakthrough) and the Dutch Cancer Genomics Center.
Glossary
Abbreviations:
- 3C
Chromosome conformation capture
- ChIP-Seq
Chromatin immunoprecipitation coupled to high throughput sequencing
- Igк
Immunoglobulin к light chain locus
- Ser
Serine
- SNP
Single nucleotide polymorphism
- TF
Transcription factor
- TRN
Transcription regulatory network, TSS, Transcription start site
- RNA pol II
RNA polymerase II
Footnotes
Previously published online: www.landesbioscience.com/journals/transcription/article/20720
References
- 1.Pekowska A, Benoukraf T, Zacarias-Cabeza J, Belhocine M, Koch F, Holota H, et al. H3K4 tri-methylation provides an epigenetic signature of active enhancers. EMBO J. 2011;30:4198–210. doi: 10.1038/emboj.2011.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF, et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459:108–12. doi: 10.1038/nature07829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koch F, Andrau JC. Initiating RNA polymerase II and TIPs as hallmarks of enhancer activity and tissue-specificity. Transcription. 2011;2:263–8. doi: 10.4161/trns.2.6.18747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visel A, Blow MJ, Li Z, Zhang T, Akiyama JA, Holt A, et al. ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature. 2009;457:854–8. doi: 10.1038/nature07730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan M, Luo H, Lee S, Jin F, Yang JS, Montellier E, et al. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell. 2011;146:1016–28. doi: 10.1016/j.cell.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soler E, Andrieu-Soler C, de Boer E, Bryne JC, Thongjuea S, Stadhouders R, et al. The genome-wide dynamics of the binding of Ldb1 complexes during erythroid differentiation. Genes Dev. 2010;24:277–89. doi: 10.1101/gad.551810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson NK, Foster SD, Wang X, Knezevic K, Schütte J, Kaimakis P, et al. Combinatorial transcriptional control in blood stem/progenitor cells: genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell. 2010;7:532–44. doi: 10.1016/j.stem.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Snow JW, Trowbridge JJ, Fujiwara T, Emambokus NE, Grass JA, Orkin SH, et al. A single cis element maintains repression of the key developmental regulator Gata2. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stadhouders R, Thongjuea S, Andrieu-Soler C, Palstra RJ, Bryne JC, van den Heuvel A, et al. Dynamic long-range chromatin interactions control Myb proto-oncogene transcription during erythroid development. EMBO J. 2012;31:986–99. doi: 10.1038/emboj.2011.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt D, Wilson MD, Ballester B, Schwalie PC, Brown GD, Marshall A, et al. Five-vertebrate ChIP-seq reveals the evolutionary dynamics of transcription factor binding. Science. 2010;328:1036–40. doi: 10.1126/science.1186176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visel A, Rubin EM, Pennacchio LA. Genomic views of distant-acting enhancers. Nature. 2009;461:199–205. doi: 10.1038/nature08451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thein SL, Menzel S, Lathrop M, Garner C. Control of fetal hemoglobin: new insights emerging from genomics and clinical implications. Hum Mol Genet. 2009;18(R2):R216–23. doi: 10.1093/hmg/ddp401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visser M, Kayser M, Palstra RJ. HERC2 rs12913832 modulates human pigmentation by attenuating chromatin-loop formation between a long-range enhancer and the OCA2 promoter. Genome Res. 2012;22:446–55. doi: 10.1101/gr.128652.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sagai T, Hosoya M, Mizushina Y, Tamura M, Shiroishi T. Elimination of a long-range cis-regulatory module causes complete loss of limb-specific Shh expression and truncation of the mouse limb. Development. 2005;132:797–803. doi: 10.1242/dev.01613. [DOI] [PubMed] [Google Scholar]
- 15.Lettice LA, Heaney SJ, Purdie LA, Li L, de Beer P, Oostra BA, et al. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum Mol Genet. 2003;12:1725–35. doi: 10.1093/hmg/ddg180. [DOI] [PubMed] [Google Scholar]
- 16.Montavon T, Soshnikova N, Mascrez B, Joye E, Thevenet L, Splinter E, et al. A regulatory archipelago controls Hox genes transcription in digits. Cell. 2011;147:1132–45. doi: 10.1016/j.cell.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Ruf S, Symmons O, Uslu VV, Dolle D, Hot C, Ettwiller L, et al. Large-scale analysis of the regulatory architecture of the mouse genome with a transposon-associated sensor. Nat Genet. 2011;43:379–86. doi: 10.1038/ng.790. [DOI] [PubMed] [Google Scholar]
- 18.Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295:1306–11. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- 19.de Wit E, de Laat W. A decade of 3C technologies: insights into nuclear organization. Genes Dev. 2012;26:11–24. doi: 10.1101/gad.179804.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palstra RJ, Tolhuis B, Splinter E, Nijmeijer R, Grosveld F, de Laat W. The beta-globin nuclear compartment in development and erythroid differentiation. Nat Genet. 2003;35:190–4. doi: 10.1038/ng1244. [DOI] [PubMed] [Google Scholar]
- 21.Ramsay RG, Gonda TJ. MYB function in normal and cancer cells. Nat Rev Cancer. 2008;8:523–34. doi: 10.1038/nrc2439. [DOI] [PubMed] [Google Scholar]
- 22.Jing H, Vakoc CR, Ying L, Mandat S, Wang H, Zheng X, et al. Exchange of GATA factors mediates transitions in looped chromatin organization at a developmentally regulated gene locus. Mol Cell. 2008;29:232–42. doi: 10.1016/j.molcel.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noordermeer D, Leleu M, Splinter E, Rougemont J, De Laat W, Duboule D. The dynamic architecture of Hox gene clusters. Science. 2011;334:222–5. doi: 10.1126/science.1207194. [DOI] [PubMed] [Google Scholar]
- 24.Kolovos P, Knoch TA, Grosveld FG, Cook PR, Papantonis A. Enhancers and silencers: an integrated and simple model for their function. Epigenetics Chromatin. 2012;5:1. doi: 10.1186/1756-8935-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ong CT, Corces VG. Enhancer function: new insights into the regulation of tissue-specific gene expression. Nat Rev Genet. 2011;12:283–93. doi: 10.1038/nrg2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribeiro de Almeida C, Stadhouders R, de Bruijn MJ, Bergen IM, Thongjuea S, Lenhard B, et al. The DNA-binding protein CTCF limits proximal Vκ recombination and restricts κ enhancer interactions to the immunoglobulin κ light chain locus. Immunity. 2011;35:501–13. doi: 10.1016/j.immuni.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Splinter E, Heath H, Kooren J, Palstra RJ, Klous P, Grosveld F, et al. CTCF mediates long-range chromatin looping and local histone modification in the beta-globin locus. Genes Dev. 2006;20:2349–54. doi: 10.1101/gad.399506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wendt KS, Yoshida K, Itoh T, Bando M, Koch B, Schirghuber E, et al. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature. 2008;451:796–801. doi: 10.1038/nature06634. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro de Almeida C, Stadhouders R, Thongjuea S, Soler E, Hendriks RW. DNA-binding factor CTCF and long-range gene interactions in V(D)J recombination and oncogene activation. Blood. 2012 doi: 10.1182/blood-2012-03-402586. [DOI] [PubMed] [Google Scholar]
- 30.Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–5. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drissen R, Palstra RJ, Gillemans N, Splinter E, Grosveld F, Philipsen S, et al. The active spatial organization of the beta-globin locus requires the transcription factor EKLF. Genes Dev. 2004;18:2485–90. doi: 10.1101/gad.317004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song SH, Hou C, Dean A. A positive role for NLI/Ldb1 in long-range beta-globin locus control region function. Mol Cell. 2007;28:810–22. doi: 10.1016/j.molcel.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vakoc CR, Letting DL, Gheldof N, Sawado T, Bender MA, Groudine M, et al. Proximity among distant regulatory elements at the beta-globin locus requires GATA-1 and FOG-1. Mol Cell. 2005;17:453–62. doi: 10.1016/j.molcel.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 34.Xu J, Sankaran VG, Ni M, Menne TF, Puram RV, Kim W, et al. Transcriptional silencing of gamma-globin by BCL11A involves long-range interactions and cooperation with SOX6. Genes Dev. 2010;24:783–98. doi: 10.1101/gad.1897310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawado T, Halow J, Bender MA, Groudine M. The beta -globin locus control region (LCR) functions primarily by enhancing the transition from transcription initiation to elongation. Genes Dev. 2003;17:1009–18. doi: 10.1101/gad.1072303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song SH, Kim A, Ragoczy T, Bender MA, Groudine M, Dean A. Multiple functions of Ldb1 required for beta-globin activation during erythroid differentiation. Blood. 2010;116:2356–64. doi: 10.1182/blood-2010-03-272252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong P, Hattangadi SM, Cheng AW, Frampton GM, Young RA, Lodish HF. Gene induction and repression during terminal erythropoiesis are mediated by distinct epigenetic changes. Blood. 2011;118:e128–38. doi: 10.1182/blood-2011-03-341404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bulger M, Groudine M. Functional and mechanistic diversity of distal transcription enhancers. Cell. 2011;144:327–39. doi: 10.1016/j.cell.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]