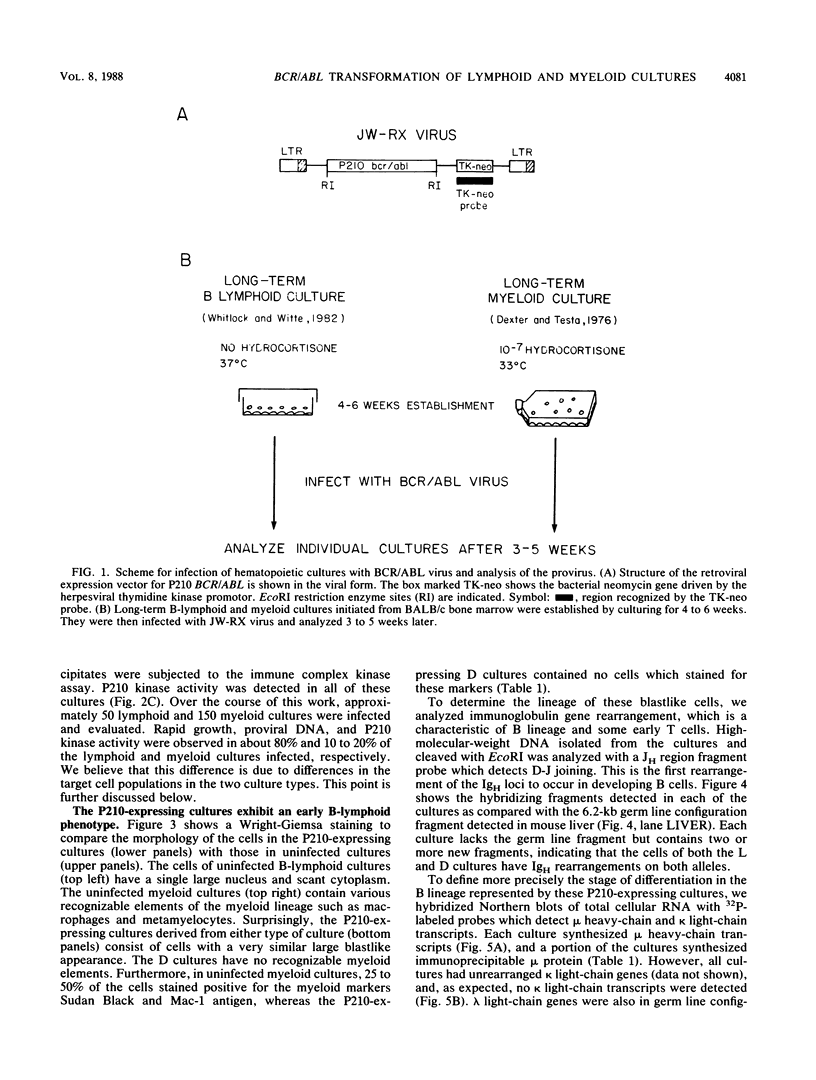
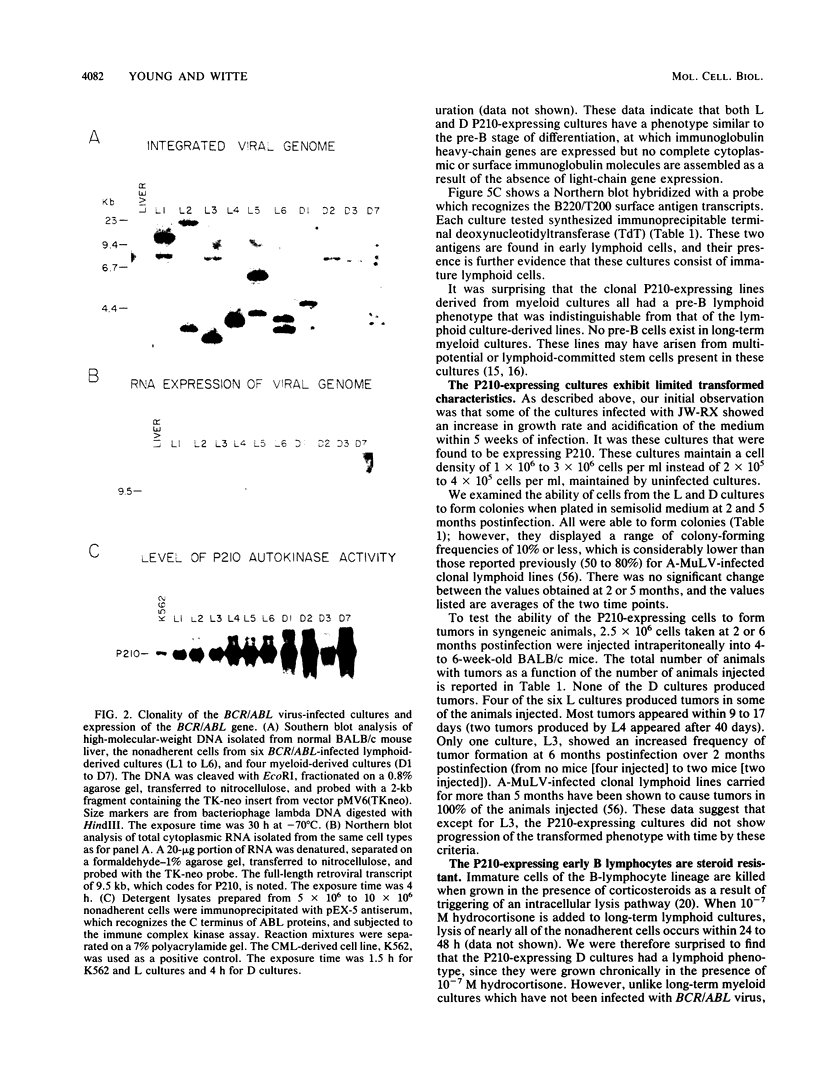
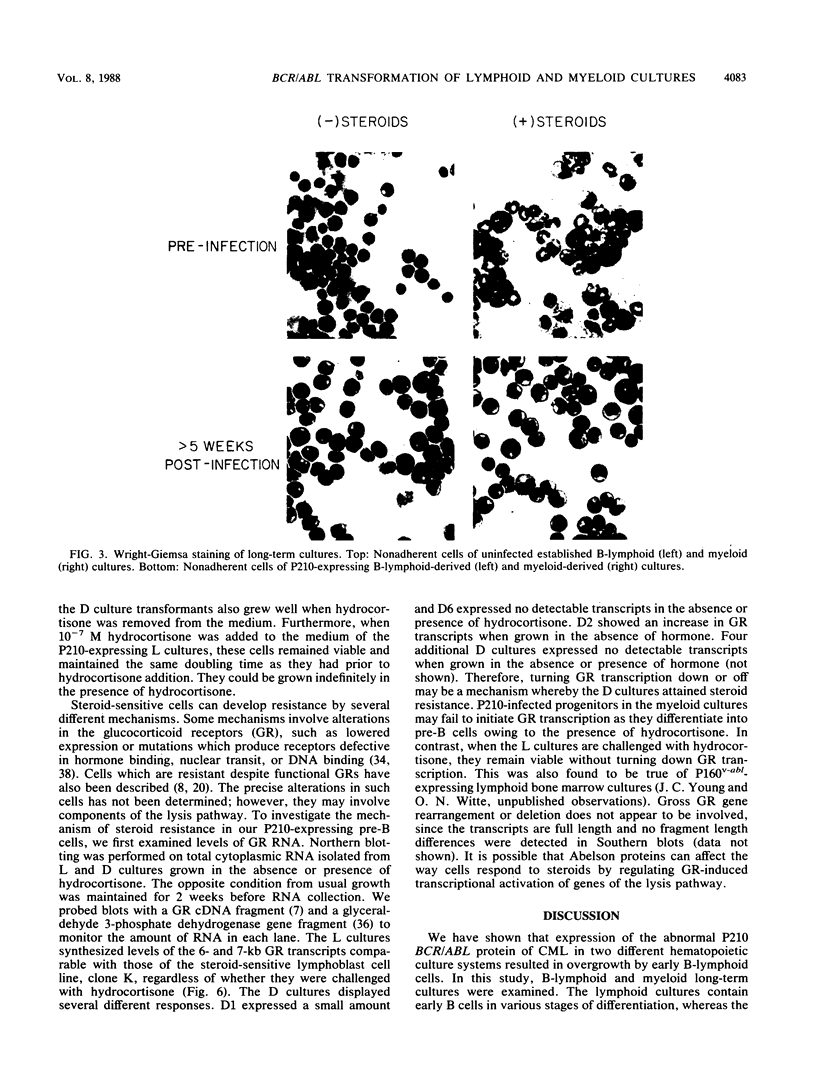
Abstract
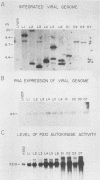
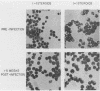
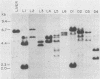
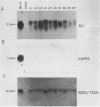
The BCR/ABL gene, formed by the Philadelphia chromosome translocation (Ph1) of human chronic myelogenous leukemia, encodes an altered ABL gene product, P210. P210 is strongly implicated in the malignant process of chronic myelogenous leukemia, but it precise role is unknown. Infection of long-term bone marrow cultures enriched for B-lymphoid cell types with a Moloney murine leukemia virus retroviral vector containing the BCR/ABL cDNA resulted in clonal outgrowths of immature B-lymphoid cells which expressed abundant P210 kinase activity. Surprisingly, infection of long-term myeloid lineage-enriched cultures also resulted in clonal outgrowths of immature B-lymphoid cells. The P210-expressing lymphoid cell lines resulting from either type of culture were resistant to the lethal effects of corticosteroids. These findings indicate that high levels of P210 expressed from a Moloney murine leukemia virus long terminal repeat preferentially stimulate the growth of immature B-lineage cells, and this effect is apparent even in myeloid lineage-enriched cultures, in which few if any lymphoid cells can be detected prior to infection.
Full text
PDF

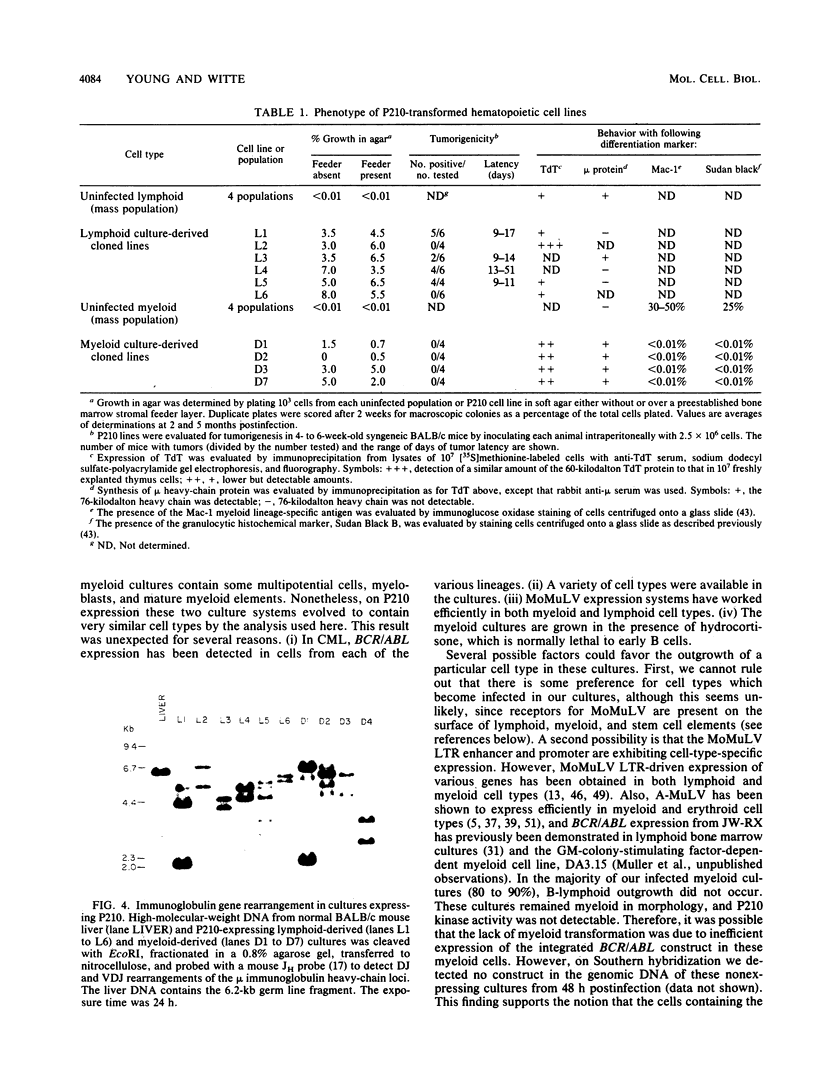
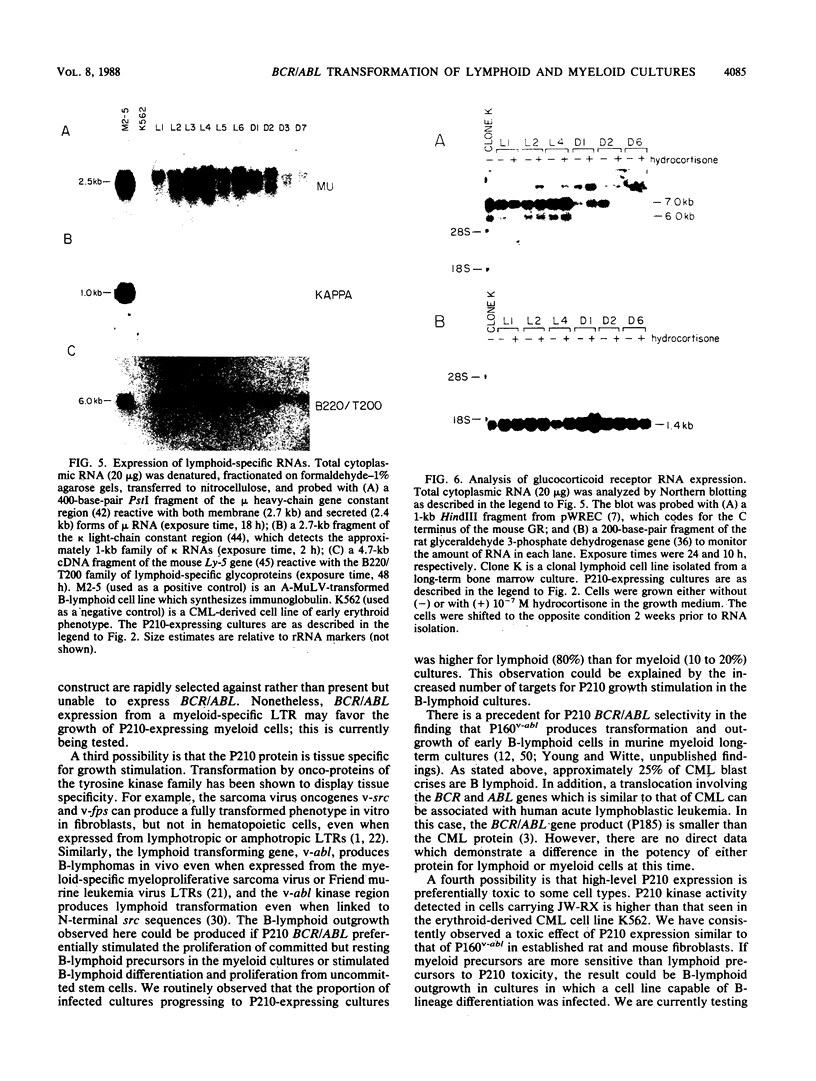


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boettiger D., Anderson S., Dexter T. M. Effect of src infection on long-term marrow cultures: increased self-renewal of hemopoietic progenitor cells without leukemia. Cell. 1984 Mar;36(3):763–773. doi: 10.1016/0092-8674(84)90356-8. [DOI] [PubMed] [Google Scholar]
- Champlin R. E., Golde D. W. Chronic myelogenous leukemia: recent advances. Blood. 1985 May;65(5):1039–1047. [PubMed] [Google Scholar]
- Clark S. S., McLaughlin J., Timmons M., Pendergast A. M., Ben-Neriah Y., Dow L. W., Crist W., Rovera G., Smith S. D., Witte O. N. Expression of a distinctive BCR-ABL oncogene in Ph1-positive acute lymphocytic leukemia (ALL). Science. 1988 Feb 12;239(4841 Pt 1):775–777. doi: 10.1126/science.3422516. [DOI] [PubMed] [Google Scholar]
- Collins S., Coleman H., Groudine M. Expression of bcr and bcr-abl fusion transcripts in normal and leukemic cells. Mol Cell Biol. 1987 Aug;7(8):2870–2876. doi: 10.1128/mcb.7.8.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D., Metcalf D., Nicola N. A., Burgess A. W., Walker F. Malignant transformation of a growth factor-dependent myeloid cell line by Abelson virus without evidence of an autocrine mechanism. Cell. 1985 Jul;41(3):677–683. doi: 10.1016/s0092-8674(85)80048-9. [DOI] [PubMed] [Google Scholar]
- Daley G. Q., McLaughlin J., Witte O. N., Baltimore D. The CML-specific P210 bcr/abl protein, unlike v-abl, does not transform NIH/3T3 fibroblasts. Science. 1987 Jul 31;237(4814):532–535. doi: 10.1126/science.2440107. [DOI] [PubMed] [Google Scholar]
- Danielsen M., Northrop J. P., Ringold G. M. The mouse glucocorticoid receptor: mapping of functional domains by cloning, sequencing and expression of wild-type and mutant receptor proteins. EMBO J. 1986 Oct;5(10):2513–2522. doi: 10.1002/j.1460-2075.1986.tb04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbre P. D., King R. J. Progression to steroid insensitivity can occur irrespective of the presence of functional steroid receptors. Cell. 1987 Nov 20;51(4):521–528. doi: 10.1016/0092-8674(87)90121-8. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Konopka J. B., Witte O. N. Activation of the c-abl oncogene by viral transduction or chromosomal translocation generates altered c-abl proteins with similar in vitro kinase properties. Mol Cell Biol. 1985 Jan;5(1):204–213. doi: 10.1128/mcb.5.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis K. A., Witte O. N. In vitro development of B lymphocytes from long-term cultured precursor cells. Proc Natl Acad Sci U S A. 1986 Jan;83(2):441–445. doi: 10.1073/pnas.83.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Lajtha L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977 Jun;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Teich N. M. Modification of the proliferative and differentiation capacity of stem cells following treatment with chemical and viral leukemogens. Haematol Blood Transfus. 1979;23:223–229. doi: 10.1007/978-3-642-67057-2_28. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Testa N. G. Differentiation and proliferation of hemopoietic cells in culture. Methods Cell Biol. 1976;14:387–405. doi: 10.1016/s0091-679x(08)60498-7. [DOI] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Dorshkind K. In vitro differentiation of B lymphocytes from primitive hemopoietic precursors present in long-term bone marrow cultures. J Immunol. 1986 Jan;136(2):422–429. [PubMed] [Google Scholar]
- Dorshkind K., Phillips R. A. Characterization of early B lymphocyte precursors present in long-term bone marrow cultures. J Immunol. 1983 Nov;131(5):2240–2245. [PubMed] [Google Scholar]
- Dorshkind K., Phillips R. A. Maturational state of lymphoid cells in long-term bone marrow cultures. J Immunol. 1982 Dec;129(6):2444–2450. [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J., Martin P. J., Najfeld V., Penfold G. K., Jacobson R. J., Hansen J. A. Evidence for a multistep pathogenesis of chronic myelogenous leukemia. Blood. 1981 Jul;58(1):158–163. [PubMed] [Google Scholar]
- Gasson J. C., Bourgeois S. A new determinant of glucocorticoid sensitivity in lymphoid cell lines. J Cell Biol. 1983 Feb;96(2):409–415. doi: 10.1083/jcb.96.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. L., Kaehler D. A., McKearn J., Risser R. Substitution of the LTR of Abelson murine leukemia virus does not alter the cell type of virally induced tumors. Oncogene. 1988 Jun;2(6):585–592. [PubMed] [Google Scholar]
- Kahn P., Frykberg L., Brady C., Stanley I., Beug H., Vennström B., Graf T. v-erbA cooperates with sarcoma oncogenes in leukemic cell transformation. Cell. 1986 May 9;45(3):349–356. doi: 10.1016/0092-8674(86)90320-x. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Clark S., McLaughlin J., Nitta M., Kato Y., Strife A., Clarkson B., Witte O. N. Variable expression of the translocated c-abl oncogene in Philadelphia-chromosome-positive B-lymphoid cell lines from chronic myelogenous leukemia patients. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4049–4052. doi: 10.1073/pnas.83.11.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka J. B., Davis R. L., Watanabe S. M., Ponticelli A. S., Schiff-Maker L., Rosenberg N., Witte O. N. Only site-directed antibodies reactive with the highly conserved src-homologous region of the v-abl protein neutralize kinase activity. J Virol. 1984 Jul;51(1):223–232. doi: 10.1128/jvi.51.1.223-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Ku G., Witte O. N. Corticosteroid-resistant bone marrow-derived B lymphocyte progenitor for long term in vitro cultures. J Immunol. 1986 Nov 1;137(9):2802–2807. [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Liu E., Hjelle B., Bishop J. M. Transforming genes in chronic myelogenous leukemia. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1952–1956. doi: 10.1073/pnas.85.6.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddon P. J., Littman D. R., Godfrey M., Maddon D. E., Chess L., Axel R. The isolation and nucleotide sequence of a cDNA encoding the T cell surface protein T4: a new member of the immunoglobulin gene family. Cell. 1985 Aug;42(1):93–104. doi: 10.1016/s0092-8674(85)80105-7. [DOI] [PubMed] [Google Scholar]
- Martin P. J., Najfeld V., Fialkow P. J. B-lymphoid cell involvement in chronic myelogenous leukemia: implications for the pathogenesis of the disease. Cancer Genet Cytogenet. 1982 Aug;6(4):359–368. doi: 10.1016/0165-4608(82)90092-9. [DOI] [PubMed] [Google Scholar]
- Mathey-Prevot B., Baltimore D. Recombinants within the tyrosine kinase region of v-abl and v-src identify a v-abl segment that confers lymphoid specificity. Mol Cell Biol. 1988 Jan;8(1):234–240. doi: 10.1128/mcb.8.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J., Chianese E., Witte O. N. In vitro transformation of immature hematopoietic cells by the P210 BCR/ABL oncogene product of the Philadelphia chromosome. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6558–6562. doi: 10.1073/pnas.84.18.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mes-Masson A. M., McLaughlin J., Daley G. Q., Paskind M., Witte O. N. Overlapping cDNA clones define the complete coding region for the P210c-abl gene product associated with chronic myelogenous leukemia cells containing the Philadelphia chromosome. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9768–9772. doi: 10.1073/pnas.83.24.9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Sieburg C. E., Whitlock C. A., Weissman I. L. Isolation of two early B lymphocyte progenitors from mouse marrow: a committed pre-pre-B cell and a clonogenic Thy-1-lo hematopoietic stem cell. Cell. 1986 Feb 28;44(4):653–662. doi: 10.1016/0092-8674(86)90274-6. [DOI] [PubMed] [Google Scholar]
- Northrop J. P., Danielsen M., Ringold G. M. Analysis of glucocorticoid unresponsive cell variants using a mouse glucocorticoid receptor complementary DNA clone. J Biol Chem. 1986 Aug 25;261(24):11064–11070. [PubMed] [Google Scholar]
- Pendergast A. M., Traugh J. A., Witte O. N. Normal cellular and transformation-associated abl proteins share common sites for protein kinase C phosphorylation. Mol Cell Biol. 1987 Dec;7(12):4280–4289. doi: 10.1128/mcb.7.12.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechaczyk M., Blanchard J. M., Marty L., Dani C., Panabieres F., El Sabouty S., Fort P., Jeanteur P. Post-transcriptional regulation of glyceraldehyde-3-phosphate-dehydrogenase gene expression in rat tissues. Nucleic Acids Res. 1984 Sep 25;12(18):6951–6963. doi: 10.1093/nar/12.18.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. H., Di Fiore P. P., Aaronson S. A., Potter M., Pumphrey J., Scott A., Ihle J. N. Neoplastic transformation of mast cells by Abelson-MuLV: abrogation of IL-3 dependence by a nonautocrine mechanism. Cell. 1985 Jul;41(3):685–693. doi: 10.1016/s0092-8674(85)80049-0. [DOI] [PubMed] [Google Scholar]
- Rabindran S. K., Danielsen M., Stallcup M. R. Glucocorticoid-resistant lymphoma cell variants that contain functional glucocorticoid receptors. Mol Cell Biol. 1987 Dec;7(12):4211–4217. doi: 10.1128/mcb.7.12.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke W. C., Baird S., Ralph P., Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978 Sep;15(1):261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- Rennick D., Yang G., Gemmell L., Lee F. Control of hemopoiesis by a bone marrow stromal cell clone: lipopolysaccharide- and interleukin-1-inducible production of colony-stimulating factors. Blood. 1987 Feb;69(2):682–691. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rogers J., Early P., Carter C., Calame K., Bond M., Hood L., Wall R. Two mRNAs with different 3' ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980 Jun;20(2):303–312. doi: 10.1016/0092-8674(80)90616-9. [DOI] [PubMed] [Google Scholar]
- Schwartz R. C., Stanton L. W., Riley S. C., Marcu K. B., Witte O. N. Synergism of v-myc and v-Ha-ras in the in vitro neoplastic progression of murine lymphoid cells. Mol Cell Biol. 1986 Sep;6(9):3221–3231. doi: 10.1128/mcb.6.9.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman J. G., Leder P. The arrangement and rearrangement of antibody genes. Nature. 1978 Dec 21;276(5690):790–795. doi: 10.1038/276790a0. [DOI] [PubMed] [Google Scholar]
- Shen F. W., Saga Y., Litman G., Freeman G., Tung J. S., Cantor H., Boyse E. A. Cloning of Ly-5 cDNA. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7360–7363. doi: 10.1073/pnas.82.21.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short M. K., Okenquist S. A., Lenz J. Correlation of leukemogenic potential of murine retroviruses with transcriptional tissue preference of the viral long terminal repeats. J Virol. 1987 Apr;61(4):1067–1072. doi: 10.1128/jvi.61.4.1067-1072.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtivelman E., Gale R. P., Dreazen O., Berrebi A., Zaizov R., Kubonishi I., Miyoshi I., Canaani E. bcr-abl RNA in patients with chronic myelogenous leukemia. Blood. 1987 Mar;69(3):971–973. [PubMed] [Google Scholar]
- Silverstone A., Sun L., Witte O. N., Baltimore D. Biosynthesis of murine terminal deoxynucleotidyltransferase. J Biol Chem. 1980 Jan 25;255(2):791–796. [PubMed] [Google Scholar]
- Smith L. J., Benchimol S. Introduction of new genetic material into human myeloid leukemic blast stem cells by retroviral infection. Mol Cell Biol. 1988 Feb;8(2):974–977. doi: 10.1128/mcb.8.2.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teich N., Boss M., Dexter T. M. Infection of mouse bone marrow cells with Abelson murine leukemia virus and establishment of producer cell lines. Haematol Blood Transfus. 1979;23:487–490. doi: 10.1007/978-3-642-67057-2_63. [DOI] [PubMed] [Google Scholar]
- Waneck G. L., Keyes L., Rosenberg N. Abelson virus drives the differentiation of Harvey virus-infected erythroid cells. Cell. 1986 Jan 31;44(2):337–344. doi: 10.1016/0092-8674(86)90768-3. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Robertson D., Witte O. N. Murine B cell lymphopoiesis in long term culture. J Immunol Methods. 1984 Mar 16;67(2):353–369. doi: 10.1016/0022-1759(84)90475-7. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Witte O. N. Abelson virus-infected cells can exhibit restricted in vitro growth and low oncogenic potential. J Virol. 1981 Nov;40(2):577–584. doi: 10.1128/jvi.40.2.577-584.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock C. A., Witte O. N. Long-term culture of B lymphocytes and their precursors from murine bone marrow. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3608–3612. doi: 10.1073/pnas.79.11.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock C. A., Ziegler S. F., Witte O. N. Progression of the transformed phenotype in clonal lines of Abelson virus-infected lymphocytes. Mol Cell Biol. 1983 Apr;3(4):596–604. doi: 10.1128/mcb.3.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock C., Denis K., Robertson D., Witte O. In vitro analysis of murine B-cell development. Annu Rev Immunol. 1985;3:213–235. doi: 10.1146/annurev.iy.03.040185.001241. [DOI] [PubMed] [Google Scholar]
- Witte O. N. Functions of the abl oncogene. Cancer Surv. 1986;5(2):183–197. [PubMed] [Google Scholar]