Abstract
Background:
Stereotactic brain biopsies are widely used for establishing the diagnosis of intracranial lesions. Here we examine whether stereotactic biopsy of smaller brain lesions, defined for this study as being less than 1 cubic centimeter (1 cc) in volume, are associated with lowered diagnostic yield.
Methods:
We conducted a retrospective analysis of 267 consecutive patients who underwent stereotactic brain biopsy between 2007 and 2011. Lesion volumes were calculated and were stratified by <1 or >1 cc.
Results:
A total of 13 of 246 (5.2%) biopsies for lesions >1 cc resulted in nondiagnostic tissue or an incorrect diagnosis. In contrast, 5 of 21 (23.8%) biopsies for <1 cc lesions yielded nondiagnostic or incorrect diagnosis. Posthoc review of tissue from the <1 cc lesions suggests the neuropathologist's expertise in the handling and analysis of limited specimen as a critical parameter of successful diagnosis. The operative morbidities were low for both the <1 and >1 cc biopsies (0% and 1%, respectively).
Conclusion:
This study demonstrates that stereotactic cerebral biopsy of lesions less than a cubic centimeter in volume results in a lower diagnostic yield versus larger lesions (76.2% versus 94.8%). While auxiliary measures may be taken to improve diagnostic yield, these patients may be best managed in a specialized center with experienced stereotactic neurosurgeons and neuropathologists.
Keywords: Biopsy, diagnostic yield, size, stereotactic, tumor
INTRODUCTION
Stereotactic-guided needle biopsy is a well-accepted method for obtaining tissue diagnosis for intracranial lesions that are not amenable to surgical resection. The accuracy, safety, and diagnostic yield of stereotactic needle-biopsies have been well established in the neurosurgical literature.[2,4,9,12,20,22,23] In terms of mechanical precision, stereotactically guided techniques have demonstrated an accuracy ranging from 1.2 to 2.8 mm.[4,24] In terms of safety, complications related to stereotactic biopsy ranged 1-8%.[1,2,5,8,10,21,23] In terms of diagnostic yield, several large series suggest that tissue diagnosis can be attained in >90% of the biopsies.[2,5,8,9,21,23]
Despite this extended literature, there has not been a study that looked specifically at how the size of the target influenced diagnostic yield. There are many reasons to expect lowered diagnostic yield for the smaller lesions. First, a smaller target will magnify the effect of any degree of mechanical deviation, however slight, within the stereotactic system. Second, tissues attained in the biopsy of smaller lesions are typically more limited in quantity, and the diagnostic yield directly correlates with the quantity of pathologic specimen secured and the expertise of the neuropathologist.[2,9,14,23] Finally, the limited specimens may be more demanding in terms of the pathologist's expertise.[15] Here we report our experience in stereotactic brain biopsies of the smaller lesions (defined as < 1 cm3) and compared the diagnostic yield of such biopsies to the larger lesions (defined as > 1 cm3).
METHODS
Study population
Electronic records from 267 consecutive patients who underwent stereotactic needle-biopsy from 2007 to 2011 by PW and CC were retrospectively reviewed. Information was collected regarding final pathology, morbidity, and indications. Magnetic resonance (MR) images for each case were imported into the Inomed system (Stereoplan Plus, Germany) for volumetric calculation. Based on this calculation, lesions were stratified into <1 cm3 or >1 cm3. For each case, postoperative computed tomography (CT) was evaluated for evidence of new hyper-density at the biopsy site. For clinical follow-up, transient neurologic deficit was defined as an altered postoperative neurologic examination that resolved within a month of surgery. This study was approved by the institutional review boards under IRB#2010-P-000134.
Surgical procedure
After induction of anesthesia, a Riechert/Mundinger stereotactic head frame (Inomed GmbH, Emmendingen, Germany) was secured onto the patient's cranium. A 1.25 mm slice-thickness contrast-enhanced CT imaging of the head was subsequently acquired using either an intraoperative scanner (Ceretom, Neurologica, MA) or a conventional CT scanner. CT images were processed to yield three-dimensional reconstructions using software by Inomed (Stereoplan Plus, Germany). Using these reconstructions, an optimal trajectory through the lesion was planned to avoid intersection of vasculature or sulci. In select cases, a positron emission tomographic (PET) scan was performed to define the hyper-metabolic area as a target. Image fusion of the CT, MRI, and PET where available was then performed.
After mounting the aiming bow to the head frame, a scalp incision (approximately 3 cm in length) and burr hole were placed at the planned entry site. Biopsy forceps (1.0 or 1.4 mm) were then advanced to the lesion under the guidance of the aiming bow. Serial biopsy through the entire lesion was performed. Depending on the size of the lesion, 2 to >10 biopsies were taken in 1 mm intervals.
Posthoc pathology review
For the <1 cm3 lesions that were biopsied and yielded nondiagnostic tissue or misdiagnosis, we retrieved the original slides as well as slides from the repeat biopsy or resection. The slides were reviewed with an independent neuropathologist (HR) who was not involved in the initial diagnosis. “Limited tissue” was used to designate situations where the pathologist felt that the tissue obtained during the first biopsy was lesional but definitive diagnosis was not possible due to tissue limitation.
RESULTS
Of the 267 patients, 21 (7.9%) had lesions that were <1 cm3. A summary of the final pathologic diagnosis for the 267 biopsied cases as stratified by <1 cm3 and >1 cm3 volume is presented in Table 1. A total of 246 (92%) of the biopsies were performed for lesions >1 cm3 and 21 (8%) were for lesions <1 cm3. Irrespective of lesion volume, the most frequently encountered category of diagnosis was tumor.
Table 1.
Final pathologic diagnosis of biopsied cases
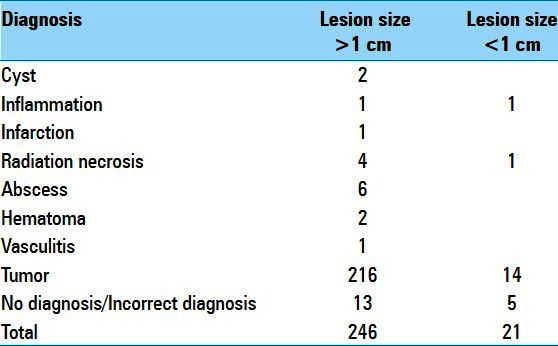
Of the >1 cm3 biopsies, 5.2% (13/246) resulted in nondiagnostic tissue or incorrect diagnosis [Table 2]. All lesions were contrast enhancing on MRI. Of these, six biopsies (6/246 or 2.4%) yielded a diagnosis that was revised upon resection of the lesion, and seven biopsies (7/246 or 2.8%) yielded nondiagnostic tissues. Cases of misdiagnosis most often involved mis-staged glial tumors. The remaining nondiagnostic biopsies were performed for presumptive diagnoses related to inflammatory or infectious diseases [Table 2].
Table 2.
Incorrect diagnosis
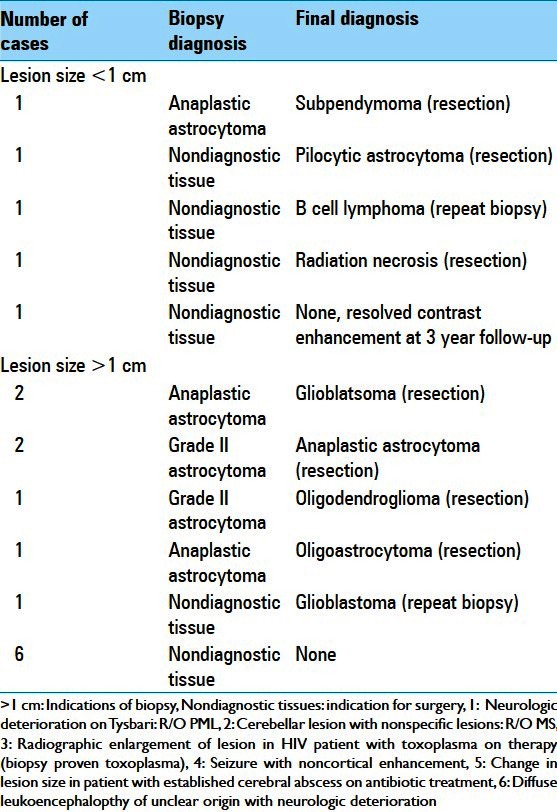
All 21 lesions <1 cm3 were also contrast enhancing. Figure 1a–i illustrates representative radiographs from cases with a definitive diagnosis for lesions <1 cm3. The indications for biopsy of sub-cubic centimeter lesions are listed in Table 3. A total of 23.8% (5/21) of the <1 cm3 biopsies resulted in nondiagnostic or incorrect diagnosis. Of these cases, one biopsy (1/21 or 4.7%) yielded the diagnosis of anaplastic astrocytoma that was revised to sub-ependymoma upon resection (patient 21 in Table 3, Figure 2a). Four biopsies (4/21 or 19%) yielded nondiagnostic tissues. In one case, a second biopsy was performed to yield the diagnosis of a B cell lymphoma (patient 17 in Table 3, Figure 2b). In two other cases, surgical resection was performed due to enlargement of the lesions on serial MRI. Definitive diagnosis was made using the resected tissues (radiation necrosis and pilocytic astrocytoma, patients 8 and 12 in Table 3, Figure 2c and d). In the last case, the patient with the nondiagnostic biopsy was followed by serial MRIs. The patient initially presented with seizure and was treated with antiseizure medications without further neurologic events. The contrast enhancement resolved spontaneously at the 3-year follow-up (patient 13 in Table 3, Figure 2e).
Figure 1.
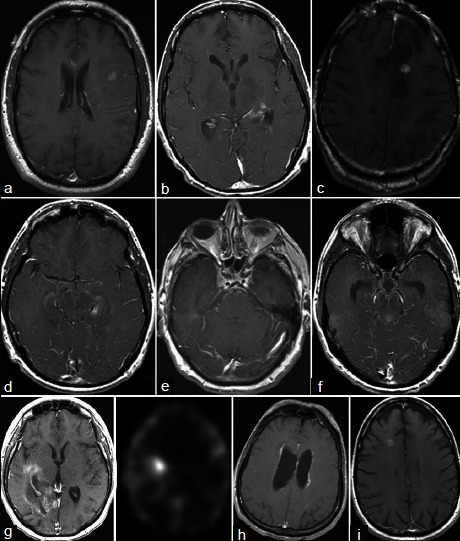
Illustrative cases with definitive diagnosis (a) Grade III oligoastrocytoma (b) Anaplastic astrocytoma (c) Grade III oligoastrocytoma (d) Anaplastic astrocytoma (e) Metastatic squamous cell carcinoma (f) Anaplastic astrocytoma (g) Metastatic carcinoma (h) Hodgkin's lymphoma (i) Metastasis
Table 3.
Indications for the sub-centimeter biopsies
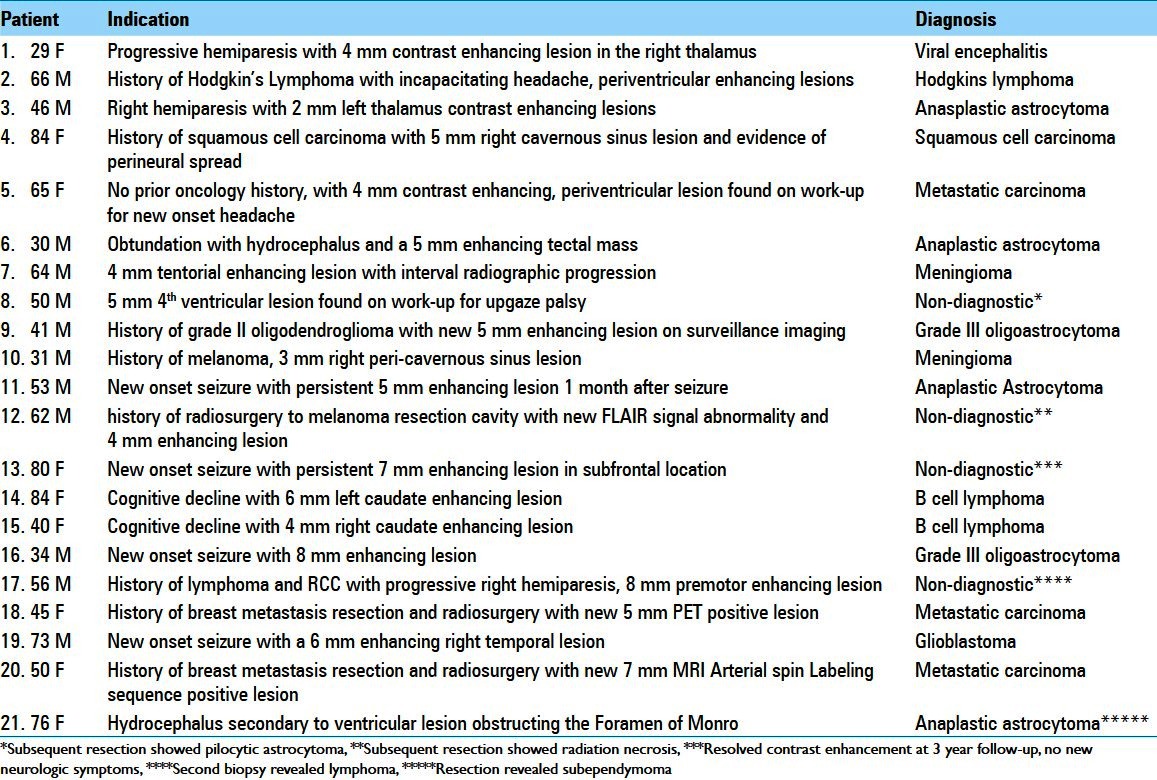
Figure 2.
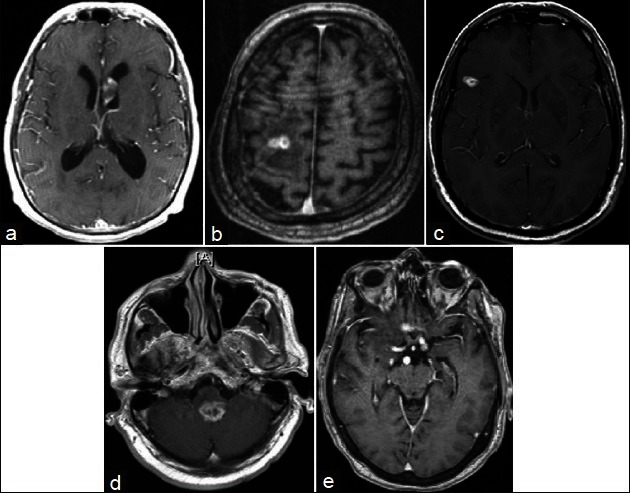
Cases of misdiagnosis (a) Misdiagnosed subependymoma (initial biopsy showed anaplastic astrocytoma, subsequent resection showed subependymoma. (b) Initial biopsy nondiagnostic. Repeat biopsy showed lymphoma (c) Radiation necrosis (d) Nondiagnostic (resection showed pilocytic astrocytoma) (e) Nondiagnostic
In terms of achieving a definitive diagnosis, the gross diagnostic yield was 94.8% and 76.2% for the >1 cm3 and the <1 cm3 lesions, respectively. The difference in diagnostic yield was statistically significant (P = 0.0081, Fisher's Exact Test).
Posthoc analysis of the tissue specimens from these lesions suggests that the primary cause for diagnostic failure was due to the limited amount of tissue [Table 4]. For all four cases, lesional tissue was obtained on the initial biopsies, but the limited specimens led to incorrect or indefinite diagnosis (patients 8, 12, 17, and 21 in Tables 3 and 4). For instance, the initial pathology report for patient 8 [Table 4] was nondiagnostic, but included pilocytic astrocytoma as a potential diagnosis. This diagnosis was confirmed based on the larger specimens secured through an open resection. Similar situations were encountered for cases 12 and 17. Case 21, where a subependymoma was initially misdiagnosed as an anaplastic astrocytoma, warrants additional comment. The initial diagnosis was made based on focal hypercellular regions thought to represent anaplastic astrocytoma. On subsequent resection, the lesion showed pathologic findings classic for subependymoma, without evidence of hypercellularity. The pathologic findings of the original specimen were subsequently attributed to artifact related to sample processing.
Table 4.
Reasons for mis-diagnosis in the biopsy of <1 cc lesions
Morbidities associated with the biopsies did not significantly differ based on the lesion size [Table 5]. No patients suffered from permanent neurologic deficit or death in either group. A total of 3 (0.8%) of 246 patients with >1 cm3 lesions had a transient neurologic deficit postbiopsy that resolved completely by 1 month follow-up. All three of these lesions were located in eloquent cortex.
Table 5.
Morbidities associated with stereotactic biopsies of lesions >1 cc or <1 cc in size
Postoperative CT demonstrated hyper-density at the site of biopsy in 15% (37/246) of the patients with >1 cm3 lesion and 9.5% (2/21) of the patients with <1 cm3 lesion (P = 0.7485, Fisher's Exact test). With the exception of the above noted cases, the hyper-density did not contribute to clinically detectable changes in neurologic examination.
DISCUSSION
This study represents the first to our knowledge to evaluate the diagnostic yield of stereotactic brain biopsy of small lesions (<1 cm3) relative to larger lesions. In our series, diagnostic yield from biopsies of lesions >1 cm3 (94.8%) was comparable to previously published rates of 90-96% for stereotactic brain biopsies without size stratification.[5,8,21] Biopsies of the <1 cm3 lesions, in contrast, were associated with a lowered diagnostic yield relative to the >1 cm3 lesions (76.2%, P = 0.0081).
Our study contributes to the field of neurosurgical oncology in two ways. First, our study suggest that the risk of a nondiagnostic biopsy for <1 cm3 lesions are significantly higher than most biopsies in general.[5,8,21] This is an issue that should be carefully reviewed during the informed consent for biopsy of <1 cm3 intracranial lesions. Second, given the inherent technical challenges associated with such biopsies, referral to centers where experienced stereotactic neurosurgeons and neuro-pathologists are available warrants consideration.
Posthoc review of the tissue specimen for the mis-diagnostic or nondiagnostic cases offered an opportunity to investigate the underlying cause. The observations that lesional tissue was obtained during the first biopsy and that the differential diagnosis generated using these specimens included the final diagnosis support the accuracy of the frame-based stereotactic biopsy. In this context, the major determinant of diagnosis for the <1 cm3 lesion appeared to be the familiarity of the pathologist in the handling and analysis of limited specimens. A smaller lesion necessarily translated into less tissue for examination, and the published literature suggested that diagnostic yield is a function of the amount of available specimen.[14] Further, sub-optimal processing of limited specimens may lead to artifacts prohibitive of definitive diagnosis.[15] Overall, our results suggest the neuropathologist's expertise in the handling and analysis of limited specimen as a critical determinant of diagnosis.
There are several limitations to this study. First and foremost, this study included patients treated by two surgeons (CC and PW) at a single institution. As such, the results presented here may not be broadly applicable. Second, the size criteria for small versus large lesions using a 1 cm3 volumetric cut off was somewhat arbitrary. Third, the number of <1 cm3 biopsies performed are fairly limited, constituting only 7.9% of all biopsies performed. Realizing the small sample size, we nevertheless performed this analysis of the patients accumulated over the 3-year interval with the goal of assessing the diagnostic yield in a timely manner. Finally, the retrospective design means that patient selection bias cannot be entirely excluded as the cause of the differential diagnostic yield. Despite these limitations, the implications of our findings contribute to the management of patients with a small intracranial lesion.
Many adjuncts to the biopsy technique and recent advances in technology may improve diagnostic yield in the biopsy of small lesions.[3,6,7,11,12,16–20] Notably, intraoperative MRI-based biopsies afford the opportunity to directly visualize the region of biopsy relative to the lesion as well as ascertainment of potential hemorrhage at the biopsy site.[3] Such information may be helpful in redirecting biopsy sites or may enable a greater number of biopsy specimens and thereby improve the diagnostic yield. Diagnosis through molecular analysis of limited specimen may also afford opportunities to enhance diagnostic yield.[13] The application of such technology to augment the diagnostic yield of challenging intracranial targets awaits investigation.
CONCLUSIONS
This study demonstrates that stereotactic cerebral biopsy of < 1 cm3 intracranial lesions is associated with a lower diagnostic yield relative to the >1 cm3 lesions (76.2% versus 94.8%, respectively). Morbidities associated with biopsy in both groups are comparable at approximately 1%. Our findings identify the neuropathologist's expertise in the handling and analysis of limited specimen as a critical determinant of tissue diagnosis. Our findings also support the accuracy of frame-based stereotactic biopsy in tissue acquisition. Given the technical challenges associated with these biopsies, consideration should be given for referral of patients with such lesions to centers where experienced stereotactic neurosurgeons and neuropathologists are available.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2013/4/4/176/110677
Contributor Information
J. Dawn Waters, Email: j.dawn.waters@gmail.com.
David D. Gonda, Email: dgonda@ucsd.edu.
Hasini Reddy, Email: hreddy1@bidmc.harvard.edu.
Ekkehard M. Kasper, Email: ekasper@bidmc.harvard.edu.
Peter C. Warnke, Email: pwarnke@partners.org.
Clark C. Chen, Email: clarkchen@ucsd.edu.
REFERENCES
- 1.Air EL, Leach JL, Warnick RE, McPherson CM. Comparing the risks of frameless stereotactic biopsy in eloquent and noneloquent regions of the brain: A retrospective review of 284 cases. J Neurosurg. 2009;111:820–4. doi: 10.3171/2009.3.JNS081695. [DOI] [PubMed] [Google Scholar]
- 2.Apuzzo ML, Chandrasoma PT, Cohen D, Zee CS, Zelman V. Computed imaging stereotaxy: Experience and perspective related to 500 procedures applied to brain masses. Neurosurgery. 1987;20:930–7. doi: 10.1227/00006123-198706000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Bernays RL, Kollias SS, Khan N, Brandner S, Meier S, Yonekawa Y. Histological yield, complications, and technological considerations in 114 consecutive frameless stereotactic biopsy procedures aided by open intraoperative magnetic resonance imaging. J Neurosurg. 2002;97:354–62. doi: 10.3171/jns.2002.97.2.0354. [DOI] [PubMed] [Google Scholar]
- 4.Bjartmarz H, Rehncrona S. Comparison of accuracy and precision between frame-based and frameless stereotactic navigation for deep brain stimulation electrode implantation. Stereotact Funct Neurosurg. 2007;85:235–42. doi: 10.1159/000103262. [DOI] [PubMed] [Google Scholar]
- 5.Chen CC, Hsu PW, Erich Wu TW, Lee ST, Chang CN, Wei KC, et al. Stereotactic brain biopsy: Single center retrospective analysis of complications. Clin Neurol Neurosurg. 2009;111:835–9. doi: 10.1016/j.clineuro.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Chernov MF, Muragaki Y, Ochiai T, Taira T, Ono Y, Usukura M, et al. Spectroscopy-supported frame-based image-guided stereotactic biopsy of parenchymal brain lesions: Comparative evaluation of diagnostic yield and diagnostic accuracy. Clin Neurol Neurosurg. 2009;111:527–35. doi: 10.1016/j.clineuro.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Dammers R, Schouten JW, Haitsma IK, Vincent AJ, Kros JM, Dirven CM. Towards improving the safety and diagnostic yield of stereotactic biopsy in a single centre. Acta Neurochirurgica. 2010;152:1915–21. doi: 10.1007/s00701-010-0752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ersahin M, Karaaslan N, Gurbuz MS, Hakan T, Berkman MZ, Ekinci O, et al. The safety and diagnostic value of frame-based and ct-guided stereotactic brain biopsy technique. Turk Neurosurg. 2011;21:582–90. [PubMed] [Google Scholar]
- 9.Feiden W, Steude U, Bise K, Gundisch O. Accuracy of stereotactic brain tumor biopsy: Comparison of the histologic findings in biopsy cylinders and resected tumor tissue. Neurosurg Rev. 1991;14:51–6. doi: 10.1007/BF00338192. [DOI] [PubMed] [Google Scholar]
- 10.Field M, Witham TF, Flickinger JC, Kondziolka D, Lunsford LD. Comprehensive assessment of hemorrhage risks and outcomes after stereotactic brain biopsy. J Neurosurg. 2001;94:545–51. doi: 10.3171/jns.2001.94.4.0545. [DOI] [PubMed] [Google Scholar]
- 11.Firlik KS, Martinez AJ, Lunsford LD. Use of cytological preparations for the intraoperative diagnosis of stereotactically obtained brain biopsies: A 19-year experience and survey of neuropathologists. J Neurosurg. 1999;91:454–8. doi: 10.3171/jns.1999.91.3.0454. [DOI] [PubMed] [Google Scholar]
- 12.Frati A, Pichierri A, Bastianello S, Raco A, Santoro A, Esposito V, et al. Frameless stereotactic cerebral biopsy: Our experience in 296 cases. Stereotact Funct Neurosurg. 2011;89:234–45. doi: 10.1159/000325704. [DOI] [PubMed] [Google Scholar]
- 13.Hunt JL. Molecular pathology in anatomic pathology practice: A review of basic principles. Arch Pathol Lab Med. 2008;132:248–60. doi: 10.5858/2008-132-248-MPIAPP. [DOI] [PubMed] [Google Scholar]
- 14.Jain D, Sharma MC, Sarkar C, Deb P, Gupta D, Mahapatra AK. Correlation of diagnostic yield of stereotactic brain biopsy with number of biopsy bits and site of the lesion. Brain Tumor Pathol. 2006;23:71–5. doi: 10.1007/s10014-006-0204-y. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Chang WS, Kim JP, Minn YK, Choi J, Chang JW, et al. Peripheral compressing artifacts in brain tissue from stereotactic biopsy with sidecutting biopsy needle: A pitfall for adequate glioma grading. Clin Neuropathol. 2011;30:328–32. doi: 10.5414/NP300404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levivier M, Goldman S, Pirotte B, Brucher J, Balériaux D, Luxen A, et al. Diagnostic yield of stereotactic brain biopsy guided by positron emission tomography with [18F] fluorodeoxyglucose. J Neurosurg. 1995;82:445–52. doi: 10.3171/jns.1995.82.3.0445. [DOI] [PubMed] [Google Scholar]
- 17.Moriuchi S, Yamada K, Dehara M, Teramoto Y, Soda T, Imakita M, et al. Use of 5-aminolevulinic acid for the confirmation of deep-seated brain tumors during stereotactic biopsy. J Neurosurg. 2011;115:278–80. doi: 10.3171/2011.4.JNS102137. [DOI] [PubMed] [Google Scholar]
- 18.Ooba H, Abe T, Momii Y, Fujiki M. Stereotactic biopsy with electrical monitoring for deep-seated brain tumors. World Neurosurg. 2013;79:207.e1–5. doi: 10.1016/j.wneu.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 19.Powell S. Intraoperative consultation, cytologic preparations, and frozen section in the central nervous system. Arch Pathol Lab Med. 2005;129:1635–52. doi: 10.5858/2005-129-1635-ICCPAF. [DOI] [PubMed] [Google Scholar]
- 20.Shooman D, Belli A, Grundy PL. Image-guided frameless stereotactic biopsy without intraoperative neuropathological examination. J Neurosurg. 2010;113:170–8. doi: 10.3171/2009.12.JNS09573. [DOI] [PubMed] [Google Scholar]
- 21.Silva EU, Vasconcellos LP, Lara NA, Jr, Veiga JC, Lancellotti CL, Shiozawa P. Stereotactic Biopsy for Intracranial Lesions. Arq Neuropsiquiatr. 2009;67:1062–5. doi: 10.1590/s0004-282x2009000600019. [DOI] [PubMed] [Google Scholar]
- 22.Smith JS, Quiñones-Hinojosa A, Barbaro NM, McDermott MW. Frame-based stereotactic biopsy remains an important diagnostic tool with distinct advantages over frameless stereotactic biopsy. J Neurooncol. 2005;73:173–9. doi: 10.1007/s11060-004-4208-3. [DOI] [PubMed] [Google Scholar]
- 23.Tilgner J, Herr M, Ostertag C, Volk B. Validation of intraoperative diagnoses using smear preparations from stereotactic brain biopsies: Intraoperative versus final diagnosis-influence of clinical factors. Neurosurgery. 2005;56:257–65. doi: 10.1227/01.neu.0000148899.39020.87. [DOI] [PubMed] [Google Scholar]
- 24.Zacest A, Berk C, Burchiel KJ. Precision and accuracy of stereotactic targeting in patients undergoing repeat stereotactic surgery. Stereotact Funct Neurosurg. 2009;87:168–73. doi: 10.1159/000215932. [DOI] [PubMed] [Google Scholar]


