Abstract
Eating disorders (EDs) are a group of severely impaired eating behaviors, which include three subgroups: anorexia nervosa (AN), bulimia nervosa (BN), and ED not otherwise specified (EDNOS). The precise mechanism of EDs is still unclear and the disorders cause remarkable agony for the patients and their families. Although there are many available treatment methods for EDs today, such as family therapy, cognitive behavioral therapy, medication, psychotherapy, and so on, almost half of the patients are refractory to all current medical treatment and never fully recover. For treatment-refractory EDs, stereotactic surgery may be an alternative therapy. This review discusses the history of stereotactic surgery, the modern procedures, and the mostly used targets of stereotactic surgery in EDs. In spite of the limited application of stereotactic surgery in ED nowadays, stereotactic lesion and deep brain stimulation (DBS) are promising treatments with the development of modern functional imaging techniques and the increasing understanding of its mechanism in the future.
Keywords: Anorexia nervosa, eating disorders, surgical treatment, stereotactic neurosurgery
INTRODUCTION
Eating disorders (EDs), which are characterized by severely impaired eating behavior, are one of the most common health problems afflicting female adolescents and young women and have been reported worldwide both in developed countries and emerging economies such as Brazil and China.[12,16,24] EDs are divided into three subgroups: Anorexia nervosa (AN), bulimia nervosa (BN), and ED not otherwise specified (EDNOS) according to the Diagnostic Criteria of Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV). The most common symptoms in EDs are restrictive food intake, binge eating, excessive exercise, and body image disturbance. In addition, psychiatric comorbidity, such as obsessive and compulsive disorders (OCDs), affective disorders, and anxiety disorders, could be found in most of the ED patients.
Lifetime prevalence of EDs (including the three major EDs) worldwide is estimated at about 4-6%.[24,45] The lifetime prevalence was about 1.75-3 times higher among women than men and the age of onset of AN and BN has decreased in younger generations.[18,24] EDs, which have one of the highest excessive mortality rates of all psychiatric disorders, cause remarkable agony for the patients and their families. Suicide or medical complications are the major causes of mortality for those with EDs.[6,19]
Risk factors such as genetic, environmental, and developmental factors have been well established. The interaction between genetic factors and environmental factors play a key role in the etiology of the disease.[9,27,44] Twin and family studies indicate that EDs including AN, BN, and EDNOS are complex genetic diseases, and the genetic factors contribute 50-83% of the variance in AN, BN, and EDNOS.[10,13,25,38] Besides, linkage studies further confirmed that about a third of genetic risk for EDs and depression, anxiety disorders, and addictive disorders are shared.[20,28,48]
The neurocircuitry underlying food intake is complex and the precise mechanism of EDs is still unclear. However, it is believed that the reward system and hypothalamus play critical roles in the progression of the disease. The hypothalamus has projections directly to the nucleus accumbens (NAcc). The NAcc is of interest because of its indication in the reward of natural behaviors, such as exercise, sex, and feeding.[52,54] Besides the NAcc, there are other brain regions engaged in EDs such as the anterior cingulate cortex (ACC), insula, and striatum.[22]
Although there are many available treatment methods for EDs today, such as family therapy, cognitive behavioral therapy, medication, psychotherapy, and so on, almost 50% of the patients are refractory to all current medical treatment and never fully recover. The standardized mortality ratio over the first 10 years is about 10%.[33] For treatment-refractory EDs, stereotactic surgery may be an alternative therapy.
STEREOTACTIC SURGERY FOR EATING DISORDERS
Early in the 1960s, White Le et al. found that lesions in the lateral hypothalamus could produce a variety of levels of feeding response, which indicates the close relationship between EDs and the hypothalamus.[50] This finding was confirmed by many reports from different medical centers over the next 30 years.[15,22,23,29,40,46] In particular, Barbier et al. reported an interesting case with comorbid AN and OCD. They suggested that bilateral anterior capsulotomy can be a therapeutic option for patients with comorbid AN and OCD.[4] All these findings expanded the knowledge of the neurocircuit associated with EDs and provided the potential targets for stereotactic surgery.
Before the application of computed tomography (CT) and magnetic resonance imaging (MRI), the target of stereotactic surgery for EDs was mainly based on the empirical findings on brain lesions. At first, limited by the understanding of neural circuit for EDs and the stereotactic surgery method, lobotomy was the most common surgical intervention for the treatment of EDs. For example, Sifneos presented a successful treatment of one case of AN by a unilateral lower quadrant leucotomy in 1952.[41] In that same year, Carmody et al. reported a refractory case of AN treated by prefrontal lobotomy.[11] Zamboni et al. described two patients suffering from an extremely severe, chronic, and refractory anorectic syndrome. Both patients underwent bilateral stereotactic thalamotomy and subsequently regained weight.[55] In spite of the fact that the leucotomy and thalamotomy were successful in these sporadic cases, considering the complication and irreversible invasive procedure, it was believed that such operations should be considered only after other forms of treatment had failed.
MODERN PROCEDURES
The modern psychosurgery consisted of lesions and deep brain stimulation (DBS), which were guided by either CT or MRI. The most common lesions were the anterior capsulotomy, anterior cingulotomy, subcaudate tractotomy, and limbic leucotomy.[8] As neuroimaging methods became widely applied (e.g., CT, MRI, Positron Emission Tomography-Computed Tomography, (PET-CT), and functional Magnetic Resonance Imaging (fMRI)), modern psychosurgery became more accurate and minimally invasive. In particular, the DBS was accepted worldwide because of its reversibility. In recent decades, DBS has had great success in treatment of movement disorders and some psychiatric disorders such as OCD and depression. Hence, a resurgence of psychosurgery has been recognized in the treatment of many psychiatric disorders. Sun reported on DBS of NAcc for medical treatment of AN. (13th North American Neuromodulation Congress, Las Vegas 2006.12) In 2011, Barbier et al. reported an interesting case with comorbid AN and OCD treated with capsulotomy.[4] In 2012, Wu et al. showed that the use of DBS to treat AN may be a valuable option for weight restoration in otherwise-refractory cases.[53] Moreover, Sun et al. reported a long-term follow-up results of surgical treatment and a grading for AN that indicated DBS being only helpful for patients without bulimia, otherwise patients with severe AN could get excellent results after bilateral capsulotomies (American society for stereotactic and functional neurosurgery (ASSFN), San Francisco, 2012.6). These studies lead the way in exploiting the potential role of stereotactic surgery in the treatment of EDs.
THE TARGETS FOR EATING DISORDERS
The target of the stereotactic surgery for psychiatric disorders has been discussed in the literature. For lesion sites, cingulate gyrus and anterior limb of internal capsule are the most important targets according to the proposal of an anatomic basis of emotions in 1937 by Papez.[35] Anterior cingulotomy and anterior capsulotomy showed acceptable results in the treatment of mental disorders.[5] Compared with lesions, DBS has more flexible targets because of its reversibility and adaptability.[5] The most commonly used targets for DBS are the Globus Pallidus Internus (GPi), Subthalamic Nucleus (STN), Anterior Hypothalamus, and NAcc. Although little published data are yet available in EDs, all of these potential targets have been suggested based on preliminary evidence in animal tests and clinical case reports.
Globus pallidus internus
The globus pallidus, also known as paleostriatum, is a sub-cortical structure of the brain. It is located just inside the putamen, with an outer part and an inner part. GPi was mainly used as the target for the treatment of movement disorders. However, there are some reports indicating that GPi is related with the weight changes.[31,34,43] For example, Ondo et al. showed significant weight gain in patients with Parkinson's disease (PD) who have undergone unilateral pallidotomy in their study.[34]
Subthalamic nucleus
This is currently the preferred target because of its effectiveness on the treatment of dopaminergic symptoms of PD. At the same time, the body weight gain in PD patients who underwent DBS sparked researcher interest.[32,43] Walker et al. studied the weight changes in 39 patients with PD undergoing unilateral STN DBS and found a significant weight gain after the surgery.[49] Bannier et al. also reported that, 16 months after the surgery, 82% of DBS patients followed were overweight.[3]
Anterior hypothalamus
It is believed that the neural structures in the anterior hypothalamic area are involved in the control of feeding behavior and metabolism of food. Lacan et al. found that total food consumption increased after the 3-month bilateral implant of electrodes and subsequent periods of high-frequency ventromedial hypothalamus (VMH) stimulation.[30]
Nucleus accumbens
NAcc, as part of the reward center, is thought to play an important role in reward, pleasure, addition, and placebo effect. Animal experimental data have suggested that the NAcc might be a potential target for AN either alone or combined with anterior capsulotomy.[26,42,47] Van der Plasse et al. examined the effect of DBS-NAcc on food-directed behavior. Their data revealed a functional dissociation between the Lateral Shell (lShell) and Medial Shell (mShell). On one hand, DBS of the lShell reduced motivation to respond for sucrose under a progressive ratio schedule of reinforcement, while on the other hand, mShell DBS profoundly and selectively increased the intake of food, which indicates that the intake of food and motivation to get palatable food can be independently modulated by DBS in subregions of the NAcc shell.[47] In 2012, Sun et al. reported a NAcc targeted DBS study of four patients with refractory AN [Figure 1]. This is the first study targeting the NAcc for EDs in humans.[53]
Figure 1.
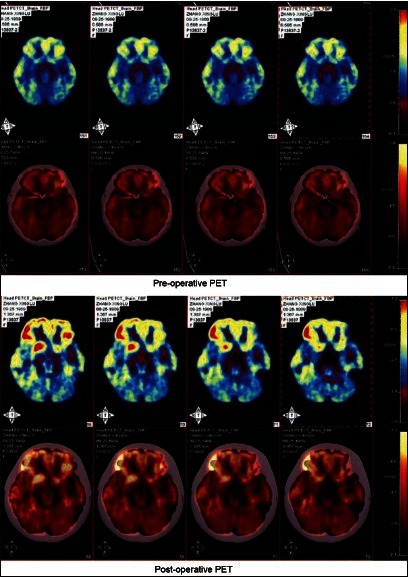
Notice the decrease in fluro-deoxy-glucose uptake after DBS of the nucleus accumbens in a patient with nervous anorexia
Anterior capsule
Many publications have shown excellent effects using lesion or DBS in the anterior capsule in patients with OCD and other psychiatric disorders. Because AN belongs to obsessive-compulsive spectrum disorders, it is reasonable that the anterior capsule also is affected in AN. Sun et al. reported perfect long-term follow-up results in patients with severe anorexia patients who underwent bilateral anterior capsulotomy (ASSFN 2001).
SURGICAL TECHNIQUE
Minimal invasion of the brain and maximal efficacy are the principles of stereotactic neurosurgery. With rapid advancements made in functional neuroimaging methods, the lesions have become more accurate and less invasive. Anterior capsulotomy and anterior cingulotomy are currently the most commonly employed neurosurgical procedures for psychiatric disease. Despite the lack of research for capsulotomy and cingulotomy focused on the EDs, these two safe procedures are still potential surgical techniques.
ANTERIOR CAPSULOTOMY
Anterior capsulotomy was first performed by Tailarach in the late 1940s and further developed by Lars Leksell.[21] In this procedure, lesions are placed within the anterior limb of the internal capsule to cut the connective fibers between prefrontal cortex and subcortical nuclei (dorsomedial thalamus included). The lesions are made using thermocoagulation through stereotactic burr holes in the skull guided by CT or MRI. Because there is a large individual difference in anterior capsule, MRI targeting became the best modality to ensure accuracy when making lesions or implant DBS electrodes in the anterior capsule target.
ANTERIOR CINGULOTOMY
The procedure was first performed in the early 1950s and was subsequently made popular in America.[51] Ballantine et al. subsequently showed the safety of anterior cingulotomy and studied its efficacy for a broad range of psychiatric indications.[2] The functional and stereotactic neurosurgery group in Massachusetts General Hospital has had rich experience with anterior cingulotomy and is still engaged in this procedure. Lesions are made on each side by thermocoagulation with MRI.
DEEP BRAIN STIMULATION
Comparing to the lesions, DBS offers the prospect of a reversible method for effective neuromodulation to relieve suffering in severe and treatment-refractory EDs. In 1954, Pool et al. attempted to treat a woman with anorexia and depression by stimulating the caudate nucleus.[39] In the next 50 years, DBS was mostly used in the animals to explore the possible mechanism of EDs and later observed in a study of four patients with refractory AN by Sun et al.,[34] but the good surgical results of DBS were found only in patients with anorexia who did not exhibit bulimia and vomiting (ASSFN 2012).
EFFECTS OF STEREOTACTIC SURGERY FOR EATING DISORDERS
Most of the studies about the effects of stereotactic surgery for EDs are based on the animal experiments and sporadic case reports. Montaurier et al. explored the weight changes in PD patients treated with DBS-STN implantation and they found that the stimulation of STN area might favor body weight gain in PD patients.[32] The same phenomena were found in other targets of DBS treatment. In 2012, Sun et al. reported a NAcc targeted DBS study of four patients with refractory AN. They reported an average of 65% increase in body weight in four severe and refractory patients with AN after the DBS procedure in a 3 years follow-up. Compared with BMI improvement, comorbidities such as body image disturbance and personality disorders had less improvement after surgical treatment. In spite of abundant findings of the weight gain in clinical reports, the exact effect of the stereotactic surgery in EDs is still equivocal due to the lack of controlled and well-designed studies in large samples.
COMPLICATIONS AND SIDE EFFECTS OF STEREOTACTIC SURGERY FOR ANOREXIA NERVOSA
Very few publications of surgical treatment for anorexia are available. In general, the complications of psychosurgery include serious complications such as coma, hemorrhage in the brain, paralysis, seizures, and infection. Some of these may be fatal for the patients. However, the incidence of these complications is very low. Cosgrove and Rauch reported on more than 800 cingulotomies performed at the Massachusetts General Hospital over a 40-year period. There were no deaths and only two infections.[14] Side effects, no matter short-term or long-term, are usually not lethal. Short-term side effects include incontinence, disorientation, sleep-disorder, and headache. These symptoms usually disappeared in one month after the operation. A few patients experienced the long-term side effects including memory loss, fatigue, and personality changes.[8,36,53] DBS has hardware-related problems besides the surgical complications such as lead or wire fracture, skin infection, malfunction of IPG, and lead migration.[1,37] Bhatia et al. reviewed a total of 191 patients who received 330 electrode implants and found that overall incidence of hardware-related problems were 4.2% based on the total number of systems implanted. The mean duration between implantation and complication was 1.8 years.[7] Doshi reported similar results in their study.[17]
CONCLUSION
EDs are complex and severe, sometimes life-threatening, psychiatric disorders with high relapse rates under standard treatments. In spite of the limited application of stereotactic surgery in ED nowadays, stereotactic lesion and DBS are promising treatments awaiting further controlled studies in larger samples. There are several concerns to address in order to spread the application of stereotactic surgery in EDs. First, precise targeting confirmed with the help of modern functional imaging techniques await definition based on functional imaging such as PET-CT, fMRI. Furthermore, a deeper understanding of the exact etiology and pathogenesis of ED must be researched. Second, the continuing evolution of stereotactic and functional techniques should be made to reduce the damage to the brain as much as possible. And last, more specific psychometric testing methods could be used to better define positive and negative ED outcomes.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2013/4/4/164/110668
Contributor Information
Bomin Sun, Email: bominsun@sh163.net.
Wei Liu, Email: doctorliuwei@yahoo.cn.
REFERENCES
- 1.Allert N, Markou M, Miskiewicz AA, Nolden L, Karbe H. Electrode dysfunctions in patients with deep brain stimulation: A clinical retrospective study. Acta Neurochir (Wien) 2011;153:2343–9. doi: 10.1007/s00701-011-1187-y. [DOI] [PubMed] [Google Scholar]
- 2.Ballantine HT, Jr, Bouckoms AJ, Thomas EK, Giriunas IE. Treatment of psychiatric illness by stereotactic cingulotomy. Biol Psychiatry. 1987;22:807–19. doi: 10.1016/0006-3223(87)90080-1. [DOI] [PubMed] [Google Scholar]
- 3.Bannier S, Montaurier C, Derost PP, Ulla M, Lemaire JJ, Boirie Y, et al. Overweight after deep brain stimulation of the subthalamic nucleus in parkinson disease: Long term follow-up. J Neurol Neurosurg Psychiatry. 2009;80:484–8. doi: 10.1136/jnnp.2008.158576. [DOI] [PubMed] [Google Scholar]
- 4.Barbier J, Gabriels L, van Laere K, Nuttin B. Successful anterior capsulotomy in comorbid anorexia nervosa and obsessive-compulsive disorder: Case report. Neurosurgery. 2011;69:E745–51. doi: 10.1227/NEU.0b013e31821964d2. [DOI] [PubMed] [Google Scholar]
- 5.Benabid AL, Torres N. New targets for dbs. Parkinsonism Relat Disord. 2012;18(Suppl 1):S21–3. doi: 10.1016/S1353-8020(11)70009-8. [DOI] [PubMed] [Google Scholar]
- 6.Berkman ND, Lohr KN, Bulik CM. Outcomes of eating disorders: A systematic review of the literature. Int J Eat Disord. 2007;40:293–309. doi: 10.1002/eat.20369. [DOI] [PubMed] [Google Scholar]
- 7.Bhatia S, Oh M, Whiting T, Quigley M, Whiting D. Surgical complications of deep brain stimulation. A longitudinal single surgeon, single institution study. Stereotact Funct Neurosurg. 2008;86:367–72. doi: 10.1159/000175799. [DOI] [PubMed] [Google Scholar]
- 8.Binder DK, Iskandar BJ. Modern neurosurgery for psychiatric disorders. Neurosurgery. 2000;47:9–21. doi: 10.1097/00006123-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Bird A. Perceptions of epigenetics. Nature. 2007;447:396–8. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 10.Bulik CM, Slof-Op’t Landt MC, van Furth EF, Sullivan PF. The genetics of anorexia nervosa. Annu Rev Nutr. 2007;27:263–75. doi: 10.1146/annurev.nutr.27.061406.093713. [DOI] [PubMed] [Google Scholar]
- 11.Carmody JT, Vibber FL. Anorexia nervosa treated by prefrontal lobotomy. Ann Intern Med. 1952;36:647–52. doi: 10.7326/0003-4819-36-2-647. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Jackson T. Prevalence and sociodemographic correlates of eating disorder endorsements among adolescents and young adults from china. Eur Eat Disord Rev. 2008;16:375–85. doi: 10.1002/erv.837. [DOI] [PubMed] [Google Scholar]
- 13.Clarke TK, Weiss AR, Berrettini WH. The genetics of anorexia nervosa. Clin Pharmacol Ther. 2012;91:181–8. doi: 10.1038/clpt.2011.253. [DOI] [PubMed] [Google Scholar]
- 14.Cosgrove GR, Rauch SL. Stereotactic cingulotomy. Neurosurg Clin N Am. 2003;14:225–35. doi: 10.1016/s1042-3680(02)00115-8. [DOI] [PubMed] [Google Scholar]
- 15.De Luca B, Monda M, Pellicano MP, Zenga A. Cortical control of thermogenesis induced by lateral hypothalamic lesion and overeating. Am J Physiol. 1987;253:R626–33. doi: 10.1152/ajpregu.1987.253.4.R626. [DOI] [PubMed] [Google Scholar]
- 16.de Souza Ferreira JE, da Veiga GV. Eating disorder risk behavior in brazilian adolescents from low socio-economic level. Appetite. 2008;51:249–55. doi: 10.1016/j.appet.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Doshi PK. Long-term surgical and hardware-related complications of deep brain stimulation. Stereotact Funct Neurosurg. 2011;89:89–95. doi: 10.1159/000323372. [DOI] [PubMed] [Google Scholar]
- 18.Favaro A, Caregaro L, Tenconi E, Bosello R, Santonastaso P. Time trends in age at onset of anorexia nervosa and bulimia nervosa. J Clin Psychiatry. 2009;70:1715–21. doi: 10.4088/JCP.09m05176blu. [DOI] [PubMed] [Google Scholar]
- 19.Forcano L, Alvarez E, Santamaria JJ, Jimenez-Murcia S, Granero R, Penelo E, et al. Suicide attempts in anorexia nervosa subtypes. Compr Psychiatry. 2011;52:352–8. doi: 10.1016/j.comppsych.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Franko DL, Dorer DJ, Keel PK, Jackson S, Manzo MP, Herzog DB. Interactions between eating disorders and drug abuse. J Nerv Ment Dis. 2008;196:556–61. doi: 10.1097/NMD.0b013e31817d0153. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg BD, Price LH, Rauch SL, Friehs G, Noren G, Malone D, et al. Neurosurgery for intractable obsessive-compulsive disorder and depression: Critical issues. Neurosurg Clin N Am. 2003;14:199–212. doi: 10.1016/s1042-3680(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 22.Harris F, Illingworth RS. Congenital hypothalamic lesion leading to growth hormone deficiency and destruction of appetite satiety centre. Proc R Soc Med. 1973;66:217. [PMC free article] [PubMed] [Google Scholar]
- 23.Hebebrand J, Siemon P, Lutcke A, Mari BG, Remschmidt H. A putaminal lesion in an adolescent with obsessive-compulsive disorder and atypical anorexia nervosa. J Nerv Ment Dis. 1993;181:520–1. doi: 10.1097/00005053-199308000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the national comorbidity survey replication. Biol Psychiatry. 2007;61:348–58. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Javaras KN, Laird NM, Reichborn-Kjennerud T, Bulik CM, Pope HG, Jr, et al. Familiality and heritability of binge eating disorder: Results of a case-control family study and a twin study. Int J Eat Disord. 2008;41:174–9. doi: 10.1002/eat.20484. [DOI] [PubMed] [Google Scholar]
- 26.Jean A, Conductier G, Manrique C, Bouras C, Berta P, Hen R, et al. Anorexia induced by activation of serotonin 5-ht4 receptors is mediated by increases in cart in the nucleus accumbens. Proc Natl Acad Sci U S A. 2007;104:16335–40. doi: 10.1073/pnas.0701471104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karwautz AF, Wagner G, Waldherr K, Nader IW, Fernandez-Aranda F, Estivill X, et al. Gene-environment interaction in anorexia nervosa: Relevance of non-shared environment and the serotonin transporter gene. Mol Psychiatry. 2011;16:590–2. doi: 10.1038/mp.2010.125. [DOI] [PubMed] [Google Scholar]
- 28.Keel PK, Klump KL, Miller KB, McGue M, Iacono WG. Shared transmission of eating disorders and anxiety disorders. Int J Eat Disord. 2005;38:99–105. doi: 10.1002/eat.20168. [DOI] [PubMed] [Google Scholar]
- 29.Kelly D, Mitchell-Heggs N. Stereotactic limbic leucotomy--a follow-up study of thirty patients. Postgrad Med J. 1973;49:865–82. doi: 10.1136/pgmj.49.578.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacan G, De Salles AA, Gorgulho AA, Krahl SE, Frighetto L, Behnke EJ, et al. Modulation of food intake following deep brain stimulation of the ventromedial hypothalamus in the vervet monkey. Laboratory investigation. J Neurosurg. 2008;108:336–42. doi: 10.3171/JNS/2008/108/2/0336. [DOI] [PubMed] [Google Scholar]
- 31.Locke MC, Wu SS, Foote KD, Sassi M, Jacobson CE, Rodriguez RL, et al. Weight changes in subthalamic nucleus vs globus pallidus internus deep brain stimulation: Results from the compare parkinson disease deep brain stimulation cohort. Neurosurgery. 2011;68:1233–7. doi: 10.1227/NEU.0b013e31820b52c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montaurier C, Morio B, Bannier S, Derost P, Arnaud P, Brandolini-Bunlon M, et al. Mechanisms of body weight gain in patients with parkinson's disease after subthalamic stimulation. Brain. 2007;130:1808–18. doi: 10.1093/brain/awm113. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen S. Epidemiology and mortality of eating disorders. Psychiatr Clin North Am. 2001;24:201–14. doi: 10.1016/s0193-953x(05)70217-3. [DOI] [PubMed] [Google Scholar]
- 34.Ondo WG, Ben-Aire L, Jankovic J, Lai E, Contant C, Grossman R. Weight gain following unilateral pallidotomy in parkinson's disease. Acta Neurol Scand. 2000;101:79–84. doi: 10.1034/j.1600-0404.2000.101002079.x. [DOI] [PubMed] [Google Scholar]
- 35.Papez JW. A proposed mechanism of emotion.1937. J Neuropsychiatry Clin Neurosci. 1995;7:103–12. doi: 10.1176/jnp.7.1.103. [DOI] [PubMed] [Google Scholar]
- 36.Patel SR, Aronson JP, Sheth SA, Eskandar EN. Lesion procedures in psychiatric neurosurgery. (01327-7).World Neurosurg. 2012;(12):S1878–8750. doi: 10.1016/j.wneu.2012.11.038. [DOI] [PubMed] [Google Scholar]
- 37.Piacentino M, Pilleri M, Bartolomei L. Hardware-related infections after deep brain stimulation surgery: Review of incidence, severity and management in 212 single-center procedures in the first year after implantation. Acta Neurochir (Wien) 2011;153:2337–41. doi: 10.1007/s00701-011-1130-2. [DOI] [PubMed] [Google Scholar]
- 38.Pinheiro AP, Root T, Bulik CM. The genetics of anorexia nervosa: Current findings and future perspectives. Int J Child Adolesc Health. 2009;2:153–64. [PMC free article] [PubMed] [Google Scholar]
- 39.Pool JL. Psychosurgery in older people. J Am Geriatr Soc. 1954;2:456–66. doi: 10.1111/j.1532-5415.1954.tb02138.x. [DOI] [PubMed] [Google Scholar]
- 40.Rowland NE, Miceli MO, Malsbury CW, Baile CA, Della-Fera MA, Gingerich RL, et al. Medial hypothalamic lesions in syrian hamsters: Characterization of hyperphagia and weight gain. Physiol Behav. 1986;36:513–21. doi: 10.1016/0031-9384(86)90324-0. [DOI] [PubMed] [Google Scholar]
- 41.Sifneos PE. A case of anorexia nervosa treated successfully by leucotomy. Am J Psychiatry. 1952;109:356–60. doi: 10.1176/ajp.109.5.356. [DOI] [PubMed] [Google Scholar]
- 42.Soria-Gomez E, Matias I, Rueda-Orozco PE, Cisneros M, Petrosino S, Navarro L, et al. Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-fos expression in the hypothalamus. Br J Pharmacol. 2007;151:1109–16. doi: 10.1038/sj.bjp.0707313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strowd RE, Cartwright MS, Passmore LV, Ellis TL, Tatter SB, Siddiqui MS. Weight change following deep brain stimulation for movement disorders. J Neurol. 2010;257:1293–7. doi: 10.1007/s00415-010-5509-4. [DOI] [PubMed] [Google Scholar]
- 44.Thornton LM, Mazzeo SE, Bulik CM. The heritability of eating disorders: Methods and current findings. Curr Top Behav Neurosci. 2011;6:141–56. doi: 10.1007/7854_2010_91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Treasure J, Claudino AM, Zucker N. Eating disorders. Lancet. 2010;375:583–93. doi: 10.1016/S0140-6736(09)61748-7. [DOI] [PubMed] [Google Scholar]
- 46.Uher R, Treasure J. Brain lesions and eating disorders. J Neurol Neurosurg Psychiatry. 2005;76:852–7. doi: 10.1136/jnnp.2004.048819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Plasse G, Schrama R, van Seters SP, Vanderschuren LJ, Westenberg HG. Deep brain stimulation reveals a dissociation of consummatory and motivated behaviour in the medial and lateral nucleus accumbens shell of the rat. PLoS One. 2012;7:e33455. doi: 10.1371/journal.pone.0033455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wade TD, Bulik CM, Neale M, Kendler KS. Anorexia nervosa and major depression: Shared genetic and environmental risk factors. Am J Psychiatry. 2000;157:469–71. doi: 10.1176/appi.ajp.157.3.469. [DOI] [PubMed] [Google Scholar]
- 49.Walker HC, Lyerly M, Cutter G, Hagood J, Stover NP, Guthrie SL, et al. Weight changes associated with unilateral stn dbs and advanced pd. Parkinsonism Relat Disord. 2009;15:709–11. doi: 10.1016/j.parkreldis.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 50.White LE, Hain RF. Anorexia in association with a destructive lesion of the hypothalamus. Arch Pathol. 1959;68:275–81. [PubMed] [Google Scholar]
- 51.Whitty CW, Duffield JE, Tov PM, Cairns H. Anterior cingulectomy in the treatment of mental disease. Lancet. 1952;1:475–81. doi: 10.1016/s0140-6736(52)90051-2. [DOI] [PubMed] [Google Scholar]
- 52.Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361:1149–58. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu H, Van Dyck-Lippens PJ, Santegoeds R, van Kuyck K, Gabriels L, Lin G, et al. Deep-brain stimulation for anorexia nervosa. World Neurosurg. 2012 doi: 10.1016/j.wneu.2012.06.039. [In press] [DOI] [PubMed] [Google Scholar]
- 54.Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–61. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zamboni R, Larach V, Poblete M, Mancini R, Mancini H, Charlin V, et al. Dorsomedial thalamotomy as a treatment for terminal anorexia: A report of two cases. Acta Neurochir Suppl (Wien) 1993;58:34–5. doi: 10.1007/978-3-7091-9297-9_7. [DOI] [PubMed] [Google Scholar]


