Abstract
The inducible nitric oxide (NO) synthase and the cytokine transforming growth factor-β1 (TGF-β1), both central modulators of wound healing, interact reciprocally: TGF-β1 generally suppresses iNOS expression, while NO can induce and activate latent TGF-β1. We have shown that chemical NO activates recombinant human latent TGF-β1 by S-nitrosation of the latency-associated peptide (LAP), a cleaved portion of pro-TGF-β1 that maintains TGF-β1 in a biologically-inactive state. We hypothesized that cell-associated TGF-β1 could be activated by NO via known NO-inducible signaling pathways (soluble guanylate cyclase [sGC] and mitogen-activated protein [MAP] kinases). Treatment of mouse RAW 264.7 macrophage-like cells with the NO donor S-nitroso-N-acetyl-D,L-penicillamine (SNAP) led to a dose- and time-dependent increase in cell-associated active and latent TGF-β1, as assessed by quantitative immunocytochemistry for active TGF-β1 vs. LAP and partially validated by western blot analysis. Treatment with the sGC inhibitor 1,H-[1,2,4]oxadiazole[4,3-a]quinoxalon-1-one (ODQ) reduced both active and latent TGF-β1 dose-dependently. SNAP, in the presence or absence of ODQ or the MAP kinase inhibitors, did not affect steady-state TGF-β1 mRNA levels. Treatment with inhibitors specific for JNK1/2, ERK1/2, and p38 MAP kinases suppressed SNAP-induced active and latent TGF-β1. Treatment with the cell-permeable cGMP analog 8-Br-cGMP increased both active and latent TGF-β1. However, TGF-β1 activation induced by 8-Br-cGMP was not blocked by MAP kinase inhibitors. Our findings suggest that NO activates latent TGF-β1 via activation of sGC and generation of cGMP and separately via MAP kinase activation, and may shed insight into the mechanisms by which both cGMP production and MAP kinase activation enhance wound healing.
Wound healing is a process that involves both inflammation and the resolution of the inflammatory response.1 Although many cell types participate in this coordinated response, wound macrophages carry out key functions in the initial inflammatory response, the down-regulation of that response through the production of anti-inflammatory mediators, and the cascade of events involved in early remodeling events.1 Among the many agents produced by macrophages and involved in wound healing is nitric oxide (NO),2 produced through the actions of both the inducible and the endothelial NO synthases (iNOS and eNOS, respectively).2 Another agent central to the wound-healing response and produced by macrophages as well as other cells involved in wound healing is transforming growth factor β1 (TGF-β1).3 TGF-β1 is a cytokine that belongs to a family of three related isoforms, all of which exert crucial biological functions.3 All three isoforms are secreted in their latent form, which is characterized by its association with latency-associated peptide (LAP). The active cytokine is formed from two 25 kDa homodimers that are produced from a 100 kDa homodimer precursor. The precursor is cleaved intracellularly (in the Trans Golgi) by furin enzymes (proteases) to yield the 25 kDa active cytokine, which remains associated with LAP (the remaining portion of its own proform). The latent TGF-β can be bound by other proteins, such as latent TGF-β-binding protein (LTBP), which attaches itself to the latent cytokine by disulfide linkages between specific cysteine residues on the LTBP and the LAP. This complex is called the large latent complex (LLC), which attaches to the extracellular matrix, thus localizing the molecule to that area. In order for the cytokine to be considered active, it must be freed from the LLC, which consists of the active cytokine, the LAP, and the LTBP. There are many methods of post-translational activation, such as low pH, heat, proteases (such as plasmin), and both nitrogen and oxygen free radicals.4 Once active, the TGF-β regulates cellular processes by binding to three types of high-affinity cell surface receptors, types I, II, III, and then downstream via phosphorylation-dependent activation of Smad family members proteins and eventual gene transcription.5
Both NO and TGF-β1 play central roles in wound healing, and can cross-regulate each other.2 We have shown that induction of iNOS, or treatment with a NO donor to mimic iNOS induction, leads to the activation of latent TGF-β1.6 In cell-free experiments, NO activates TGF-β1 through the S-nitrosation of LAP, preventing it from binding the active cytokine.6 This S-nitrosation of LAP can occur relatively quickly (within 30 minutes in vitro),6 similar to the time scale reported for TGF-β1 activation induced by ionizing radiation and reactive oxygen species.7 However, NO is also known to activate several different signaling processes within the cell, including the soluble guanylate cyclase (sGC)/cyclic guanosine monophosphate (cGMP) and the mitogen-activated protein kinases (MAPK’s) p38, ERK1/2, and JNK1/2.8,9 We hypothesized that upon exposure to NO, some of these signaling systems may play additional roles in the induction and activation of TGF-β1. We examined the role of the sGC and MAPK signaling pathways in the activation of TGF-β1 in RAW 264.7 mouse macrophage-like cells. Our studies suggest that NO-stimulated TGF-β1 activation is driven by the activation of sGC, production of cGMP, and separately via the activation of MAPK.
MATERIALS AND METHODS
Reagents
S-Nitroso-N-acetyl-d,l-penicillamine (SNAP) was purchased from Alexis Biochemicals (San Diego, CA). Stock solutions (100 mM) were prepared in ethanol and kept in the dark. Decomposed solutions of SNAP were prepared by either incubating freshly prepared SNAP solutions at 37 °C for 24 hours (heat treatment) or by adding NaOH solution and adjusting the pH back to 7.4 with HCl solution (pH change). The chemical inhibitors ODQ, PD98059, SP600125, and SB203580 were all from Sigma-Aldrich (St. Louis, MO) and were dissolved in dimethyl sulfoxide (American Type Culture Collection, Manassas, VA). The DMEM and HEPES buffer used for cell culture was purchased from BioWhittaker–Lonza (Baltimore, MD). The penicillin/streptomycin and the l-glutamine media additives were purchased from Invitrogen. Fetal bovine serum (FBS) was purchased from Gemini BioProducts (West Sacramento, CA), and Gemini Standard™ (Gemini BioProducts) was used due to its low TGF-β1 content (Table 1). Antibodies to human TGF-β1 and human LAP antibody were purchased from R&D Systems (Minneapolis, MN). The secondary antibodies, the normal serum, the ABC enzyme conjugate kit, and the DAB brown stain kit were all purchased from Vector Laboratories (Burlingame, CA).
Table 1.
Levels of active and latent TGF-β1 in fetal bovine serum
| Latent TGF-β1 |
Active TGF-β1 |
|
|---|---|---|
| Samples | Concentration TGF-β1 (pg/mL) |
Concentration TGF-β1 (pg/mL) |
| Gemini Standard™-10% | 476.20268 | 19.629 |
| Gemini Standard™-1% | 104.7179 | 15.02 |
| Gemini Standard™-0.1% | 41.97662 | 13.781 |
| Gemini Neugem™-10% | 1167.52968 | 22.354 |
| Gemini Neugem™-1% | 205.50808 | 19.926 |
| Gemini Neugem™-0.1% | 50.40574 | 15.02 |
| Gemini Research™-10% | 895.23048 | 24.768 |
| Gemini Research™-1% | 154.60108 | 24.786 |
| Gemini Research™-0.1% | 50.40574 | 16.252 |
Fetal bovine serum samples (Gemini BioProducts, West Sacramento, CA) were diluted as indicated in ELISA assay medium and tested for the presence of latent and active TGF-β1 using a specific ELISA (R&D Systems, Minneapolis, MN) as per the manufacturer’s recommendations. In all the studies presented herein, RAW 264.7 cells were cultured using Gemini Standard™ at 1% (shaded).
ELISA, enzyme-linked immunosorbent assay; TGF-β1, transforming growth factor-β1.
Cell culture and experimental treatments
RAW 264.7 mouse macrophage-like cells (American Type Culture Collection) were cultured and plated in DMEM11% FBS containing l-glutamine and penicillin/streptomycin. The FBS used was previously determined to have the lowest levels of TGF-β1 of various manufacturers and lots (data not shown), in order to minimize the exposure of cells to TGF-β1 that could auto-induce expression of TGF-β1.10 The cells were cultured in 1% FBS because this was the lowest concentration of serum that allowed for cell proliferation while minimizing exposure to TGF-β1. The passage number was maintained < 18 for the same reason: as the passage number increased, the basal immunocytochemical expression of both active and latent TGF-β1 increased (Table 1). For each experiment, the cells were plated in eight-well Lab Tek™ Chamber Slide™ tissue culture plates (Nalge Nunc International, Rochester, NY), which allowed for several experimental conditions per well and also for immunocytochemistry at the end of the culture period. The cells were plated at a concentration of 2 × 105 cells/mL in 500 μL, and were maintained in 5% CO2 in a humidified atmosphere. The cells were incubated at 37 °C for 24 hours, and were then treated with the NO donor SNAP at various concentrations, in the presence or absence of specific inhibitors of sGC (ODQ), p38 (SB203580), JNK1/2 (SP600125), or ERK1/2 (PD98059). All of the inhibitors were dissolved in DMSO and were administered at concentrations of 0, 0.3, 3, or 30 μM 15 minutes before the addition of SNAP. Each treatment was carried out in duplicate, in order to allow for the eventual parallel immunocytochemical detection of active and latent TGF-β1 (see below). The cells were then incubated for a further 24 hours and then processed for immunocytochemistry.
Immunocytochemistry
At the conclusion of the incubation period, the cells were fixed in 70% ethanol for 20 minutes, followed by a 5-min wash in phosphate-buffered saline (PBS). Endogenous peroxidases were inhibited using 0.3% hydrogen peroxide solution for 30 minutes. Nonspecific antibody reactivity was blocked as follows: the wells that were stained for active TGF-β1 were blocked with 1.5% goat serum, while the wells stained for latent TGF-β1 were blocked with 1.5% rabbit serum; all blocking was performed for 20 minutes at room temperature. The cells were then incubated with primary antibody specific for either active TGF-β1 (chicken anti-human active TGF-β1; R&D Systems) or latent TGF-β1 (goat anti-human latent TGF-β1; R&D Systems) for 30 minutes. After washing in PBS, the cells were then incubated with secondary antibodies as follows: for wells being stained for active TGF-β1, the secondary antibody solution consisted of approximately 0.5% goat anti-chicken antibody, mixed with 1.5% goat serum and 1 mL PBS. For wells being stained for latent TGF-β1, the secondary antibody solution consisted of 0.5% rabbit anti-goat antibody mixed with 1.5% rabbit serum and 1 mL PBS. The incubation time for the secondary antibody was 30 minutes at room temperature. The cells were then exposed to the ABC enzyme conjugate for 30 minutes, after which they were stained with a diaminobenzidene (DAB) brown stain for 90 seconds. The cells were then incubated with a hematoxylin blue counterstain for 20 seconds. The slides were then dehydrated and cleared in a series of graded ethanol solutions (one wash in 70%, two washes in 95%, and two washes in 100%), followed by one wash in xylene. The slides were then dried overnight and mounted with Permount™ (Fisher Scientific, Pittsburgh, PA).
Quantification of immunocytochemistry
A variation of a previously published method by El-Sherif et al.11 was used to quantify the degree of active and latent TGF-β1 immunocytochemistry. In that previous study, only grayscale images were used;11 in the present study, color images were used as described below. Three images from each well were captured using a Motic microscope (Motic Biological Microscopes, Richmond, BC, Canada) equipped with a digital camera and Motic Images 2000 version 1.3 software (Motic China Group). The images were captured at ×100 magnification, and were analyzed using the Image J™ 1.35c freeware (National Institutes of Health, Bethesda, MD) and the Color Inspector 3D plug-in. Because the two primary colors in each picture were brown and blue, data were gathered using a brown to blue ratio. Owing to the DAB brown stain, the color brown indicated the presence of TGF-β1, while the color blue, from the hematoxylin counterstain, indicated the absence of TGF-β1. The histogram setting in Color Inspector 3D was used in order to cluster similar color values, thus simplifying the quantification process. The thresholds for blue and brown were set, and the percentage of each photomicrograph taken up by pixels that fell under the “blue” category or the “brown” category was assessed. The ratio of brown positive/blue positive cells in two to three fields per image was calculated utilizing the following formula:
Each complete immunocytochemistry assessment was repeated at least three times/experiment and the mean brown positive/blue positive ratio was calculated. A higher brown positive/blue positive mean value indicates a higher cell-associated expression of either active or latent TGF-β1. The final values for active or latent TGF-β1 were determined as fold change (vs. nontreated control cells) ± SEM of at least three independent experiments as indicated.
Northern blot analysis
Total RNA was isolated from cultured RAW 264.7 cells using an UltraSpec™ RNA isolation reagent from Biotecx Laboratories Inc. (Houston, TX). Briefly, the cells were lysed directly in a culture dish by adding UltraSpec™ reagent (1 mL/3.5-cm Petri dish) and passing the cell lysate several times through a pipette. After extraction with chloroform (0.2 mL/mL of UltraSpec™), the total RNA was precipitated from the aqueous phase by addition of isopropanol, washed with 75% ethanol, and solubilized in diethyl pyrocarbonate (DEPO)-treated water. The RNA (20 μg/lane) was electrophoresed on a 0.9% agarose gel containing 12.3 M formaldehyde and transferred to nylon membranes (GeneScreen™, PerkinElmer Life Sciences, Waltham, MA) by vacuum blotting. The membranes were prehybridized for 3–4 hours at 43 °C and hybridized with a [32P]dCTP-labeled probe (106 c.p.m./mL) at 43 °C. Membranes were then washed three times with SSC/SDS at 53 °C before exposure. Membranes were then stripped and probed for 18S RNA to assess RNA loading. Northern blot analysis of TGF-β1 mRNA levels in RAW 264.7 cells was carried out using a 1.6-kb TGF-β1 cDNA probe derived from the mouse full-length sequence (Image clone #3586216 from Invitrogen, Accession #BC013738) after Xba 1 and Sal 1 digestion. Northern blots were hybridized with labeled probes that were previously sequenced. Radioactive membranes were quantified with storage phosphor screens (PhosphorImager™, Molecular Dynamics, Piscataway, NJ), and the relative amount of mRNA is presented as the ratio of mRNA to 18S RNA.
Western blot analysis
RAW 264.7 cell monolayers (5 × 106 cells/100-mm Petri dish) were washed twice with ice-cold PBS and resuspended in 1 mL of ice-cold lysis buffer containing 2 mM Tris-HCl buffer (pH 7.5), 15 mM NaCl, EDTA and EGTA (both 100 μM), 0.1% Triton X-100, 250 μM sodium pyrophosphate, 100 μM β-glycerolphosphate, 100 μM Na3VO4, and the protease inhibitors leupeptin (0.1 μg/mL) and phenylmethylsulfonyl fluoride (1 mM). After a 5-minutes incubation on ice, the cells were scraped off the dish and transferred to microcentrifuge tubes. Following a 20–30-minutes incubation at 4 °C, cell debris was removed by centrifugation at 15,000 g for 10–15 minutes, and the supernatant was used as a cell lysate and stored at −80 °C when necessary. An aliquot was used to determine the protein concentration using the BCA Protein Assay kit from Pierce (Rockford, IL) with bovine serum albumin as the standard.
For detection of active and latent TGF-β1, protein samples (75 μg) were separated on 12% SDS-polyacrylamide gels, and the gels were electroblotted onto PVDF membranes. Latent TGF-β1 was detected using the same polyclonal goat anti-LAP antibodies utilized for immunocytochemistry (R&D systems, 1: 1,000 dilution), followed by HRP-conjugated anti-goat secondary antibody (1: 1,000 dilution). The bands were detected using the Supersignal West Pico Chemiluminiscent kit following the instructions of the manufacturer (Thermofisher Scientific, Rockford, IL). For normalization, the membranes were stripped and reprobed with an anti-β-actin antibody from Sigma-Aldrich followed by densitometric analysis of both the experimental (LAP or TGF-β1) and control (β-actin) blots.
For detection of MAPK, protein samples (30 μg) were separated on 15% SDS-polyacrylamide gels, and the gels were electroblotted onto nitrocellulose membranes. Immunodetection of both MAPK- and phospho-MAPK proteins was performed using the ECFTM Western blotting kit (Amersham Pharmacia Biotech, Arlington Heights, IL) and the rabbit polyclonal antibodies from Cell Signaling Technology® (Danvers, MA). Instructions for the kit were provided by the supplier. We sought to determine the proportion of total MAPK that were phosphorylated (and hence presumably enzymatically active). Accordingly, we quantified both ERK1/2 and JNK1/2 Western blots as follows: the sum of the two bands for phospho-MAPK was divided by the sum of the bands for total MAPK. In order to assess the degree of MAPK phosphorylation, we calculated the fold activation as the ratio between the normalized band density for each treatment divided by the normalized band density of the respective control at each time point.
Statistical analysis
Experiments were performed at least three times independently and data are presented as mean ± SEM. Statistical differences were assessed using Student t-test, Mann–Whitney rank sum test, or one-way analysis of variance (ANOVA), followed by the Fisher LSD post hoc test or by the Holm–Sidak method, where appropriate, using Sigma-Plot for Windows 9.01 (Systat Software Inc., San Jose, CA). Differences were considered significant at the 95% confidence interval (p < 0.05).
RESULTS
Active and latent TGF-β1 are increased in macrophages following exposure to NO
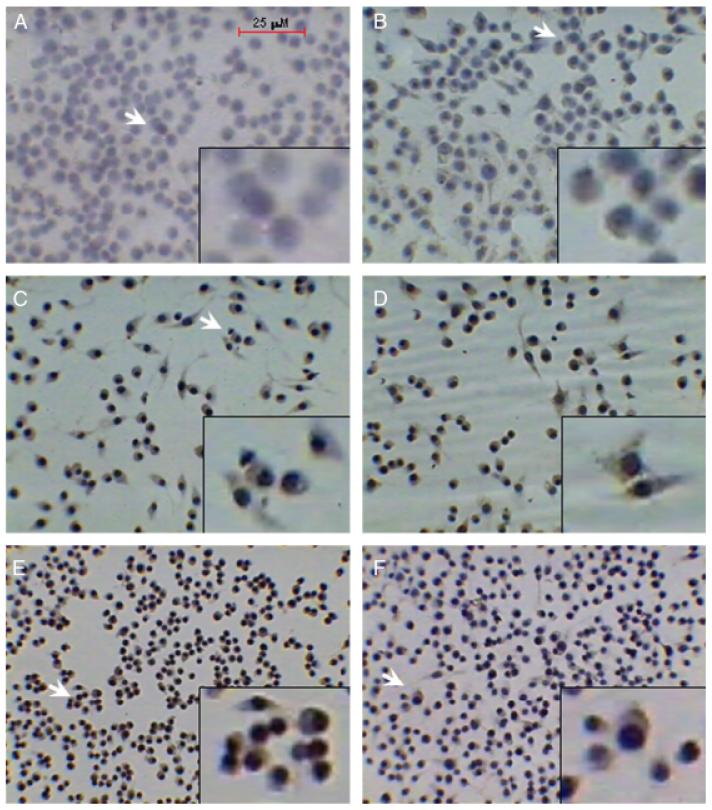
The expression of latent and active TGF-β1 can be assessed by various methods, including ELISA, cell-based bioactivity assays, Western blotting, and immunocytochemistry to detect cell-associated TGF-β1.11-16 Our original report on the effects of NO on active and latent TGF-β1 in macrophages was made using immunocytochemical detection of cell-associated active and latent TGF-β1 following stimulation with the NO donor 1,1-diethyl-2-hydroxy-2-nitroso-hydrazine (DEA/NO).6 We also demonstrated activation of TGF-β1 induced by radiation in macrophages using immunocytochemistry.17 We therefore first wished to confirm that this effect was independent of the type of macrophage and NO donor used, and so we examined the effect of a 24-hour treatment with the NO donor SNAP on RAW 264.7 mouse macrophage-like cells. As can be seen in Figure 1, treatment with 100 μM SNAP resulted in increased expression of both active and latent TGF-β1 protein (Figure 1C and D, respectively) as compared with untreated cells (Figure 1A and B, respectively). Treatment with 1,000 μM SNAP, a high dose of this NO donor that can induce cell death in RAW 264.7 cells,18 also led to TGF-β1 activation, but the appearance of the cells was rounder and less spread and suggested a detrimental effect of this high dose of SNAP (Figure 1, compare Figure 1E–F with Figure 1A–D).
Figure 1.
Increased expression of active and latent transforming growth factor-β1 (TGF-β1) protein induced by the nitric oxide donor S-nitroso-N-acetyl-d,l-penicillamine (SNAP) in RAW 264.7 macrophage-like cells. Mouse RAW264.7 macrophage-like cells were incubated with medium alone (A and B), with 100 μM SNAP (C and D), or with 1,000 μM SNAP (E and F) for 24 hours. The cells were then immunostained for active TGF-β1 (A, C, and E) or latent TGF-β1 (B, D, and F) as described in “Materials and methods,” and photomicrographs were taken at ×40 magnification. Arrows indicate the region from each respective field digitally magnified and depicted in the insets. Red bar (A) indicates 25 μm.
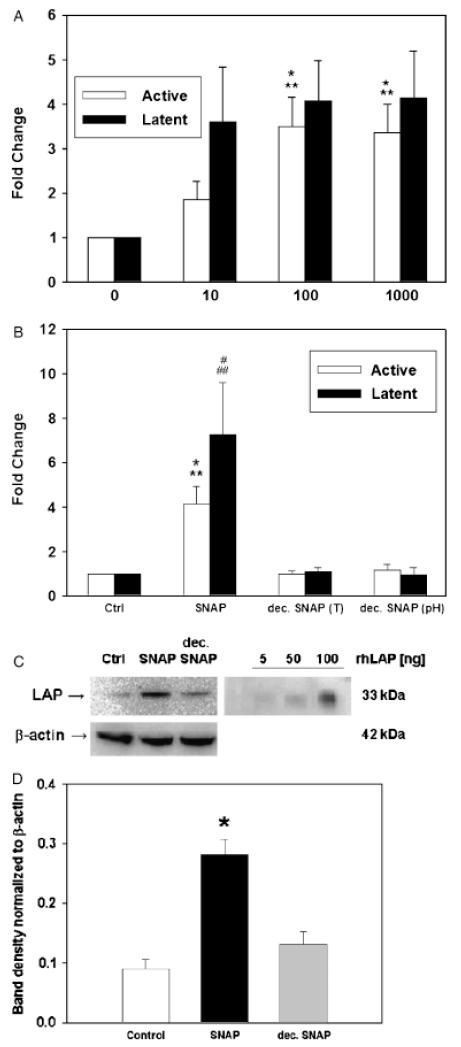
Our prior immunocytochemical studies on TGF-β1 activation in macrophages did not quantify the extent of activation.6,17 Accordingly, we attempted to quantify the effects of SNAP on active and latent TGF-β1. To do so, we developed a quantitative immunostaining assay based on DAB staining, as described in “Materials and methods.” As can be seen in Figure 2A, treatment with SNAP for 24 hours at doses of 10–1,000 μM increased the expression of both active and latent TGF-β1 in a dose-dependent fashion, with a peak of both active and latent TGF-β1 seen at 100 μM. This effect was caused by NO released from SNAP, because treatment of RAW 264.7 cells with 100 μM SNAP that was decomposed before addition to the cells (either by changing the pH or by changing the temperature) did not alter the levels of either active or latent TGF-β1 (Figure 2B).
Figure 2.
Nitric oxide induces a dose-dependent increase in active and latent transforming growth factor-β1 (TGF-β1) protein expression in RAW 264.7 macrophage-like cells. (A) RAW 264.7 cells were treated with 10–1,000 μM S-nitroso-N-acetyl-d,l-penicillamine (SNAP) for 24 hours, and the expression of TGF-β1 was analyzed as described in “Materials and methods.” Results shown are the mean ± SEM from six to seven independent experiments (*p < 0.005 vs. control; **p < 0.05 vs. 10 μM SNAP). (B) RAW 264.7 cells were treated with 100 μM SNAP or decomposed SNAP (either by heat treatment [T] or pH change) for 24 hours, and the expression of TGF-β1 was analyzed as described in “Materials and methods.” Results shown are the mean ± SEM. from four independent experiments (*p < 0.05 vs. control and decomposed SNAP (pH), **p < 0.01 vs. decomposed SNAP (T), #p < 0.05 vs. control and decomposed SNAP (T), ##p < 0.01 vs. decomposed SNAP (pH). (C) RAW 264.7 cells were untreated, treated with 100 μM SNAP, or 100 μM decomposed SNAP. Cell lysates or defined amounts (5, 50, or 100 ng) of recombinant human latency-associated peptide (LAP) were subjected to anti-LAP and β-actin Western blot as described in “Materials and methods.” The experiment is representative of three; numbers indicate quantification (mean ± SEM) of LAP expression normalized for β-actin expression.
To verify our findings, we carried out Western blotting for latent TGF-β1 (identified specifically with anti-LAP antibody in a manner analogous to prior studies by others12) of lysates of RAW 264.7 cells that were unstimulated or stimulated with 100 μM SNAP or 100 μM decomposed SNAP. As can be seen in Figure 2C, the levels of the LAP 33 kDa monomer (indicative of latent TGF-β1) present in RAW 264.7 cells stimulated with SNAP were higher than those present either in control cells or cells treated with decomposed SNAP. The specificity of the antibodies for LAP was established by inclusion of a defined amount of recombinant human LAP. Western blots of the same lysates for active TGF-β1 showed very faint bands that were impossible to quantify, even when nanogram quantities of recombinant human TGF-β1 or 100 μg of RAW 264.7 cell lysates were loaded (data not shown). Because the effect of SNAP on active and latent TGF-β1 was maximal at 100 μM (Figure 2A), and because it appeared that 1,000 μM SNAP was exerting a detrimental effect on the cells (Figure 1), we carried out the remainder of our studies on NO-mediated activation of TGF-β1 using 100 μM SNAP for 24 hours.
Inhibition of the sGC pathway suppresses NO-induced expression of latent and active TGF-β1
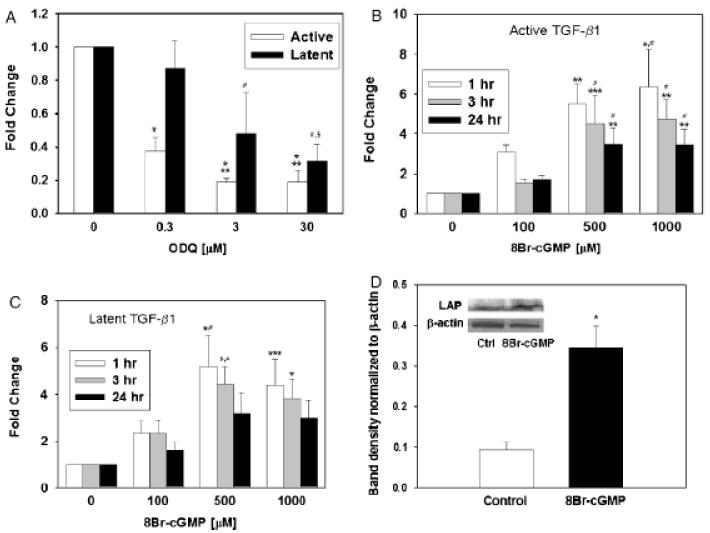
The canonical second messenger pathway associated with NO involves the activation of sGC by the binding of NO to the enzyme’s catalytic site heme, with the subsequent production of cGMP.8 We therefore sought to determine whether this pathway was involved in the increased expression of active and latent TGF-β1 induced by NO in RAW 264.7 cells. A 15-min pretreatment of RAW 264.7 cells with the sGC inhibitor ODQ led to decreased expression of both active and latent TGF-β1 induced by 100 μM SNAP at 24 hours, in a manner dependent on ODQ dose (Figure 3A). Conversely, treatment of RAW 264.7 cells with a cell-permeable analog of cGMP (8-Br-cGMP) in the absence of NO was associated with increased expression of both active (Figure 3B) and latent (Figure 3C) TGF-β1. The maximal effect of 8-Br-cGMP was seen at 1 hour of stimulation and at a dose of 500 μM. Longer incubations with 8-Br-cGMP resulted in a lower degree of TGF-β1 activation or increased latent TGF-β1 expression (Figure 3B and C). We partially validated the effect of 8-Br-cGMP on TGF-β1 using the same Western blotting technique described for Figure 2C: treatment of RAW 264.7 cells led to the significantly increased expression of latent TGF-β1 (Figure 3D). As described for Figure 2C, we could not observe a band upon Western blotting for active TGF-β1 (data not shown).
Figure 3.
Increased expression of active and latent transforming growth factor-β1 (TGF-β1) protein induced by S-nitroso-N-acetyl-d,l-penicillamine (SNAP) is mediated partially by soluble guanylate cyclase (sGC). (A) Treatment of RAW 264.7 cells with 100 μM SNAP for 24 hours led to activation of latent TGF-β1 (increased brown stain; quantification shown). Co-treatment with the sGC inhibitor 1,H-[1,2,4]oxadiazole[4,3-a]quinoxalon-1-one (ODQ) reversed the activation of TGF-β1 induced by SNAP. Results shown are the mean ± SEM from four independent experiments (*p < 0.001 vs. control, **p < 0.05 vs. 0.3 μM ODQ, #p < 0.05 vs. control; $p < 0.05 vs. 0.3 μM ODQ). (B) (active) and (C) (latent): Treatment of RAW264.7 cells with the cell-permeable cGMP analog 8-Br-cGMP (100, 500, or 1,000 μM) shows a concentration- and time-dependent effect on both active (B) and latent (C) TGF-β1. Results shown are the mean ± SEM from six to seven independent experiments ($p < 0.001, *p < 0.005, **p < 0.01 and ***p < 0.05 vs. control; #p < 0.05 vs. 100 μM 8-Br-cGMP). (D) Treatment with 8-Br-cGMP (500 μM) led to an increase in latent TGF-β1 protein levels as detected by Western blotting analysis using anti-latency-associated peptide. A representative blot (insert) and the densitometric analysis of five experiments expressed as the mean ± SEM are shown. (*p=0.002 vs. control; analyzed by Student t-test).
Inhibition of the MAPK pathway suppresses NO-induced expression of latent and active TGF-β1
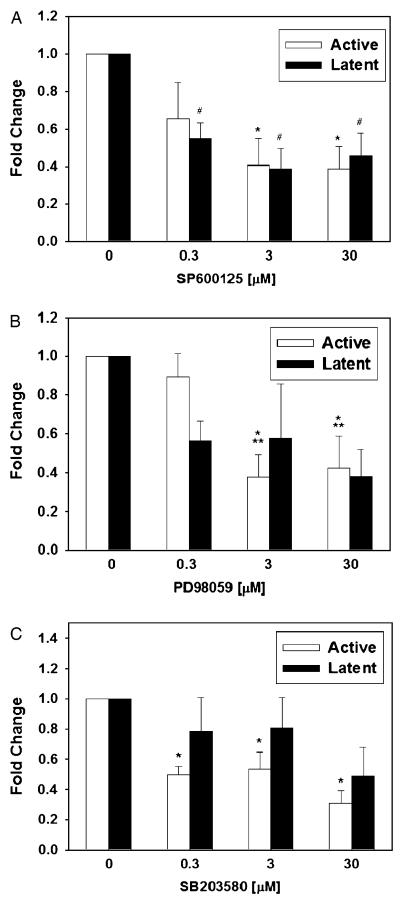
The MAPK are a group of related serine/threonine protein kinases that participate in transmitting extracellular signals to the cell nucleus. Activation of each of the three major MAPK subfamilies (ERK1/2, JNK1/2, and p38) involves dual phosphorylation of tyrosine and threonine residues within the catalytic domain of these enzymes.19 NO can either induce or suppress the MAPK family of signaling enzymes.9 Accordingly, we sought to determine whether inhibition of these three MAPK would affect NO-stimulated increases in the expression of active and latent TGF-β1. As can be seen in Figure 4A, treatment with the JNK1/2 inhibitor SP600125 led to a dose-dependent decrease in both active and latent TGF-β1 at 24 hours upon co-treatment with 100 μM SNAP. A similar, but less potent, effect was seen upon inhibition of ERK1/2 with PD98059 (Figure 4B). The effect of inhibition of p38 with SB203580 was lesser than the other two MAPK inhibitors, and was only statistically significant for inhibition of TGF-β1 activation and not for expression of latent TGF-β1 (Figure 4C).
Figure 4.
Increased expression of active and latent transforming growth factor-β1 (TGF-β1) protein induced by S-nitroso-N-acetyl-d,l-penicillamine (SNAP) is mediated partially via the mitogen-activated protein kinase pathway. (A—C) Treatment of RAW 264.7 cells with 100 μM SNAP for 24 hours led to activation of latent TGF-β1. (A) Co-treatment with the JNK1/2 inhibitor SP600125 reversed the expression of both latent and active TGF-β1 (reduced brown stain; quantification shown) induced by SNAP. Results shown are the mean ± SEM from five independent experiments (*p < 0.01 and #p < 0.005 vs. control). (B) Co-treatment with the ERK1/2 inhibitor PD98059 reversed the expression of both latent and active TGF-β1 (reduced brown stain; quantification shown) induced by SNAP. Results shown are the mean ± SEM from five independent experiments (*p < 0.005 vs. control; **p < 0.05 vs. 0.3 μM PD98059). (C) Co-treatment with the p38 inhibitor SB203580 only slightly reversed the activation of TGF-β1 and had no significant effect on the expression of latent TGF-β1. Results shown are the mean ± SEM from five independent experiments (*p < 0.001 vs. control).
Confirmation of the effects of sGC and MAPK inhibition of SNAP-induced latent TGF-β1
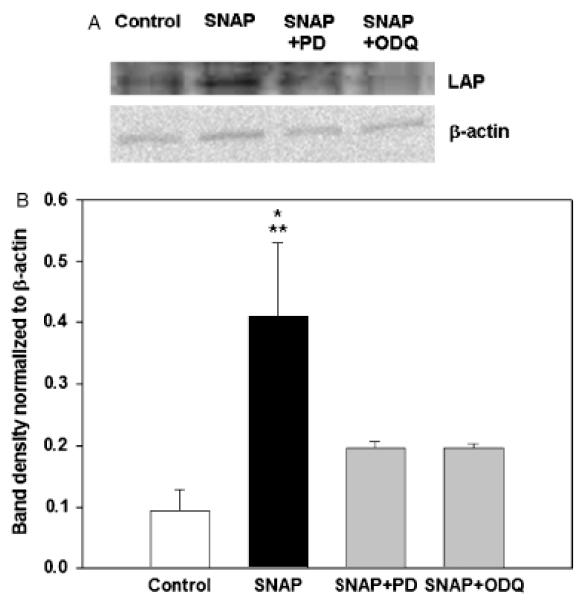
We wished to confirm the findings regarding the effects of ODQ and MAPK inhibitors depicted in Figures 3 and 4. Accordingly, we subjected RAW 264.7 cells to 100 μM SNAP in the absence or presence of ODQ or the ERK1/2 inhibitor PD98059, followed by Western blot analysis of latent TGF-β1 as in Figure 2C. As can be seen in Figure 5, and confirming the results of Figure 2C, treatment of RAW 264.7 cells with SNAP led to significantly increased latent TGF-β1 expression. Pretreatment with either ODQ or PD98059 significantly suppressed the elevated expression of latent TGF-β1 induced by SNAP.
Figure 5.
Inhibition of soluble guanylate cyclase (sGC) or mitogen-activated protein kinases reduces S-nitroso-N-acetyl-d,l-penicillamine (SNAP)-induced latent transforming growth factor-β1 (TGF-β1) expression. (A) and (B) Co-treatment with both the ERK1/2 inhibitor PD98059 (3 μM) and the sGC inhibitor 1,H-[1,2,4]oxadiazole[4,3-a]quinoxalon-1-one (ODQ) (3 μM) inhibited the elevation of latent TGF-β1 protein levels induced by SNAP as detected by Western blotting analysis using anti-latency-associated peptide. A representative blot (A) and the densitometric analysis (B) of three independent experiments expressed as the mean ± SEM are shown. (*p < 0.005 vs. control; **p < 0.05 vs. PD98059 and ODQ, analyzed by one-way analysis of variance, followed by the Fisher LSD method).
Neither NO nor inhibitors of sGC or MAPK affect steady-state TGF-β1 mRNA
Given that TGF-β1 can auto-induce its own expression,10 we next sought to determine whether the effects of SNAP on TGF-β1 protein would be manifest at the mRNA level. Additionally, we sought to determine whether inhibitors of sGC and the MAPK would antagonize any SNAP-induced effects on TGF-β1 mRNA. As can be seen in Figure 6, 100 μM SNAP exerted little effect on steady-state TGF-β1 mRNA in RAW 264.7 cells following 24 hours of treatment. Pretreatment of these cells with inhibitors of sGC or MAPK 15 minutes before the addition of 100 μM SNAP likewise did not affect steady-state TGF-β1 mRNA.
Figure 6.
Neither nitric oxide nor the soluble guanylate cyclase (sGC) and mitogen-activated protein (MAP) kinase signaling pathway affects steady-state transforming growth factor-β1 (TGF-β1) mRNA. RAW264.7 cells were treated with S-nitroso-N-acetyl-d,l-penicillamine (SNAP) (100 μM) in the absence or presence of the MAPK inhibitors (3 μM) or soluble guanylate cyclase inhibitor (3 μM) for 24 hours. Total RNA was isolated, followed by Northern blotting and analysis for TGF-β1 mRNA (20 μg total RNA/lane) as described in “Materials and methods.” A representative blot (A) and the densitometric quantification (B) of three independent experiments are shown.
Lack of cross-talk between sGC and MAPK in the regulation of latent and active TGF-β1 protein
Our indings (Figures 3 and 4) suggested that two different signaling pathways, sGC and MAPK, can affect the expression of active and latent TGF-β1 induced by NO to roughly similar degrees. Because cGMP has been reported to induce MAPK activation,20-25 we sought to determine whether a similar cross-talk can lead to increased expression of active and latent TGF-β1. As can be seen in Figure 3B, treatment of RAW 264.7 cells with 8-Br-cGMP led to the activation of latent TGF-β1 and increases in latent TGF-β1 in a dose-dependent fashion. Based on this finding, we hypothesized that the sequence of events induced by NO and culminating in TGF-β1 expression and activation could be the following: NO → sGC activation → production of cGMP → activation of MAPK.
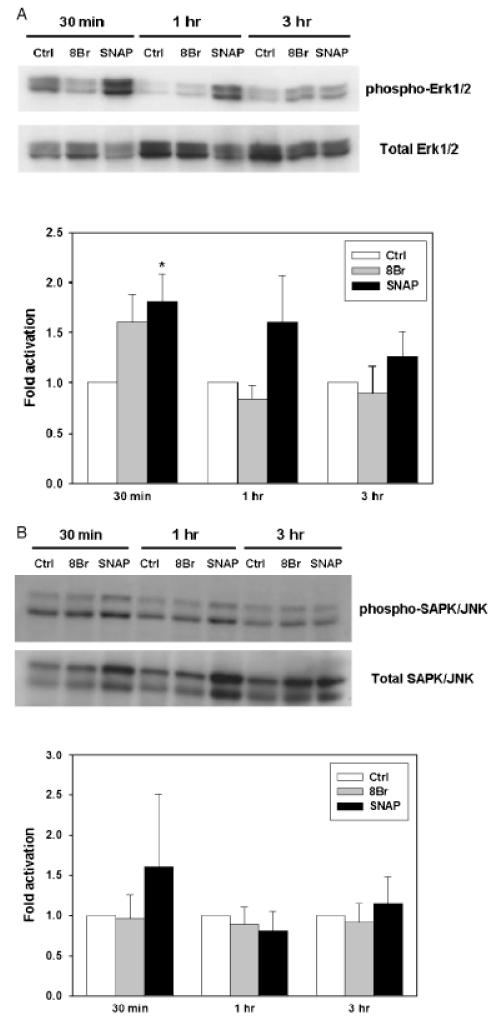
To test this hypothesis, we first examined the expression of phosphorylated (activated) MAPK in RAW 264.7 cells treated with SNAP or 8-Br-cGMP. Both SNAP and 8-Br-cGMP independently caused variable increases in phosphorylated ERK1/2 (as seen in Figure 7A) and, to a lesser degree, in JNK1/2 (Figure 7B). Treatment with SNAP resulted in phosphorylation of ERK1/2 significantly at 30 minutes but not so at 1 hour and 3 hours (Figure 7A). Treatment with 8-Br-cGMP, in contrast, resulted in generally lower levels of ERK1/2 phosphorylation at 1 hour and 3 hours, with the maximum observed at 3 hours (Figure 7A). 8-Br-cGMP led to a variable increase in ERK1/2 phosphorylation at 30 minutes, but this was not statistically significant. No changes in ERK1/2 phosphorylation were observed at 1 hour or 3 hours following treatment. We observed JNK1/2 phosphorylation only at 30 minutes of stimulation with SNAP (although this effect did not reach statistical significance), but not at 1 hour or 3 hours or with 8-Br-cGMP at any time point (Figure 7B). We could not detect phosphorylated p38 in RAW 264.7 cells treated with either SNAP or 8-Br-cGMP (n=3; data not shown). Taken together, we found that both NO and cGMP could increase the phosphorylation of ERK1/2, and to a lesser degree, JNK1/2, although this effect was variable and only reached statistical significance under limited circumstances.
Figure 7.
Effect of nitric oxide and cGMP on ERK1/2 kinase phosphorylation. RAW264.7 macrophage-like cells were incubated with medium alone (control), S-nitroso-N-acetyl-d,l-penicillamine (SNAP) (100 μM), or 8-Br-cGMP (500 μM) for 30 minutes, 1, and 3 hours as indicated. Cell lysates were prepared and protein was isolated, followed by Western blotting and analysis for phospho-ERK1/2 and total ERK1/2 (A) or phospho-JNK1/2 and total JNK1/2 (B) as described in “Materials and methods.” A representative blot of six independent experiments for each panel, followed by the densitometric analysis of those experiments, expressed as the mean ± SEM, are shown. (*p=0.010 vs. control; analyzed by the Mann–Whitney rank sum test).
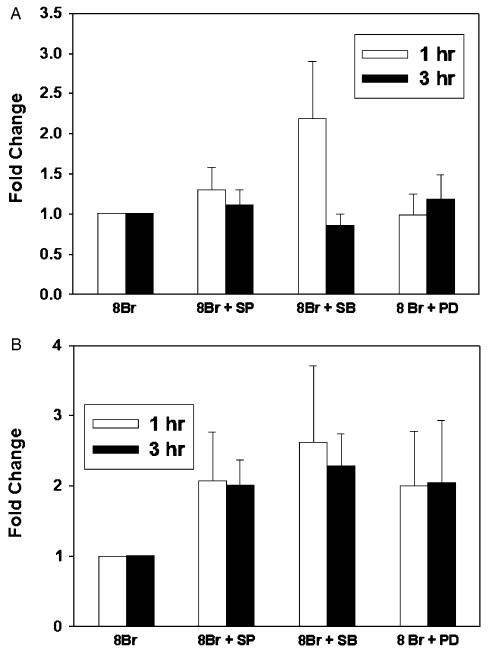
We then sought to determine whether 8-Br-cGMP would activate latent TGF-β1 via a MAP kinase-dependent mechanism, by stimulating RAW 264.7 cells with 8-Br-cGMP in the presence or absence of MAP kinase inhibitors. In 10 separate experiments, no statistically significant effect of MAP kinase inhibitors was seen on either active (Figure 8A) or latent (Figure 8B) TGF-β1 induced by 8-Br-cGMP. Thus, the effects of NO on activation of TGF-β1 appear to be mediated independently through the sGC and MAP kinase pathways.
Figure 8.
Effect of mitogen-activated protein kinases on soluble guanylate cyclase -induced expression of active and latent transforming growth factor-β1 (TGF-β1). RAW264.7 macro-phage-like cells were treated with 8-Br-cGMP (500 μM) in the absence or presence of the p38 inhibitor SB203580 (SB, 3 μM), the ERK1/2 inhibitor PD98059 (PD, 3 μM), or the JNK inhibitor SP600125 (SP, 3 μM), for 1 hour and 3 hours. The cells were then immunostained for active (A) or latent TGF-β1 (B) and analyzed as described in “Materials and methods.” Results shown are the mean ± SEM from eight independent experiments and represent fold change vs. cells treated with 8-Br-cGMP alone.
DISCUSSION
NO can exert numerous, often overlapping effects in both physiological and pathological settings, including wound healing.2 One of these effects of NO and related redox species involves the modulation of expression and activation of the cytokine TGF-β1;6 this interaction may underlie various disease processes.2 The increased expression and activation of TGF-β1 by NO may occur via the S-nitrosation of LAP, preventing the ability of LAP to bind to and hence inactivate TGF-β1.6 In the present study, we suggest that NO can lead to the increased expression and activation of TGF-β1 via both the sGC and the MAP kinase pathways in an apparently independent manner, despite previous reports of cross-talk between these pathways.20 Given the central role of TGF-β1 in promoting wound healing, our results support studies carried out over two decades ago demonstrating that cGMP promotes wound healing,26 as well as recent work showing that NO stimulates collagen type I expression in keloid fibroblasts via the activation of TGF-β1, secondary to the formation of cGMP.27 Our results are also in line with studies that suggest a crucial role for activation of MAPK in various cell types involved in wound healing.28
We embarked on our studies seeking to elucidate signal transduction pathways by which NO could activate TGF-β1 in macrophages. We had previously demonstrated that the NO donor DEANO led to increased expression of both active and latent TGF-β1.6 We found that pharmacological inhibition of sGC, JNK1/2, ERK1/2, and to a lesser extent p38, was associated with reduced expression of active TGF-β1 protein. Blocking all of these pathways, except p38, was also associated with reduced expression of latent TGF-β1 protein. From these results, it is tempting to speculate that NO first leads to the activation of TGF-β1, which in turn leads to increased transcription of TGF-β1 mRNA and subsequently to increased levels of latent TGF-β1 protein. However, two findings contradict this hypothesis. First, we found no evidence of increased TGF-β1 mRNA in response to NO donors, either in our previous study6 or in this one. Second, NO led to increases in both active and latent TGF-β1 at the same time points. We therefore hypothesize that NO may reduce the rate of degradation of latent TGF-β1, a hypothesis that is beyond the scope of the present studies.
Because we found that blocking the activity of sGC was associated with reduced expression of active and latent TGF-β1 protein, we sought to determine the effect of cGMP on TGF-β1 protein. Treatment with the cGMP analog 8-Br-cGMP replicated the effects of NO on latent and active TGF-β1. These effects were greatest after 1 hour oftreatment and declined at later time points. We consider these results to support a short-lived, second messenger role for cGMP in the effects of NO on TGF-β1.
Based on the effects of NO, cGMP, and the MAPK on TGF-β1 protein, we hypothesized an interaction between the sGC and the MAP kinase pathways in the modulation of active and latent TGF-β1 by NO. This hypothesis was supported by prior studies from other groups that have shown cross-talk between these pathways.20 We found that both NO and cGMP could increase the phosphorylation of ERK1/2, and to a lesser degree, JNK1/2, although this effect was variable and only reached statistical significance following a 30-minutes treatment with SNAP. However, we could not demonstrate any cGMP-stimulated changes in TGF-β1 protein that could be blocked by MAP kinase inhibitors in a large number of experiments. Thus, we conclude that the effects of NO on TGF-β1 are mediated separately by the sGC and MAP kinase pathways.
Studies dating back over 15 years have demonstrated that TGF-β1 suppresses the expression of iNOS at multiple regulatory levels, including DNA transcription, mRNA stability, mRNA translation, protein stability, and probably also iNOS enzymatic activity.29 In turn, NO and related redox species can lead to the activation of TGF-β1,6 raising the possibility that these reciprocal interactions represent a sensor for redox and other stress, as part of the more general sensor property of the TGF-β1 regulatory system.15
It is logical for a sensor activity that involves such central mediators as NO and TGF-β1 to invoke diverse signaling pathways. This interlocking cascade of events may be involved in the feedback regulation of iNOS, given that TGF-β1 is a potent negative regulator of this enzyme2 and that iNOS expression itself is in part regulated via the MAP kinase pathway.9 TGF-β1 signaling occurs in many settings via the MAP kinase pathway.30 Our findings substantiate previous reports showing that certain agents that induce TGF-β1 expression do so via activation of the ERK1/231 or the p3832 pathway.
Our studies have several limitations. Chief among them is the primary use of immunocytochemistry to detect active and latent TGF-β1. ALthough various methods have been used to quantify TGF-β1,15 the immunocytochemical detection of TGF-β1 has been demonstrated to be both extremely sensitive and verifiable by other assay methods, especially given the apparent cell-surface association of activated TGF-β1.6,11,17,33 We confirmed key aspects of our studies using Western blot analysis for latent TGF-β1; repeated attempts to detect active TGF-β1 by Western blot failed (data not shown). We used only RAW264.7 macrophages in the present study, as we have in the past to examine radiation-induced TGF-β1 activation,17 although in other prior studies we have used ANA-1 macrophages to study NO-mediated TGF-β1 activation.6 We used only the NO donor SNAP in the present study, although in a prior study we used the NO donor DEANO to demonstrate activation of latent TGF-β1.6 Thus, we feel that the results depicted herein are representative of the responses of macrophages to NO. We observed variable effects of NO and 8-Br-cGMP on the steady-state levels of TGF-β1 mRNA and likewise variable phosphorylation of ERK1/2 and JNK1/2, which may require further study.
In conclusion, we suggest that the reciprocal interactions between NO and TGF-β1 can be mediated independently via the sGC and MAP kinase signaling pathways. Further studies in our laboratory are aimed at determining the downstream effects of this interaction, the mechanisms by which NO can lead to simultaneous increases in both active and latent TGF-β1, and the ultimate effects on wound healing.
ACKNOWLEDGMENTS
The authors would like to thank Binnie Betten and Hemamalini Vedagiri for their expert technical assistance. This work was supported by National Institute of Allergy and Infectious Disease grant R01-AI-50663-03.
Glossary
- 8-Br-cGMP
8-Bromo cyclic guanosine monophosphate
- cGMP
Cyclic guanosine monophosphate
- LAP
Latency-associated peptide
- NO
Nitric oxide
- ODQ
1,H-[1,2,4]oxadiazole[4,3-a]quinoxalon-1-one
- sGC
Soluble guanylate cyclase
- SNAP
S-nitroso-N-acetyl-d,l-penicillamine
- TGF-β1
Transforming growth factor-β1
Footnotes
Dr. Metukuri’s current addrress is Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania.
REFERENCES
- 1.Hart J. Inflammation. 1: its role in the healing of acute wounds. J Wound Care. 2002;11:205–9. doi: 10.12968/jowc.2002.11.6.26411. [DOI] [PubMed] [Google Scholar]
- 2.Schwentker A, Vodovotz Y, Weller R, Billiar TR. Nitric oxide and wound repair: role of cytokines? Nitric Oxide. 2002;7:1–10. doi: 10.1016/s1089-8603(02)00002-2. [DOI] [PubMed] [Google Scholar]
- 3.Roberts AB, Sporn MB. Transforming growth factor-β. In: Clark RAF, editor. The molecular and cellular biology of wound repair. Plenum Press; New York: 1996. pp. 275–308. [Google Scholar]
- 4.Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem. 2005;280:7409–12. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- 5.Ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci. 2004;29:265–73. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Vodovotz Y, Chesler L, Chong H, Kim SJ, Simpson JT, DeGraff W, Cox GW, Roberts AB, Wink DA, Barcellos-Hoff MH. Regulation of transforming growth factor-β1 by nitric oxide. Cancer Res. 1999;59:2142–9. [PubMed] [Google Scholar]
- 7.Barcellos-Hoff MH, Dix TA. Redox mediated activation of latent transforming growth factor-β1. Mol Endocrinol. 1996;10:1077–83. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- 8.Murad F. Shattuck lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N Engl J Med. 2006;355:2003–11. doi: 10.1056/NEJMsa063904. [DOI] [PubMed] [Google Scholar]
- 9.Levonen AL, Patel RP, Brookes P, Go YM, Jo H, Parthasarathy S, Anderson PG, Darley-Usmar VM. Mechanisms of cell signaling by nitric oxide and peroxynitrite: from mitochondria to MAP kinases. Antioxid Redox Signal. 2001;3:215–29. doi: 10.1089/152308601300185188. [DOI] [PubMed] [Google Scholar]
- 10.Kim S-J, Denhez F, Kim KY, Holt JT, Sporn MB, Roberts AB. Activation of the second promoter of the transforming growth factor-beta1 gene by transforming growth factor-Beta1 and phorbol ester occurs through the same target sequences. J Biol Chem. 1989;264:19373–8. [PubMed] [Google Scholar]
- 11.El-Sherif AM, Seth R, Tighe PJ, Jenkins D. Decreased synthesis and expression of TGF-beta1, beta2, and beta3 in epithelium of HPV 16-positive cervical precancer: a study by microdissection, quantitative RT-PCR, and immunocytochemistry. J Pathol. 2000;192:494–501. doi: 10.1002/1096-9896(200012)192:4<494::AID-PATH760>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 12.Horimoto M, Kato J, Takimoto R, Terui T, Mogi Y, Nitsu Y. Identification of a transforming growth factor beta-1 activator derived from a human gastric cancer cell line. Br J Cancer. 1995;72:676–862. doi: 10.1038/bjc.1995.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barcellos-Hoff MH. Latency and activation in the control of TGF-beta. J Mammary Gland Biol Neoplasia. 1996;1:353–63. [PubMed] [Google Scholar]
- 14.Mazzieri R, Munger JS, Rifkin DB. Measurement of active TGF-beta generated by cultured cells. Methods Mol Biol. 2000;142:13–27. doi: 10.1385/1-59259-053-5:13. [DOI] [PubMed] [Google Scholar]
- 15.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGF beta activation. J Cell Sci. 2003;116(Part 2):217–24. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 16.Zamora R, Vodovotz Y. Transforming growth factor-β in critical illness. Crit Care Med. 2005;33:S478–81. doi: 10.1097/01.ccm.0000191725.59611.14. [DOI] [PubMed] [Google Scholar]
- 17.Chong H, Vodovotz Y, Cox GW, Barcellos-Hoff MH. Immunocytochemical detection of latent TGF-β activation in cultured macrophages. J Cell Physiol. 1999;178:275–83. doi: 10.1002/(SICI)1097-4652(199903)178:3<275::AID-JCP1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 18.Messmer UK, Ankarcrona M, Nicotera P, Brune B. p53 expression in nitric oxide-induced apoptosis. FEBS Lett. 1994;355:23–6. doi: 10.1016/0014-5793(94)01161-3. [DOI] [PubMed] [Google Scholar]
- 19.Cano E, Mahadevan LC. Parallel signal processing among mammalian MAPKs. Trends Biochem Sci. 1995;20:117–22. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- 20.Komalavilas P, Shah PK, Jo H, Lincoln TM. Activation of mitogen-activated protein kinase pathways by cyclic GMP and cyclic GMP-dependent protein kinase in contractile vascular smooth muscle cells. J Biol Chem. 1999;274:34301–9. doi: 10.1074/jbc.274.48.34301. [DOI] [PubMed] [Google Scholar]
- 21.Kim SO, Xu Y, Katz S, Pelech S. Cyclic GMP-dependent and -independent regulation of MAP kinases by sodium nitroprusside in isolated cardiomyocytes. Biochim Biophys Acta. 2000;1496:277–84. doi: 10.1016/s0167-4889(00)00026-4. [DOI] [PubMed] [Google Scholar]
- 22.Varma S, Breslin JW, Lal BK, Pappas PJ, Hobson RW, Duran WN. p42/44MAPK regulates baseline permeability and cGMP-induced hyperpermeability in endothelial cells. Microvasc Res. 2002;63:172–8. doi: 10.1006/mvre.2001.2381. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira CJ, Schindler F, Ventura AM, Morais MS, Arai RJ, Debbas V, Stern A, Monteiro HP. Nitric oxide and cGMP activate the Ras–MAP kinase pathway-stimulating protein tyrosine phosphorylation in rabbit aortic endothelial cells. Free Radic Biol Med. 2003;35:381–96. doi: 10.1016/s0891-5849(03)00311-3. [DOI] [PubMed] [Google Scholar]
- 24.Hodges RR, Shatos MA, Tarko RS, Vrouvlianis J, Gu J, Dartt DA. Nitric oxide and cGMP mediate alpha1D-adrenergic receptor-stimulated protein secretion and p42/p44 MAPK activation in rat lacrimal gland. Invest Ophthalmol Vis Sci. 2005;46:2781–9. doi: 10.1167/iovs.05-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshioka Y, Yamamuro A, Maeda S. Nitric oxide/cGMP signaling pathway protects RAW264 cells against nitric oxide-induced apoptosis by inhibiting the activation of p38 mitogen-activated protein kinase. J Pharmacol Sci. 2006;101:126–34. doi: 10.1254/jphs.fpj06001x. [DOI] [PubMed] [Google Scholar]
- 26.Dudnikova GN, Zaidenberg MV. Effect of cyclic guanidine monophosphate on experimental wound healing. Biull Eksp Biol Med. 1980;89:543–5. [PubMed] [Google Scholar]
- 27.Hsu YC, Hsiao M, Chien YW, Lee WR. Exogenous nitric oxide stimulated collagen type I expression and TGF-beta1 production in keloid fibroblasts by a cGMP-dependent manner. Nitric Oxide. 2007;16:258–65. doi: 10.1016/j.niox.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Hirano S, Rees RS, Gilmont RR. MAP kinase pathways involving hsp27 regulate fibroblast-mediated wound contraction. J Surg Res. 2002;102:77–84. doi: 10.1006/jsre.2001.6315. [DOI] [PubMed] [Google Scholar]
- 29.Vodovotz Y. Control of nitric oxide production by transforming growth factor-b1: mechanistic insights and potential relevance to human disease. Nitric Oxide. 1997;1:3–17. doi: 10.1006/niox.1996.0105. [DOI] [PubMed] [Google Scholar]
- 30.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–78. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 31.Otsuka M, Tsuchiya S, Aramaki Y. Involvement of ERK, a MAP kinase, in the production of TGF-beta by macrophages treated with liposomes composed of phosphatidylserine. Biochem Biophys Res Commun. 2004;324:1400–5. doi: 10.1016/j.bbrc.2004.09.198. [DOI] [PubMed] [Google Scholar]
- 32.Hanafusa H, Ninomiya-Tsuji J, Masuyama N, Nishita M, Fujisawa J, Shibuya H, Matsumoto K, Nishida E. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. J Biol Chem. 1999;274:27161–7. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- 33.Barcellos-Hoff MH, Ehrhart EJ, Kalia M, Jirtle R, Flanders K, Tsang ML-S. Immunohistochemical detection of active transforming growth factor-β in situ using engineered tissue. Am J Pathol. 1995;147:1228–37. [PMC free article] [PubMed] [Google Scholar]