Abstract
Many children adversely affected by maternal drinking during pregnancy cannot be identified early in life using current diagnostic criteria for fetal alcohol spectrum disorder (FASD). We conducted a preliminary investigation to determine whether ethanol-induced alterations in placental gene expression may have some utility as a diagnostic indicator of maternal drinking during pregnancy and as a prognostic indicator of risk for adverse neurobehavioral outcomes in affected offspring. Pregnant Long-Evans rats voluntarily consumed either a 0 or 5% ethanol solution 4 h each day throughout gestation. Ethanol consumption produced a mean maternal daily intermittent peak serum ethanol concentration of 84 mg/dL. Placentas were harvested on gestational day 20 for gene expression studies. Microarray analysis of more than 28,000 genes revealed that the expression of 304 known genes was altered twofold or greater in placenta from ethanol-consuming dams compared with controls. About 76% of these genes were repressed in ethanol-exposed placentas. Gene expression changes involved proteins associated with central nervous system development; organ morphogenesis; immunological responses; endocrine function; ion homeostasis; and skeletal, cardiovascular, and cartilage development. To date, quantitative real-time polymerase chain reaction analysis has confirmed significant alterations in gene expression for 22 genes, including genes encoding for three calcium binding proteins, two matrix metalloproteinases, the cannabinoid 1, galanin 2 and toll-like receptor 4, iodothyronine deiodinase 2, 11-β hydroxysteroid dehydrogenase 2, placental growth factor, transforming growth factor alpha, gremlin 1, and epithelial growth factor (EGF)-containing extracellular matrix protein. These results suggest that the expression of a sufficiently large number of placental mRNAs is altered after moderate drinking during pregnancy to warrant more detailed investigation of the placenta as a biomarker system for maternal drinking during pregnancy and as an early indicator of FASD. Furthermore, these results provide new insights into novel mechanisms on how ethanol may directly or indirectly mediate its teratogenic effects through alterations in placental function during pregnancy.
Keywords: Fetal alcohol spectrum disorder, Ethanol, Placenta, Microarray, qRT-PCR, Biomarker
Introduction
Heavy or binge patterns of drinking during pregnancy can cause profound morphological and neurological aberrations in offspring called fetal alcohol syndrome (FAS; Clarren and Smith, 1978; Jones et al., 1973; Jones and Smith, 1973; Lemoine et al., 1968). Increasing evidence indicates that moderate drinking during pregnancy can cause subtle, long-term behavioral and cognitive impairments in the absence of the birth defects associated with FAS (Abel, 1995; Hanson et al., 1978; Shaywitz et al., 1980). These behavioral deficits may not become apparent until the educational years (Conry 1990; Hamilton et al., 2003; Jacobson et al., 1998; Streissguth et al., 1990) and may increase in severity as the child matures (Jacobson et al., 2004; Streissguth et al., 1991, 1994). Collectively, these observations led to an expansion of the diagnostic classification of prenatal alcohol-related effects to include alcohol-related neurodevelopmental disorder (Stratton et al., 1996) and, subsequently, fetal alcohol spectrum disorder (FASD) to encompass the entire range of fetal ethanol-affected children.
Current estimates suggest that at least 1% of the pediatric population has FASD (May et al., 2007, 2008; Sampson et al., 1997), and that a large majority of this group may have no physical evidence of prenatal alcohol effects at birth. In such cases, adverse neurobehavioral consequences may not be diagnosed for years, diminishing the beneficial prospect of earlier interventional opportunities. Thus, one of the critical challenges for the fetal alcohol research community is to develop more sensitive and reliable means to detect moderate drinking during pregnancy. Ideally, a reliable indicator of prenatal ethanol exposure may also serve as a predictor of functional damage to newborns, allowing earlier identification of children at risk for longer-term adverse neurobehavioral outcomes.
One approach to this clinical challenge has focused on the identification of biomarkers of alcohol consumption as a means to confirm maternal drinking during pregnancy. A relatively small number of studies on biomarkers of drinking during pregnancy have been reported (see review by Bearer, 2001a). Most of these efforts have been clinical studies of serum biomarkers and have focused either on measurements of ethanol, ethanol metabolites, compounds that chemically interact with ethanol, or a variety of proteins either directly involved in ethanol metabolism or impacted indirectly as a consequence of ethanol metabolism. A recurring theme in most of these clinical studies is that the sensitivity of these biomarkers is generally limited to heavy drinking, these ethanol-induced changes are often short-lived with abstinence, and specificity is impacted by confounding variables present in subject populations (Bearer, 2001).
Another biomarker that appears to have greater sensitivity and specificity for detecting drinking during pregnancy are the fatty acid ethyl esters (FAEEs) ethyl linolate, ethyl oleate, and ethyl arachidonate, which accumulate in maternal liver, placenta, fetal tissues, meconium, and hair after ethanol consumption (Bearer et al., 1999, 2003a; Bearer, 2001a; Kulaga et al., 2006). FAEEs have a half-life of approximately 7 days in mouse placenta (Bearer et al., 1992) and have been detected in umbilical cord blood and meconium from newborns of alcoholic mothers (Bearer et al., 1999, 2003b, 2005). However, FAEEs have also been found in some abstinent groups (Bearer, 2001a) and their sensitivity for moderate drinking during pregnancy is not firmly established at present, possibly indicating a need for more sensitive analytical approaches for detecting these compounds in clinical samples (Pichini et al., 2008).
One alternative strategy to the challenge of diagnosing drinking during pregnancy is to use a bottom-up approach, where biomarkers are first identified and validated in animal models of drinking during pregnancy and then, based on this information, pursue parallel human studies to assess clinical utility. This approach has four distinct advantages. First, it increases the prospects of identifying novel markers without the confounding variables associated with patient populations. Second, biomarker validation can proceed in a more systematic and controlled fashion over a shorter period of time and in a more cost-effective manner. Third, an animal model system provides an opportunity to assess more directly how a biomarker signature may change as a function of ethanol dosing, patterns of ethanol exposure, and the influence of other interacting risk factors during pregnancy, such as concomitant exposure to nicotine, other drugs of abuse, stress, malnutrition, or heavy metals. How a biosignature pattern is altered by concurrent exposure to other risk factors would be critically important to the interpretation of data from clinical studies. Finally, an animal model system allows for direct correlation of biomarker patterns with markers of functional damage to the fetus, longer-term adverse neurobehavioral outcomes and, in the best-case scenario, provide insights about the mechanistic basis for the teratogenic damage—assessments that would be more difficult, if not impossible, to examine in a clinical study.
In the present study, we used a recently developed rat model of voluntary drinking during pregnancy that produces offspring with deficits in hippocampal synaptic plasticity and learning to study the effects of moderate drinking during pregnancy on placental gene expression. From a clinical standpoint, placenta has a number of advantages in consideration as a biomarker tissue given that relatively large quantities are readily obtainable by minimally invasive and inexpensive procedures that are generally ethically acceptable to both the mother and in clinical practice. To date, we have confirmed that moderate drinking during pregnancy significantly altered the expression of nearly two dozen genes, of which the protein products play important roles in placental function and fetal development.
Materials and methods
Materials
All reagents were acquired from Sigma Chemical Company unless indicated otherwise in parenthetical text.
Voluntary drinking paradigm
All procedures involving the use of live rats were approved by the University of New Mexico Health Sciences Center Institutional Animal Care and Use Committee. Four-month-old Long-Evans rat breeders (Harlan Industries, Indianapolis, IN) were single housed in plastic cages at 22°C and kept on a “reverse” 12-h dark/12-h light schedule (lights on from 2100–0900 h) with Harlan Teklad rodent chow and tap water ad libitum. After 1 week of acclimation to the animal facility, all female rats were provided 0.066% saccharin in tap water for 4 h each day from 1000 to 1400 h. The saccharin water contained 0% ethanol on the first and second day, 2.5% ethanol (vol/vol) on the third and fourth day, and 5% ethanol on the fifth day and thereafter. Daily 4-h consumption of ethanol was monitored for at least 2 weeks, and then, the mean daily ethanol consumption was determined for each female breeder. At the end of 2 weeks of daily ethanol consumption, females that drank less than 1 standard deviation below the mean of the entire group were removed from the study. The remainder of the females were assigned to either a saccharin control or 5% ethanol-drinking group and matched such that the mean prepregnancy ethanol consumption by each group was similar.
Subsequently, females were placed with proven male breeders until pregnant, as indicated by the presence of a vaginal plug. Female rats did not consume ethanol during the breeding procedure. Beginning on gestational day 1, rat dams were provided saccharin water containing either 0 or 5% ethanol for 4 h a day. The volume of 0% ethanol saccharin water provided to the controls was matched with the mean volume of saccharin water consumed by the 5% ethanol-drinking group. Daily 4-h ethanol consumption was recorded for each dam.
Maternal serum ethanol levels
A separate set of 12 rat dams was used to determine serum ethanol concentrations. These dams were run through the same voluntary drinking paradigm as described earlier, except that at the end of the 4-h ethanol consumption episode on each of three alternate days during the third week of gestation, each rat dam was briefly anesthetized with isoflurane. One hundred microlitres of whole blood was collected from the tail vein and immediately mixed with 0.2 mL of 6.6% perchloric acid, was frozen, and was stored at –20°C until assayed. Serum ethanol standards were created by mixing rat whole blood from untreated rats with known amounts of ethanol ranging from 0 to 240 mg ethanol/dL and then mixing 100-μL aliquots of each standard with perchloric acid and storing the standards frozen with the samples. Serum ethanol samples were assayed using a modification of the method of Lundquist (1959).
Tissue harvesting and RNA preparative procedures
On gestational day 20, rat dams were sacrificed, Caesarian sections were performed, and placental tissue was harvested rapidly. The position of each placenta within the uterine horn and the gender of the associated fetus were noted. The placenta was perfused with ice-cold saline to remove blood, frozen in liquid nitrogen and stored at –80°C. Total RNA was isolated from the frozen tissue using the RNeasy kit following the manufacturer's instructions (Qiagen, Valencia, CA), and the yield was determined by spectrophotometry (Nanodrop, Wilmington, DE). Total RNA was assessed with an Agilent 2001 Bioanalyzer using RNA 6000 nanochips (Agilent Technologies, Santa Clara, CA). All samples had a RNA integrity number of 9.8 or higher, indicating high quality RNA (Schroeder et al., 2006).
Microarray analysis
RNA samples from individual placenta were labeled and analyzed separately on GeneChip Rat Genome 230 2.0 Arrays (Affymetrix Inc., Santa Clara, CA). Equal amounts of total RNA (5 μg) were converted into double-stranded cDNA using Superscript II (Invitrogen, Carlsbad, CA). The resulting cDNA was used for the in vitro synthesis of biotin-labeled cRNA using the ENZO Bioarray High Yield RNA Transcript Labeling Kit T7 (Enzo Diagnostics Inc., Farmingdale, NY). After a cleanup step, 15 μg of the antisense cRNA was fragmented for 35 min at 94°C and then used as a probe on the microarray. Immediately following incubation for 16 h at 45°C, the chips were washed and stained with streptavidin–phycoerythrin using a GeneChip Fluidics Station 400 (Affymetrix Inc.). Washing, staining, and scanning were carried out according to the standard Affymetrix protocol.
The raw data were analyzed with the Affymetrix Microarray Analysis Suite (MAS 5.0) and GeneSpring GX 7.3 software (Agilent Technologies, Santa Clara, CA), starting with a per-chip normalization. The microarray data are available from the National Center for Biotechnology Information's Gene Expression Omnibus at http://www.ncbi.nlm.gov/geo/ (Barrett et al., 2005; Edgar et al., 2002) under series accession number GSE18162. All samples had a scaling factor of less than 20 to achieve the same overall intensity (500 RFU). Raw data were adjusted using a pergene normalization step to the median to compare the relative expression profiles of genes that might be expressed at very different absolute levels. Next, samples from the ethanol-exposed group were normalized to the saccharin controls. Normalized data was prefiltered by expression level (> 100 RFU). In addition, only genes that were called “present” (i.e., by intensity of signal and specificity of hybridization to all of the corresponding oligonucleotide probes per set of each gene on the chip) in at least three of the seven samples were analyzed, thereby reducing false-positive calls and removing genes that were not reliably detected (McClintick and Edenberg, 2006). A principal component analysis of the samples demonstrated that all of them passed this quality control step (data not shown). Significant changes in gene expression were defined using two filters: first by fold change of more than 2 and then by a Student's t-test multiple testing correction with a threshold of P < .05 for false discovery rate (Benjamini and Hochberg, 1995).
A global characterization of significant genes in gene ontology (GO) categories of biological processes, molecular function, and cellular compartment (Ashburner, Ball et al., 2000; Harris, Clark et al., 2004) was performed using the Gene Ontology Tree Machine tool of Vanderbilt University in Nashville, TN (http://bioinfo.vanderbilt.edu/gotm). Briefly, a list of differentially expressed genes was compared with a list of all genes represented on the Rat Genome 230 2.0 Array. Relatively enriched genes were identified using the GO hypergeometric distribution analysis. Categories were considered significant at P < .01.
Real-time quantitative polymerase chain reaction analysis
Total RNA was isolated and quantified as described earlier and stored in aliquots at –80°C until use. First-strand cDNA synthesis from 1 μg of total RNA was performed using Superscript II reverse transcriptase and oligo(dT) primer (Invitrogen, Carlsbad, CA). Gene expression levels in all samples were examined by quantitative real-time polymerase chain reaction (qRT-PCR) reactions using SYBR® green Supermix (BioRad, Hercules, CA) on an ABI 7300 system (Applied Biosystems, Foster City, CA). Using Primer 3 software (Rozen and Skaletsky, 2000), the primer pairs were designed to be exon-spanning if possible to ensure that no product was amplified from genomic DNA and were created to be specific for each gene (as verified by a BLAST search) to a region different from the one used by the oligonucleotides on the Affymetrix chip. Table A1 provides detailed information of the primer sets used in the qRT-PCR studies. In preliminary studies, the optimal concentration for each primer set was determined using 5 ng of template per reaction, and a dissociation curve analysis was performed to ensure that specific amplification was achieved. The amplification conditions consisted of an initial step at 50°C for 2 min, denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Controls included analysis of template-free reactions (both in the reverse transcription and in the PCR reaction), RNA not being reverse transcribed (to detect contamination with DNA in the RNA preparation) and samples treated with RNase A before reverse transcription reaction.
RNA samples were run in triplicate for the genes of interest and for the reference gene within the same experiment. Each experiment was performed three times. Triplicate cycle thresholds (Ct's) of all the experiments were averaged for each sample. The size of the amplicons and specificity of the primer set was verified on a 2% agarose Tris-acetate-EDTA (TAE) gel.
All data were normalized against β-actin as a reference gene. The expression of β-actin was similar in the saccharin and ethanol-exposed groups both in the microarray data and the qRT-PCR experiments. The mean Ct values for all samples were similar, making β-actin an appropriate control. Relative quantification of gene expression, that is, the relative amount of target RNA, was determined using the 2–ΔΔCt method (Livak and Schmittgen, 2001).
Results
Voluntary drinking paradigm
Rat dams stably consumed an average of 2.82 ± 0.13 g of ethanol/kg body weight over the 4-h interval each day (approximately 16 mL of 5% ethanol in 0.066% saccharin water). This pattern and level of ethanol consumption produced a mean maternal serum ethanol concentration of 84.0 ± 5.5 mg/dL during the third week of gestation. Ethanol consumption did not affect maternal weight gain during pregnancy (Table 1). Litter sizes and placental wet weights at harvesting were not different between the two experimental groups.
Table 1.
Effects of the voluntary ethanol consumption paradigm
| Outcome measure | Saccharin control | 5% Ethanol |
|---|---|---|
| Maternal weight gain during pregnancy | 120 ± 3a (44) | 115 ± 4 (51) |
| Daily 4-h ethanol consumption | NA | 2.82 ± 0.13b (51) |
| Maternal serum ethanol concentration | NA | 84.0 ± 5.5c (24) |
| Fetal litter size | 12.7 ± 0.8d (9) | 12.9 ± 0.9 (13) |
| Placental wet weight | 0.533 ± 0.049e (6) | 0.513 ± 0.045 (6) |
NA = not applicable; Numbers in parentheses indicate sample size; S.E.M. = standard error of mean.
Mean ± S.E.M. grams increase in body weight from gestational day 1 through 21.
Mean ± S.E.M. grams ethanol consumed/kg body weight/day.
Mean ± S.E.M. mg ethanol/dL serum, 30 min after a 4-h drinking period.
Mean ± S.E.M. number of fetuses/litter at gestational day 20.
Mean ± S.E.M. grams/placenta (averaged from four placentas in each litter).
Microarray analysis of placental gene expression
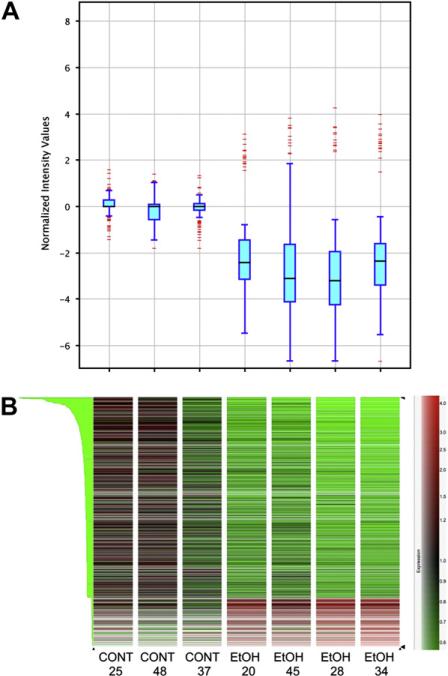
The Rat Genome 230 2.0 Expression Array contains more than 31,000 probe sets (30,000 transcripts and variants) from more than 28,000 rat genes. Of those, about 53% were detected as being expressed in placental tissue, that is, “present” in at least three of seven samples. After applying our criteria of a minimum twofold change in expression and statistical significance (P < .05) based on a Student's t-test, 649 genes were identified as significantly altered in the placenta of ethanol-consuming dams compared with the saccharin controls. The whisker box plots shown in Fig. 1A illustrate that the distribution of signal intensities of altered genes among the placental samples within each of the two experimental groups was similar, with the alcohol-exposed samples showing an overall reduction in expression levels. Figure 1B shows an unsupervised hierarchical cluster analysis of expression profile similarity between the two experimental conditions for the 649 identified genes, indicating that the two groups can be clearly distinguished. After excluding expressed sequence tags and unidentified genes, 304 of the identified genes remained. A compilation of the 304 identified genes is available in Table A2.
Fig. 1.
A. Box whisker plots of placental gene expression in control and ethanol-exposed placentas representing the minimum (end of the bottom whisker), the first quartile (bottom border of the box), the median (line through the box), the third quartile (top border of the box), and the maximum (end of the top whisker) of the distribution. The separately drawn points are outliers. Points are regarded as outliers if the minimum of their distance to the first and the third quartile is greater than 1.5 times the interquartile range (IQR = third quartile–second quartile). B. Heat map plots illustrating unsupervised hierarchical clustering on similarity in expression profiles across conditions of 643 genes list, filtered by twofold expression changes and statistical significance (Student's t-test). CONT = saccharin control group and EtOH = 5% ethanol treatment group. The numbers below the group labels indicate the litter number from which the placenta tissue was harvested. Each heat map corresponds to the whisker plot directly above it.
In general, ethanol consumption repressed placental gene expression. About 76% of the 304 identified genes were downregulated in the placentas harvested from ethanol-consuming dams compared with the controls. Of the 304 selected genes altered by ethanol consumption, 147 were differentially regulated between two- to threefold; 115 genes displayed a three- to fivefold difference in expression; and 40 genes showed differences in expression greater than fivefold (Table 2), including genes encoding proteins involved in a wide array of biological processes associated with placental and fetal development.
Table 2.
Differentially regulated placental genes, filtered by statistical significance and fold change of more than five
| Affymetrix ID | Symbol | RefSeq | Downregulated gene name | Fold Δ |
|---|---|---|---|---|
| 1369113_at | GREM1 | NM_019282 | Gremlin 1, cysteine knot superfamily homolog | –25.3 |
| 1385568_at | DIO2 | NM_031720 | Deiodinase, iodothyronine type 2 | –25.2 |
| 1387088_at | GAL | NM_033237 | Galanin | –21.4 |
| 1368731_at | ORM1 | NM_053288 | Orosomucoid 1 | –17.0 |
| 1388269_at | HBG1 | NM_172093 | Hemoglobin, gamma A | –16.2 |
| 1390112_at | EFEMP1 | NM_001012039 | EGF-containing fibulin-like extracellular matrix protein 1 | –15.8 |
| 1369677_at | CNR1 | NM_012784 | Cannabinoid receptor 1 | –15.5 |
| 1368304_at | FMO3 | NM_053433 | Flavin-containing monooxygenase 3 | –14.2 |
| 1368394_at | SFRP4 | NM_053544 | Secreted frizzled-related protein 4 | –13.9 |
| 1368912_at | TRH | NM_013046 | Thyrotropin-releasing hormone | –13.3 |
| 1398688_at | SPINK5 | XM_341607 | Serine peptidase inhibitor, Kazal type 5 | –10.7 |
| 1374558_at | ICOSLG | XM_574731 | Inducible T-cell co-stimulator ligand | –9.31 |
| 1387563_at | PGR | NM_022847 | Progesterone receptor | –9.21 |
| 1369625_at | AQP1 | NM_012778 | Aquaporin 1 | –9.15 |
| 1367846_at | S100A4 | NM_012618 | S100 calcium binding protein A4 | –8.78 |
| 1367627_at | GATM | NM_031031 | Glycine amidinotransferase | –8.26 |
| 1370843_at | GNG8 | NM_139185 | Guanine nucleotide binding protein (G-protein), gamma 8 | –8.25 |
| 1369695_at | WT1 | NM_031534 | Wilms tumor 1 | –8.14 |
| 1393069_at | SFRP5 | XM_219887 | Secreted frizzled-related protein 5 | –7.86 |
| 1369164_a_at | TRPC4 | NM_080396 | Transient receptor potential cation channel 4C | –7.63 |
| 1387450_at | TGFA | NM_012671 | Transforming growth factor, alpha | –7.62 |
| 1371102_x_at | HBD | NM_033234 | Hemoglobin, delta | –7.35 |
| 1369817_at | HAND2 | NM_022696 | Heart and neural crest derivatives expressed 2 | –6.97 |
| 1388270_at | HBE1 | NM_001008890 | Hemoglobin, epsilon 1 | –6.91 |
| 1368919_at | PGF | NM_053595 | Placental growth factor | –6.46 |
| 1396407_at | GAS8 | NM_001039030 | Growth arrest-specific 8 | –6.43 |
| 1380206_at | KIF5C | XM_221307 | Kinesin family member 5C | –6.32 |
| 1367600_at | DES | NM_022531 | Desmin | –6.23 |
| 1370157_at | PLN | NM_022707 | Phospholamban | –6.03 |
| 1388138_at | THBS4 | XM_342172 | Thrombospondin 4 | –5.69 |
| 1367794_at | A2M | NM_012488 | Alpha-2-macroglobulin | –5.62 |
| 1387656_at | SLC4A1 | NM_012651 | Solute carrier family 4, anion exchanger, member 1 | –5.60 |
| 1368713_at | MMP10 | NM_133514 | Matrix metalloproteinase 10 (Stromelysin 2) | –5.49 |
| 1370956_at | DCN | NM_024129 | Decorin | –5.37 |
| 1380285_at | CHRD | NM_001024273 | Chordin | –5.36 |
| 1368342_at | AMPD3 | NM_031544 | Adenosine monophosphate deaminase (isoform E) | –5.18 |
| Affymetrix ID | Symbol | RefSeq | Upregulated gene name | Fold Δ |
|---|---|---|---|---|
| 1380134_at | VTCN1 | NM_001024244 | V-set domain containing T-cell activation inhibitor 1 | 5.08 |
| 1370594_at | IGSF1 | NM_175763 | Immunoglobulin superfamily, member 1 | 6.00 |
| 1370077_at | INS | NM_019130 | Insulin | 6.67 |
| 1370165_at | SMPX | NM_053395 | Small muscle protein, X-linked | 13.7 |
Gene ontology analyses
Of 15,389 GO categories surveyed by GO analyses, 77 were significantly enriched in altered placental genes, that is, the odds ratio of experimentally observed differentially transcribed genes to expected genes in a given GO category was greater than 1 with P values less than .01. Those enriched categories were divided into three main groups (GO level 1): biological processes, molecular function, and cellular component (localization). The vast majority of enriched gene categories were found within the biological processes component. After applying an exclusion criterion of at least four ethanol-altered genes per category, several categories were significantly overpopulated with alcohol-altered gene products, including nervous system development; organ morphogenesis; immunological responses; ion homeostasis; and skeletal, cardiovascular, and cartilage development (Fig. 2). A prominent category of particular interest to us, within the context of biomarkers for fetal alcohol-related synaptic plasticity and learning deficits, was the nervous system development category, where 31 genes were significantly altered greater than twofold (Table 3) with an enrichment factor of 1.8 (P < .002).
Fig. 2.
Selected enriched “biological processes” gene ontology (GO) level 5 categories. Data were analyzed using Gene Ontology Tree Machine GOTM software (Zhang et al., 2000). The distribution of differentially regulated genes (gray bars) in each GO category was compared with all genes on the Affymetrix Rat 230 2.0 array (black bars). Statistical significance was analyzed using the hypergeometric statistical test; *P < .05, **P < .005.
Table 3.
Thirty-one differentially regulated genes in the “Nervous System Development Ontology Category”
| Affymetrix ID | Symbol | Fold Δ | Gene |
|---|---|---|---|
| 1387088_at | GAL | –21.4 | Galanin |
| 1370843_at | GNG8 | –8.25 | G-protein gamma 8 |
| 1380172_at | KIF5C | –6.36 | Kinesin 5C |
| 1388138_at | THBS4 | –5.70 | Thrombospondin 4 |
| 1380285_at | CHRD | –5.36 | Chordin |
| 1386903_at | S100B | –4.93 | S100 protein B |
| 1387659_at | GDA | –4.33 | Guanine deaminase |
| 1368914_at | RUNX1 | –3.93 | Runt-related transcription factor 1 |
| 1377336_at | SEMA3B | –3.31 | Sema domain, immunoglobulin domain (IG) |
| 1367668_a_at | SCD2 | –2.90 | Stearoyl-coenzyme A desaturase 2 |
| 1368721_at | ASCL2 | –2.67 | Achaete-scute complex homolog 2 |
| 1370236_at | PPT1 | –2.58 | Palmitoyl-protein thioesterase |
| 1376973_at | SDCBP2 | –2.44 | Syndecan binding protein 2 |
| 1369977_at | UCHL1 | –2.40 | Ubiquitin carboxy-terminal hydrolase 11 |
| 1369012_at | INHBA | –2.38 | Inhibin beta A |
| 1388427_at | MXRA8 | –2.24 | Limitrin |
| 1385013_at | WNT1 | –2.22 | Wingless-related MMTV integration site 1 |
| 1390233_at | Gli2 | –2.20 | GLI-Kruppel Family member GLI2 |
| 1390882_at | Heyl | –2.20 | Hairy/enhancer-of-split related with YRPW motif-like |
| 1387271_at | PHYH | –2.13 | Phytanoyl-CoA hydroxylase |
| 1369640_at | GJA1 | –2.11 | Gap junction protein, alpha 1 |
| 1382511_at | E2F1 | –2.07 | E2F transcription factor 1 |
| 1384667_x_at | GALR2 | –2.05 | Galanin receptor 2 |
| 1368254_a_at | SPHK1 | –2.04 | Sphingosine kinase 1 |
| 1372690_at | RTN4RL1 | –2.02 | Reticulon 4 receptor-like 1 |
| 1386943_at | PLLP | 2.03 | Transmembrane 4 superfamily, member 11 |
| 1375849_at | RGMA | 2.05 | Rgm domain family, member A |
| 1387232_at | BMP4 | 2.13 | Bone morphogenic protein 4 |
| 1383981_at | TRP53BP | 2.16 | Transformation-related binding protein 53 |
| 1389066_at | DSCR1L1 | 2.22 | Regulator of calcineurin 2 |
| 1382965_at | AMIGO3 | 2.68 | Amphoterin induced gene ORF 3 |
The enrichment factor for this category was 1.8, as determined by the ratio between the expected number of genes to the observed number of altered genes (P = .0016).
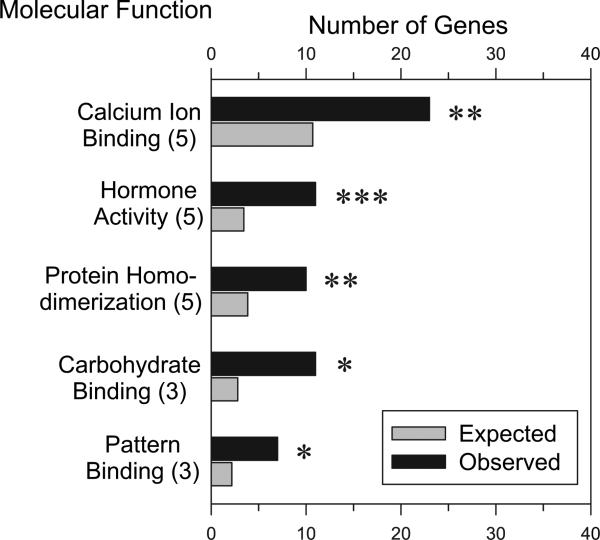
Within the molecular function GO categories, five were overpopulated by alcohol-sensitive placenta genes after application of our exclusion criterion (Fig. 3). Most significant among these were the hormone activity category (enrichment factor of 3.9) and the calcium ion–binding category (enrichment factor of 2.1). It is worth mentioning some categories that failed to pass the exclusion criteria of more than four genes/term. Several functional categories consisting of only two to three members have the common theme of binding to protein families dealing with attachment, migration, and organization of cells (i.e., laminin, collagen, actinin). GO cellular component analyses revealed that altered genes coded for proteins at various cellular locations. Most of the proteins in significantly enriched GO categories are in the extracellular region (95 observed genes, 53.12 expected genes, P > .0005).
Fig. 3.
Selected enriched “molecular function” gene ontology (GO) categories. Numbers in parenthesis after the molecular function denote the GO category level. Data were analyzed using Gene Ontology Tree Machine GOTM software (Zhang et al., 2000). The distribution of differentially regulated genes (gray bars) in each GO category was compared with all genes on the Affymetrix Rat 230 2.0 array (black bars). Statistical significance was analyzed using the hypergeometric statistical test; *P < .05, **P < .005, ***P < .0005.
Real-time polymerase chain reaction confirmation of differential gene expression
Quantitative reverse-transcription (RT-PCR) measurements were conducted to verify placental gene alterations observed in the microarray studies. Thus far, 38 placental genes have been evaluated using qRT-PCR. These genes were selected based primarily on either having a relatively high microarray fold change and/or specific interest related to known placental function or putative teratologic mechanisms of ethanol action, based on the literature. Table 4 summarizes the qRT-PCR results for these genes organized by the cellular location of action of their protein product. Within cellular location, genes are listed from most to least statistically significant. Overall, the mRNA expression values obtained for each gene using qRT-PCR qualitatively mirrored the directional change in the microarray data, but the quantitative differences varied considerably and, in general, were of smaller magnitude compared with the microarray fold-change data. These differences likely reflect technical differences between the two analytical platforms.
Table 4.
Relative quantification of mRNA using the comparative cycle threshold (Ct) method
| Cellular location of protein product gene name | Relative mRNA levelsa | P b | Gene ontology categoryc |
|---|---|---|---|
| Extracellular space | |||
| Gremlin 1 | 0.05 | .002 | Organ morphogenesis |
| Matrix metalloproteinase 2 | 0.13 | .011 | Peptidase activity/blood vessel maturation |
| EGF-containing fibulin-like extracellular matrix protein 1 | 0.06 | .013 | Calcium ion binding |
| Galanin | 0.08 | .013 | Nervous system development |
| Transforming growth factor alpha | 0.12 | .013 | Regulation of cell cycle progression |
| Serine peptidase inhibitor, Kazal type 5 | 0.04 | .046 | Regulation of cell adhesion |
| Placental growth factor | 0.09 | .048 | Progression through cell cycle |
| Thyrotropin-releasing hormone | 0.06 | .061 | Neuropeptide hormone activity |
| Orosomucoid 1 | 0.03 | .069 | Acute-phase response |
| Insulin-like growth factor binding protein 6 | 0.32 | .106 | Regulation of cell growth |
| Matrix metalloproteinase 3 | 0.17 | .213 | Proteolysis |
| Matrix metalloproteinase 10 (stromelysin 2) | 0.34 | .402 | Proteolysis |
| Insulin | 3.58 | .477 | Insulin receptor signaling pathway |
| Alpha-fetoprotein | 2.27 | .707 | Progesterone metabolism |
| Plasma membrane | |||
| Cannabinoid receptor 1 | 0.13 | .001 | G-protein coupled receptor |
| Galanin receptor 2 | 0.33 | .001 | G-protein coupled receptor |
| Toll-like receptor 4 | 0.22 | .018 | Inflammatory response |
| Integrin α7 | 0.34 | .024 | Receptor activity/regulation of cell shape |
| Secreted frizzled-related protein 4 | 0.01 | .037 | Development |
| Lipopolysaccharide binding protein | 0.16 | .042 | Lipid transport |
| Transient receptor potential cation channel 4 | 0.04 | .043 | Calcium ion transport |
| Secreted frizzled-related protein 5 | 0.04 | .079 | Development |
| Nicotinic cholinergic receptor, α2 subunit | 1.39 | .220 | Neurotransmitter receptor |
| Nicotinic cholinergic receptor, α7 subunit | 0.64 | .438 | Extracellular ligand-gated ion channel |
| Cytoplasm | |||
| Hemoglobin, epsilon 1 | 0.15 | .003 | Oxygen transport |
| Hydroxysteroid (11β) dehydrogenase 2 | 0.09 | .009 | Oxidoreductase activity |
| Hemoglobin, gamma A | 0.10 | .010 | Oxygen transport |
| Deiodinase, iodothyronine type II | 0.02 | .012 | Hormone biosynthesis |
| S100 calcium binding protein A4 | 0.09 | .021 | Calcium ion binding |
| S100 calcium binding protein G | 3.10 | .029 | Calcium ion binding |
| S100 calcium binding protein B | 0.04 | .038 | Regulation of neuronal synaptic plasticity |
| Small muscle protein, X-linked | 17.8 | .058 | Striated muscle contraction |
| Heat shock 70 kDa protein 1B | 2.05 | .092 | Anti-apoptosis |
| Flavin-containing monooxygenase 3 | 0.77 | .233 | Oxidoreductase activity |
| Cytochrome P450 1A1 | 2.45 | .549 | Oxidoreductase activity |
| Cytochrome P450 2E1 | 0.95 | .910 | Oxidoreductase activity |
| Nucleus | |||
| Heart and neural crest derivatives expressed 2 | 0.18 | .012 | Neural crest cell development |
| Progesterone receptor | 0.06 | .065 | Steroid hormone receptor |
All mRNA levels were calculated relative to beta-actin using the formula 2–ΔΔCT. Values < 1 are indicative for downregulation of expression, and values > 1 signify higher expression in the experimental group compared with the control group.
The P value is calculated by comparing all control with all ethanol exposed samples and applying Student's t-test.
Shown are selected levels of gene ontology; most genes fit in more than one category. Statistically significant genes with P ≤ .05.
In general, qRT-PCR expression values less than 0.2 or greater than 5.0 correlated with statistically significant alterations in gene expression. Six exceptions to this observation were the genes for thyrotropin-release hormone (TRH), orosomucoid 1, matrix metalloproteinase 3 (MMP-3), secreted frizzled-related protein 5, small X-linked muscle protein, and the progesterone receptor. In each of these six cases, the qRT-PCR value was in the same direction as the microarray fold-change data, but greater variability among individual samples in one or both experimental groups and the small group sample sizes resulted in P values greater than .05 but less than .10.
Thus far, 22 of the genes examined were confirmed as significantly altered (P < .05) based on qRT-PCR analysis (Table 4). This group includes genes encoding for three isoforms of S100 calcium binding proteins (A4, B, and G), two isoforms of hemoglobin (ε1 and γA), galanin and the galanin 2 receptor, the cannabinoid 1 (CB1) and toll-like 4 receptors, iodothyronine deiodinase 2, 11-β hydroxysteroid dehydrogenase 2 (HSD2), placental growth factor, transforming growth factor (TGF)-α, gremlin 1, EGF-containing extracellular matrix protein, and MMP-2.
Discussion
The salient observation from this study is that intermittent consumption of moderate quantities of ethanol during pregnancy alters the expression of at least 22 placental genes. These alterations occur in the absence of any gross observable effects of ethanol on the mother's weight gain during pregnancy, the placenta at term, fetal litter size, or pup weight (Table 1). Nevertheless, adult offspring of this moderate prenatal ethanol exposure paradigm exhibits hippocampal synaptic plasticity deficits and performance deficits in learning paradigms (unpublished observations), indicating long-lasting functional brain damage in the absence of physical defects at birth. Taken together, these results suggest that placental gene expression may be a more sensitive indicator of moderate ethanol consumption than most current ethanol biomarker systems.
Although we are encouraged by the number of placental gene alterations confirmed to date, we have also identified at least five factors that may have contributed to variability in the results of this initial study that precluded the likely identification of a larger number of ethanol-induced placental gene changes. One factor is the impact of individual genetic variation in an outbred rat stock. However, the ability to identify altered genes in an outbred stock should be considered a strength of this paradigm, as it more accurately models the human condition and promises that gene alterations that stand out will be a reliable tool for the detection of maternal drinking. A second putative factor contributing to variability may be the voluntary drinking paradigm itself. We endeavored to minimize this by screening female drinking behavior during the prepregnancy period and removing females from the study whose ethanol consumption was greater than 1 standard deviation below the mean of the entire group. Furthermore, although there were some small day-to-day variations in ethanol consumption by individual rat dams, the ethanol-exposed placentas used in this study were harvested from four ethanol-consuming dams whose mean daily ethanol consumption was within the standard error of the mean range shown in Table 1. A third factor relates to intrauterine variability in ethanol's effects (Mitchell et al., 2002). We strove to minimize this factor in our initial study by only selecting placentas attached to female fetuses, from litters with greater than nine pups (average litter size was 13), where at least one of the adjacent fetuses was male and the location of the selected fetal–placental unit was a least one position away from either the proximal or distal end of a uterine horn. Even with the incorporation of these selection criteria, it is likely that ethanol has variable effects within a litter and that this putative effect on gene expression will require more detailed investigation.
A fourth issue relates to the fact that we opted to examine whole placenta in this initial study. The placenta contains multiple cell types and, in some cases, it is clear that some genes are primarily expressed in more discreet regions within the placenta (Sood et al., 2006). Thus, sampling from whole placenta diminished our “signal to noise ratio” for detecting effects of ethanol on gene expression. For example, placental gene and protein expression of the CB1 receptor is primarily located in the syncytiotrophoblast layer near the surface facing the maternal boundary (Park et al., 2003). A similar distribution has been observed for 11β-HSD2 in preliminary in situ hybridization studies (unpublished observations). Although we were able to confirm ethanol-induced gene repression of both the CB1 receptor and HSD2 in whole placenta (Table 4), the question remains as to how many genes whose expression is heterogeneously distributed across placenta were missed in an analysis of the effects of ethanol on whole placenta. Subsequent histological approaches using in situ hybridization to examine gene expression and immuno- and radiohistochemical approaches for quantitating protein will be required to better address this question. Finally, another factor contributing to variability is that we were limited in our ability to analyze a larger number of samples in a preliminary microarray analyses. It is likely that larger sample sizes would have resulted in more significantly altered genes based on the qRT-PCR analysis. Subsequent studies will use larger sample sizes to better address this point.
Considerable work remains to confirm the utility of placental gene alterations as a biomarker system, both for detecting ethanol consumption and a prognostic indicator of adverse neurobehavioral outcomes in the absence of morphological alterations. Systematic examination of altered gene expression as a function of different levels and patterns of ethanol consumption as well as the persistence of gene alterations after the last drinking episode are critical translational research questions to address. Further, how the presence of other common pregnancy risk factors impacts ethanol-induced alterations in placental gene expression patterns needs to be determined. For example, how will concomitant exposure to such factors as nicotine, other drugs of abuse, stress, malnutrition, or heavy metals modify a biomarker signature pattern? Data from such studies would be critical for interpreting altered patterns of placental gene expression in clinical studies.
The results of this initial study also provide intriguing insights into the implications of maternal drinking on placental function and putative mechanisms of ethanol teratogenesis. At a more global level, the GO analyses of biological processes (Fig. 2) indicated that ethanol has significant effects on genes associated with organ morphogenesis as well as nervous, endocrine, and immune system development and function. This observation is consistent with a wealth of literature indicating that prenatal ethanol exposure affects organ development (Weinberg, 1994, Byrnes et al., 2003; Qiang et al., 2002; Taylor et al., 1999), particularly the development of these three highly susceptible and critically interactive organ systems. Altered expression of genes associated with vascular, skeletal, and cartilage development, although less investigated in the fetal alcohol research field to date, clearly merit additional study.
Of particular interest was the observation that a number of placental genes altered by moderate ethanol exposure are known to play critical roles in pattern formation during nervous system development. For example, interactions of the members of the TGFβ family, such as bone morphogenic protein (BMP)4 and the BMP4 antagonist chordin, help regulate polarity (i.e., back to front patterning) of the developing embryo (Chesnutt et al., 2004; Millet et al., 2001). Likewise, the products of the WnT and Notch signaling–related genes ASCL2, HeYL, SFRP4, SFRP5, and WnT1 are known to regulate cell fate during the induction of both the central and peripheral nervous system (Ciani and Salinas, 2005; Nakagawa et al., 2001). Further, both the products of SEMA3B, semaphorin 3B (Falk et al., 2005) and of THBS4, thrombospondin (Arber and Caroni, 1996) are important for axonal growth and guidance. It is also important to note that similar levels of these genes occur in both placenta and fetal brain (Genomics Institute of the Novartis Research Foundation website http://biogps.gnf.org). Taken together, these observations suggest that some changes in placental gene expression may be predictive of similar changes in gene expression in fetal brain, and that the placenta could serve as a window on brain development. Follow-up studies examining both placental and fetal brain gene expression in the same placental–fetal brain unit will directly address this supposition and, in the process, could strengthen the prospects of establishing meaningful cause–effect relationships that will further our understanding of ethanol's impact on early developmental processes.
GO analyses of molecular processes also suggested important effects of maternal ethanol consumption on endocrine mechanisms (Fig. 3). Of particular note are the systems that regulate corticosterone and thyroid hormone. The expression of 11β-HSD2 mRNA was significantly reduced by maternal ethanol consumption (Table 4). Placental HSD2 inactivates corticosterone, and the enzyme plays a critical role in regulating the levels of maternal corticosterone that cross the placenta and enter fetal circulation (Michael et al., 2003; Waddel et al., 1998). If reduced gene expression results in diminished HSD2 protein or enzymatic activity, the fetus may be exposed to abnormally high levels of corticosterone, which has been shown to have deleterious effects on brain development (Holmes et al., 2006; Welberg et al., 2000; Weinstock, 2007) and longer-term consequences (see review by Seckl and Holmes, 2007). In contrast to the neuroprotective effects of placental HSD2 during development, placental iodothyronine deiodinase 2 (DIO2) is responsible for the conversion of maternal thyroxine (T4) to the triiodothyronine (T3), the physiologically active form of thyroid hormone. Placental conversion of maternal T4 to T3 provides the only source of active thyroid hormone to the fetus through most of gestation in rodents (Morreale de Escobar et al., 1987). T3 regulates the expression of a large number of molecules important in fetal development, including neurotropic factors (Alvarez-Dolado et al., 1994), cytoskeletal elements (Silva and Rudas, 1990), and extracellular matrix molecules, such as L1 (Alvarez-Dolado et al., 2000), which is also affected by relatively low levels of ethanol exposure (see review by Bearer, 2001b). Thus, if diminished DIO2 protein or activity follows from an ethanol-induced reduction in placental DIO2 gene expression (Table 4), the fetus may be subject to a broad array of immediate and prolonged neurodevelopmental consequences as a function of a hypothyroid environment during most of the prenatal period.
GO analysis of molecular processes also suggested important effects of ethanol exposure on calcium ion binding and various types of protein- and carbohydrate-binding interactions in placenta (Fig. 3). Of particular note was ethanol's impact on three of the S100 calcium binding proteins (Table 4). The members of the S100 family are multifunctional signaling proteins that influence with many cellular events. S100B, S100A4, and S100G appear to be involved in neurotrophic and/or neuroprotective processes (Donato 2007; Druse et al., 2007; Santamaria-Kisiel et al., 2006). The literature on the function of these proteins in placental tissue is sparse. However, S100B is highly abundant in the nervous system, predominantly in astroglia, exhibiting temporal and spatial concentration patterns during brain maturation. Although the mechanisms of action of these proteins are not completely understood, they have protective neurotrophic effects during brain development, and alterations may serve as early, quantitative indicators of fetal brain damage in some biological fluids, for example, cord blood (Michetti and Gazzolo, 2002). Of particular note is the observation that S100B acts as a trophic factor for the development of the brainstem serotonergic system, which is adversely affected by prenatal ethanol exposure (Druse et al., 1991; Zhou et al., 2001, 2005).
Other proteins whose gene expression was altered (Table 4) suggest that a number of additional placental functions important for fetal development may be compromised by maternal drinking during pregnancy. However, confirmation of this speculation will require quantitation of protein levels and function in placental tissues. Such studies will be challenging for a number of reasons, including the relative paucity of tools for quantitating these proteins by standard means. Many of these proteins are membrane associated and likely to be scarce enough to be difficult to detect by proteomic approaches. Further, a number of these proteins have not been studied in placental preparations and, in some cases, the function of these proteins is not well understood in any tissue type.
Nevertheless, these challenges do not diminish the diagnostic potential of altered placental gene expression as a biomarker of fetal alcohol exposure and fetal alcohol effects. Even in this preliminary report, a sufficiently large enough number of placental genes were altered by ethanol exposure to warrant more detailed investigation of placenta as a biomarker system. Given that these genes are also expressed in human placenta, it is reasonable to expect that these findings could translate into human studies of drinking during pregnancy. Further, the clinical relevance of our findings is underscored by the fact that these gene changes occur after moderate intermittent ethanol consumption during pregnancy, a level that causes functional brain damage and learning deficits in the absence of any observable dysmorphologic effects in rat offspring. With the growing realization that most of the children with FASD exhibit neurobehavioral deficits in the absence of dysmorphologic features, the discovery of more sensitive biomarkers of fetal alcohol effects becomes an increasingly important objective for earlier diagnosis and treatment of FASD.
Acknowledgments
The authors thank the technical support of Marilee Morgan and Gavin Pickett at the Keck UNM Genomics Shared Resource, which is supported by the UNM Cancer Research and TreatmentCenter, the WM Keck Foundation,and the State of New Mexico. We also thank Ms. Denise Cordaro and Ms. Christie Wilcox for their outstanding animal care support for this project. This work was supported by AA15420, AA16619, AA17068, MH19101, and Dedicated Research Funds from the UNM Health Sciences Center.
Appendix
Table A1.
Primer sets used in quantitative real-time polymerase chain reaction validation of candidate placental genes listed in Table 4
| Symbol | Gene name | Affymetrix ID | Forward primer | Reverse primer | Amplicon bp size | Positiona |
|---|---|---|---|---|---|---|
| AFP | α-Fetoprotein | 1367758_at | ACAGGGCGATGTCCATAAAC | TGCCATTGATGCTCTCTTTG | 170 | 5538 |
| ACTB | β-Actin | 1398835_at | AAGTCCCTCACCCTCCCAAAAG | AAGCAATGCTGTCACCTTCCC | 97 | 3474 |
| CNRNA7 | Nicotinic cholinergic receptor α7 | 1387419_at | TATCACCACCATGACCCTGA | CAGAAACCATGCACACCAGT | 81 | 121903 |
| CNR1 | Cannabinoid receptor 1 | 1369677_at | AGGAGCAAGGACCTGAGACA | TAACGGTGCTCTTGATGCAG | 166 | 1197 |
| CYP1A1 | Cytochrome 450 1A1 | 1370269_at | TGAGGCTCAACTGTCTTCCAA | TCTTACTGCCCAGAAAGTCTGTC | 189 | 5452 |
| CYP2E1 | Cytochrome 450 2E1 | 1367871_at | TGAGACCACCAGCACAACTC | CTTCATGGGGTAGGTTGGAA | 216 | 6552 |
| DIO2 | Iodothyronine deiodinase 2 | 1385568_at | CTTCCTGGCGCTCTATGACT | ACACTGGAATTGGGAGCATC | 189 | 69 |
| EFEMP1 | EGF-containing fibulin-like extracellular matrix protein 1 | 1390112_at | CCGGGTTCCTTTTACTGTCA | CCACTTGGTAACCCTGAGGA | 286 | 74674 |
| FMO3 | Flavin-containing monooxygenase 3 | 1368304_at | GAGAAACCAACCATGGCAGT | CTGGGGTCCTTGAGAAACAG | 284 | 15144 |
| GAL | Galanin | 1387088_at | AGAGCAATATCGTCCGCACT | GTGTTGGCTTGAGGAGTTGG | 216 | 2972 |
| GALR2 | Galanin receptor 2 | 1384667_x_at | GCTCTGCAAGGCTGTTCATT | GGGTGGCATACTGTCAGGTT | 238 | 325 |
| GREM1 | Gremlin 1 | 1369113_at | GACAAGGCTCAGCACAATGA | CAGGTATTTGCGCTCTGTCA | 159 | 108 |
| Hand2 | Heart and neural crest derivatives expressed 2 | 1369818_at | CAAGGCGGAGATCAAGAAGA | TGGTTTTCTTGTCGTTGCTG | 81 | 94561 |
| HBD | Hemoglobin, delta | 1371102_x_at | ATGGCCTGAAACACTTGGAC | GCCCAACACAATCACAATCA | 128 | 335 |
| HBE1 | Hemoglobin, gamma 1 | 1388270_at | GCCTCTGCCATAATGGGTAA | CCTGTACCTCAGCCGTGAAT | 232 | 272 |
| HBG1 | Hemoglobin, epsilon 1 | 1388269_at | TGGGAAAAAGTGGACTTGGA | CCGAACCTAGAGACGTCAGC | 178 | 45 |
| HSD11B2 | 11β-hydroxysteroid dehydrogenase type 2 | 1368102_at | GCTATTGCACTGCTCATGGA | GCAATGCCATTCTGAGTGAA | 235 | 4060 |
| HSPA1B | Heat shock 70 kDa protein 1B | 1368247_at | CAAGATCACCATCACCAACG | GCTGATCTTGCCCTTGAGAC | 193 | 3326 |
| IGFBP6 | Insulin growth factor binding protein 6 | 1387625_at | CAGAGACCGGCAAAAGAATC | CTGCTTGCGGTAGAAACCTC | 193 | 2779 |
| INS | Insulin | 1370077_at | CAGCACCTTTGTGGTTCTCA | CAGTGCCAAGGTCTGAAGGT | 165 | 83 |
| ITGA7 | Integrin α7 | 1388240_a_at | TCGGGAACCCTATGAAGAGA | ATGAAGACATGAGCCCGAAC | 160 | 19564 |
| LBP | Lippopolysaccharide binding protein | 1387868_at | AAGGCGCAAGTGAGACTGAT | AGTCGAGGTCGTGGAGCTTA | 172 | 216 |
| MMP10 | Matrix metalloproteinase protein 10 (Stromolysin2) | 1368713_at | GGATAAAGGCTTCCCGAGAC | TGTGATGATCCACGGAAGAA | 111 | 6274 |
| MMP2 | Matrix metalloproteinase protein 2 (gelatinase A) | 1370301_at | GGATACAGGTGTGCCAAGGT | TCGGTGAGAAAAATGCAGTG | 141 | 37 |
| MMP3 | Matrix metalloproteinase protein 3 | 1368657_at | GATCGATGCAGCCATTTCTT | CACTTTCCCTGCATTTGGAT | 235 | 9908 |
| ORM2 | Orosomucoid 2 | 1368731_at | TTCAGACCACAGACGACCAG | CATGCCCACATCTTTGACAG | 254 | 650 |
| PGF | Placental growth factor | 1368919_at | TGCTGGGAACAACTCAACAG | CAGCGACTCAGAAGGACACA | 159 | 1186 |
| PGR | Progesterone receptor | 1387563_at | GAGAGGCAGCTGCTTTCAGT | AAACACCATCAGGCTCATCC | 117 | 42364 |
| S1004A | S100 calcium binding protein A4 | 1367846_at | CAACGAGGGTGACAAGTTCA | TGCAGGACAGGAAGACACAG | 182 | 1281 |
| S100B | S100 calcium binding protein G | 1386903_at | GGTGACAAGCACAAGCTGAA | TGGAGACGAAGGCCATAAAC | 172 | 28294 |
| S100G | S100 calcium binding protein B | 1368339_at | CTCTGGCAGCACTCACTGAC | GCTGGGGAACTCTGACTGAA | 164 | 24 |
| SFRP4 | Secreted frizzled-related protein 4 | 1368394_at | TATGACCGTGGAGTGTGCAT | CGATCAGGGCTCAGATGTTT | 140 | 485 |
| SFRP5 | Secreted frizzled-related protein 5 | 1393069_at | TCCTCTGGACAACGACCTCT | CTTAATGCGCATCTTGACCA | 163 | 484 |
| SMPX | Small muscle protein, X-linked | 1370165_at | AGCCTCCCAGAAGGAAAGAG | CCATTGAGAAAGCACGTCAA | 212 | 15686 |
| SPINK5 | Serine peptidase inhibitor, Kazal type 5 | 1398688_at | TTAGAGCACCAGCTGAGCAA | GCCTTGTGGACATGACAGTG | 202 | 30 |
| TGFA | Transforming growth factor alpha | 1387450_at | GGTTTTTGGTGCAGGAAGAG | GGCACCACTCACAGTGCTT | 219 | 69269 |
| TLR4 | Toll-like receptor type 4 | 1387982_at | TCACAACTTCAGTGGCTGGA | GTCTCCACAGCCACCAGATT | 176 | 5708 |
| TRH | Thyrotropin releasing hormone | 1368912_at | CAGAACGTCGATTCTTGTGG | TTCTCCCAAGTCTCCCCTCT | 152 | 1515 |
| TRPC4 | Transient receptor potential cation channel C4 | 1369164_a_at | GATGGCGGACTTCAGGATTA | CAGGTGAGAATTGGCAGTGA | 240 | 100275 |
Position number refers to the first base of the target sequence from transcription start.
Table A2.
Differentially regulated genes (304) in microarray experiment filtered by statistical significance and fold change greater than two, excluding expressed sequence tags (ESTs) and not annotated genes
| Affymetrix ID | Symbol | RefSeq | Downregulated gene name | Fold Δ |
|---|---|---|---|---|
| 1369113_at | GREM1 | NM_019282 | Gremlin 1, cysteine knot SuperFamily | –25.3 |
| 1385568_at | DIO2 | NM_031720 | Deiodinase, iodothyronine type II | –25.2 |
| 1387088_at | GAL | NM_033237 | Galanin | –21.4 |
| 1368731_at | ORM1 | NM_053288 | Orosomucoid 1 | –17.0 |
| 1388269_at | HBG1 | NM_172093 | Hemoglobin, gamma A | –16.2 |
| 1390112_at | EFEMP1 | NM_001012039 | EGF-containing fibulin-like extracellular matrix protein 1 | –15.8 |
| 1369677_at | CNR1 | NM_012784 | Cannabinoid receptor 1 | –15.5 |
| 1368304_at | FMO3 | NM_053433 | Flavin-containing monooxygenase 3 | –14.2 |
| 1368394_at | SFRP4 | NM_053544 | Secreted frizzled-related protein 4 | –13.9 |
| 1368912_at | TRH | NM_013046 | Thyrotropin-releasing hormone | –13.3 |
| 1398688_at | SPINK5 | XM_341607 | Serine peptidase inhibitor, Kazal type 5 | –10.7 |
| 1374558_at | ICOSLG | XM_574731 | Inducible T-cell co-stimulator ligand | –9.31 |
| 1387563_at | PGR | NM_022847 | Progesterone receptor | –9.21 |
| 1369625_at | AQP1 | NM_012778 | Aquaporin 1 | –9.15 |
| 1367846_at | S100A4 | NM_012618 | S100 calcium binding protein A4 | –8.78 |
| 1367627_at | GATM | NM_031031 | Glycine amidinotransferase | –8.26 |
| 1370843_at | GNG8 | NM_139185 | Guanine nucleotide binding protein, gamma 8 | –8.25 |
| 1369695_at | WT1 | NM_031534 | Wilms tumor 1 | –8.14 |
| 1393069_at | SFRP5 | XM_219887 | Secreted frizzled-related protein 5 | –7.86 |
| 1369164_a_at | TRPC4 | NM_080396 | Transient receptor potential cation channel C4 | –7.63 |
| 1387450_at | TGFA | NM_012671 | Transforming growth factor alpha | –7.62 |
| 1371102_x_at | HBD | NM_033234 | Hemoglobin, delta | –7.35 |
| 1369817_at | HAND2 | NM_022696 | Heart and neural crest derivatives expressed 2 | –6.97 |
| 1388270_at | HBE1 | NM_001008890 | Hemoglobin, epsilon 1 | –6.91 |
| 1368919_at | PGF | NM_053595 | Placental growth factor, VEGF-related protein | –6.46 |
| 1396407_at | GAS8 | NM_001039030 | Growth arrest-specific protein 8 | –6.43 |
| 1380206_at | KIF5C | XM_221307 | Kinesin family member 5C | –6.36 |
| 1367600_at | DES | NM_022531 | Desmin | –6.23 |
| 1370157_at | PLN | NM_022707 | Phospholamban | –6.03 |
| 1388138_at | THBS4 | XM_342172 | Thrombospondin 4 | –5.70 |
| 1367794_at | A2M | NM_012488 | Alpha2 macroglobulin | –5.62 |
| 1387656_at | SLC4A1 | NM_012651 | Solute carrier family 4, anion exchanger 1 | –5.60 |
| 1368713_at | MMP10 | NM_133514 | Matrix metalloproteinase 10 (stromelysin 2) | –5.49 |
| 1370956_at | DCN | NM_024129 | Decorin | –5.37 |
| 1380285_at | CHRD | NM_001024273 | Chordin | –5.36 |
| 1368342_at | AMPD3 | NM_031544 | Adenosine monophosphate deaminase E | –5.18 |
| 1386903_at | S100B | NM_013191 | S100 calcium binding protein B | –4.93 |
| 1370301_at | MMP2 | NM_031054 | Matrix metalloproteinase 2 | –4.87 |
| 1387295_at | SLC6A12 | NM_017335 | Solute carrier family 6, betaine/GABA, member 12 | –4.69 |
| 1387868_at | LBP | NM_017208 | Lipopolysaccharide binding protein | –4.66 |
| 1382757_at | FOXL2 | XM_345975 | Forkhead box l2 | –4.62 |
| 1368362_a_at | ASGR2 | NM_017189 | Asialoglycoprotein receptor 2 | –4.49 |
| 1380432_at | CMAH | XM_341876 | Cytidine monophosphate-n-acetylneuraminic acid hydroxylase | –4.46 |
| 1385182_at | PKP1 | XM_222666 | Plakophilin 1 | –4.41 |
| 1387659_at | GDA | NM_031776 | Guanine deaminase | –4.33 |
| 1390596_at | MLANA | XM_215234 | Melan-A | –4.26 |
| 1387625_at | IGFBP6 | NM_013104 | Insulin-like growth factor binding protein 6 | –4.26 |
| 1383792_at | SYTL1 | NM_001025651 | Synaptotagmin-like 1 | –4.19 |
| 1389670_at | HOXA11 | XM_575479 | Homeobox A11 | –4.19 |
| 1386921_at | CPE | NM_013128 | Carboxypeptidase E | –4.17 |
| 1377311_at | EMX2 | XM_574698 | Empty spiracles homolog 2 | –4.11 |
| 1388292_at | KCNJ3 | NM_031610 | Potassium inwardly-rectifying channel J3 | –4.08 |
| 1387025_at | DYNC1I1 | NM_019234 | Dynein, cytoplasmic 1, intermediate chain 1 | –4.04 |
| 1373032_at | MUSTN1 | NM_181368 | Musculoskeletal embryonic nuclear protein 1 | –4.02 |
| 1372254_at | SERPING1 | NM_199093 | Serpin peptidase inhibitor, Clade G (C1 inhibitor) | –3.95 |
| 1368413_at | ABP1 | NM_022935 | Amiloride binding protein 1 | –3.93 |
| 1368914_at | RUNX1 | NM_017325 | Runt-related transcription factor 1 | –3.93 |
| 1372065_at | ART3 | NM_001012034 | ADP–ribosyltransferase 3 | –3.92 |
| 1387004_at | NBL1 | NM_031609 | Neuroblastoma, suppression of tumorigenicity 1 | –3.90 |
| 1388608_x_at | HBA2 | NM_001013853 | Hemoglobin, alpha 2 | –3.90 |
| 1387419_at | CHRNA7 | NM_012832 | Nicotinic cholinergic receptor alpha 7 | –3.89 |
| 1367992_at | SCG5 | NM_013175 | Secretogranin V (7B2 protein) | –3.88 |
| 1369773_at | BMP3 | NM_017105 | Bone morphogenetic protein 3 | –3.88 |
| 1376198_at | ASAM | NM_173154 | Adipocyte-specific adhesion molecule | –3.87 |
| 1389160_at | ERAF | XM_215059 | Erythroid-associated factor | –3.86 |
| 1387200_at | OLIG1 | NM_021770 | Oligodendrocyte transcription factor 1 | –3.84 |
| 1369430_at | BCMO1 | NM_022862 | Beta-carotene 15, 15’-monooxygenase 1 | –3.74 |
| 1378745_at | PER3 | NM_023978 | Period homolog 3 | –3.73 |
| 1368081_at | ABCA2 | NM_024396 | ATP-binding cassette, sub-family A (ABC1), member 2 | –3.71 |
| 1369735_at | GAS6 | NM_057100 | Growth arrest-specific 6 | –3.69 |
| 1391534_at | ELOVL2 | XM_574001 | Elongation of very long chain fatty acids-like 2 | –3.64 |
| 1377867_at | QPCT | XM_233812 | Glutaminyl-peptide cyclotransferase | –3.61 |
| 1367985_at | ALAS2 | NM_013197 | Delta-aminolevulinate, synthase 2 | –3.60 |
| 1372649_at | HSPB7 | XM_342966 | Heat shock 27 kDa protein family member 7 | –3.60 |
| 1369572_at | MCPT1 | NM_017145 | Mast cell protease 1 | –3.58 |
| 1369464_at | ZP1 | NM_133569 | Zona pellucida glycoprotein 1 | –3.57 |
| 1388569_at | SERPINF1 | NM_177927 | Serpin peptidase I, Clade F (alpha-2 antiplasmin) | –3.52 |
| 1393588_at | CLDN14 | NM_001013429 | Claudin 14 | –3.51 |
| 1377643_at | HOXD10 | XM_221510 | Homeobox D10 | –3.47 |
| 1367566_at | SCGB1A1 | NM_013051 | Secretoglobin 1A1 | –3.46 |
| 1378898_at | DDX19A | NM_001005381 | Dead box polypeptide 19A | –3.44 |
| 1368583_a_at | HRG | NM_133428 | Histidine-rich glycoprotein | –3.44 |
| 1394316_a_at | TSPAN5 | NM_001004090 | Tetraspanin 5 | –3.42 |
| 1391018_at | MYO5C | XM_236411 | Myosin VC | –3.41 |
| 1372335_at | PCGF1 | NM_001007000 | Polycomb group ring finger 1 | –3.38 |
| 1369926_at | GPX3 | NM_022525 | Glutathione peroxidase 3 | –3.35 |
| 1369520_a_at | BCAT1 | NM_017253 | Branched-chain aminotransferase 1 | –3.33 |
| 1377336_at | SEMA3B | XM_343479 | Semaphorin 3B | –3.31 |
| 1388170_at | KCTD1 | XM_214617 | Potassium channel tetramerisation domain-containing 1 | –3.32 |
| 1367847_at | NUPR1 | NM_053611 | Nuclear protein 1 | –3.27 |
| 1368102_at | HSD11B2 | NM_017081 | Hydroxysteroid (11-beta) dehydrogenase 2 | –3.22 |
| 1387982_at | TLR4 | NM_019178 | Toll-like receptor 4 | –3.21 |
| 1379039_at | CMKLR1 | NM_022218 | Chemokine-like receptor 1 | –3.21 |
| 1388204_at | MMP13 | XM_343345 | Matrix metalloproteinase 13 (collagenase 3) | –3.21 |
| 1378673_at | MITF | XM_001065525 | Microphthalmia-associated transcription Factor | –3.19 |
| 1368167_at | CTSE | NM_012938 | Cathepsin E | –3.16 |
| 1386637_at | FGL2 | NM_053455 | Fibrinogen-like 2 | –3.14 |
| 1368338_at | CD52 | NM_053983 | CD52 molecule | –3.12 |
| 1397516_at | ALG2 | XM_232987 | Asparagine-linked glycosylation 2 homolog | –3.08 |
| 1382612_at | HOXA9 | XM_001057018 | Homeobox A9 | –3.06 |
| 1393219_at | C2 | NM_172222 | Complement component 2 | –3.06 |
| 1398398_at | HOXA10 | XM_347220 | Homeobox A10 | –3.03 |
| 1377729_at | ELOVL4 | XM_236476 | Elongation of very long chain fatty acids-like 4 | –3.03 |
| 1389408_at | RRM2 | NM_001025740 | Ribonucleotide reductase M2 polypeptide | –3.02 |
| 1388240_a_at | ITGA7 | NM_030842 | Integrin, alpha 7 | –3.01 |
| 1387011_at | LCN2 | NM_130741 | Lipocalin 2 (oncogene 24p3) | –3.01 |
| 1368464_at | CLEC10A | NM_022393 | C-type lectin domain 10A | –2.99 |
| 1398253_at | KAP | NM_052802 | Kidney androgen-regulated protein | –2.99 |
| 1379345_at | COL15A1 | XM_216399 | Collagen, type XV alpha 1 | –2.99 |
| 1367919_at | NUP210 | NM_053322 | Nucleoporin 210 kDa | –2.98 |
| 1370895_at | COL5A2 | XM_343564 | Collagen, type V, alpha 2 | –2.98 |
| 1367998_at | SLPI | XM_215940 | Secretory leukocyte peptidase inhibitor | –2.97 |
| 1367960_at | ARL4A | NM_019186 | ADP-ribosylation factor-like 4A | –2.96 |
| 1370665_at | HYOU1 | NM_001034028 | Hypoxia up-regulated 1 | –2.94 |
| 1367774_at | GSTA1 | NM_031509 | Glutathione s-transferase A1 | –2.94 |
| 1391201_at | WDHD1 | XM_223933 | WD repeat and HMG-box DNA binding protein 1 | –2.92 |
| 1390547_at | ST6GALNAC1 | XM_221248 | ST6 | –2.91 |
| 1386889_at | SCD2 | NM_031841 | Stearoyl-coenzyme A desaturase 2 | –2.90 |
| 1368893_at | CAP2 | NM_053874 | CAP, adenylate cyclase-associated protein 2 | –2.87 |
| 1377772_at | TMEFF1 | NM_023020 | Transmembrane protein w/EGF-& follistatin-like domains 1 | –2.84 |
| 1368860_at | PHLDA1 | NM_017180 | Pleckstrin homology-like domain A1 | –2.83 |
| 1398304_at | FZD2 | NM_172035 | Frizzled homolog 2 | –2.82 |
| 1368490_at | CD14 | NM_021744 | CD14 molecule | –2.82 |
| 1383862_at | CLEC2D | XM_342769 | C-type lectin domain 2D | –2.79 |
| 1385665_at | ADAM19 | XM_220328 | ADAM metallopeptidase domain 19 (Meltrin beta) | –2.76 |
| 1386884_at | HTRA1 | NM_031721 | HTRA serine peptidase 1 | –2.71 |
| 1375123_at | SOX4 | XM_344594 | SRY (sex determining region y)-box 4 | –2.70 |
| 1370361_at | CGREF1 | NM_139087 | Cell growth regulator with EF-hand domain 1 | –2.69 |
| 1368657_at | MMP3 | NM_133523 | Matrix metalloproteinase 3 (stromelysin 1, progelatinase) | –2.67 |
| 1368721_at | ASCL2 | NM_031503 | Achaete-scute complex homolog 2 | –2.67 |
| 1393808_at | FA2H | XM_001073350 | Fatty acid 2-hydroxylase | –2.66 |
| 1371081_at | RAPGEF4 | XM_215985 | RAP guanine nucleotide exchange F 4 | –2.64 |
| 1371087_a_at | MAP6 | NM_017204 | Microtubule-associated protein 6 | –2.64 |
| 1382809_at | CIRBP | NM_031147 | Cold inducible RNA binding protein | –2.60 |
| 1367564_at | NPPA | NM_012612 | Natriuretic peptide precursor A | –2.60 |
| 1370236_at | PPT1 | NM_022502 | Palmitoyl-protein thioesterase 1 | –2.58 |
| 1370034_at | CDC25B | NM_133572 | Cell division cycle 25 homolog B | –2.58 |
| 1388902_at | LOXL1 | NM_001012125 | Lysyl oxidase-like 1 | –2.58 |
| 1387219_at | ADM | NM_012715 | Adrenomedullin | –2.55 |
| 1370260_at | ADD3 | NM_031552 | Adducin 3, gamma | –2.52 |
| 1368681_at | PTHLH | NM_012636 | Parathyroid hormone-like hormone | –2.51 |
| 1368021_at | ADH1C | NM_019286 | Alcohol dehydrogenase 1C gamma polypeptide | –2.51 |
| 1377950_at | IIGP1 | NM_001024884 | Interferon-inducible GTPase 1 | –2.50 |
| 1371989_at | HMGN3 | NM_001007020 | High mobility group nucleosomal binding domain 3 | –2.47 |
| 1368970_at | CDH23 | NM_053644 | Cadherin-like 23 | –2.44 |
| 1376973_at | SDCBP2 | NM_001025692 | Syndecan binding protein 2 | –2.44 |
| 1392965_a_at | SMOC2 | XM_214777 | SPARC-related modular calcium binding 2 | –2.43 |
| 1391279_at | SCIN | NM_198748 | Scinderin | –2.42 |
| 1370384_a_at | PRLR | NM_001034111 | Prolactin receptor | –2.41 |
| 1369977_at | UCHL1 | NM_017237 | Ubiquitin carboxyl-terminal esterase 11 (Ubiquitin thiolesterase) | –2.40 |
| 1384073_at | ADHFE1 | NM_001025423 | Alcohol dehydrogenase, iron-containing, 1 | –2.39 |
| 1387972_at | MUCDHL | NM_138525 | Mucin and cadherin-like protein | –2.39 |
| 1369012_at | INHBA | NM_017128 | Inhibin, beta A (activin A, activin AB alpha polypeptide) | –2.38 |
| 1367816_at | HOP | NM_133621 | Homeodomain-only protein | –2.37 |
| 1389746_at | NAGLU | XM_340905 | N-acetylglucosaminidase, alpha | –2.35 |
| 1387873_at | WFDC1 | NM_133581 | WAP four-disulfide core domain 1 | –2.34 |
| 1368503_at | GCH1 | NM_024356 | GTP cyclohydrolase 1 | –2.33 |
| 1370633_at | CXCL1 | NM_138522 | Chemokine ligand 1 | –2.33 |
| 1372042_at | CMTM3 | XM_226200 | CKLF-like marvel transmembrane domain containing 3 | –2.33 |
| 1367705_at | GLRX | NM_022278 | Glutaredoxin (thioltransferase) | –2.31 |
| 1374151_at | TM6SF1 | NM_145785 | Transmembrane 6 SuperFamily, member 1 | –2.30 |
| 1397758_at | GNPTAB | NM_001007750 | N-acetylglucosamine-1-phosphate Transferase, α & β subunits | –2.29 |
| 1379075_at | MBOAT2 | XM_234011 | Membrane bound O-acyltransferase domain containing 2 | –2.29 |
| 1367568_a_at | MGP | NM_012862 | Matrix GLA protein | –2.28 |
| 1370714_a_at | ST6GAL1 | NM_147205 | ST6 beta-galactosamide alpha-2,6-sialyltranferase 1 | –2.27 |
| 1372715_at | SFXN1 | NM_001012213 | Sideroflexin 1 | –2.27 |
| 1376697_at | CHST12 | NM_001037775 | Carbohydrate (chondroitin 4) sulfotransferase 12 | –2.27 |
| 1368367_at | CUZD1 | NM_054005 | Cub and zona pellucida-like domains 1 | –2.27 |
| 1385559_at | DNHD3 | NM_001126292 | Dynein, axonemal, heavy chain 2 | –2.26 |
| 1383363_at | DIRAS2 | XM_225214 | DIRAS family, GTP-binding RAS-like 2 | –2.25 |
| 1377573_at | CA5B | NM_001005551 | Carbonic anhydrase VB, mitochondrial | –2.24 |
| 1388427_at | MXRA8 | NM_001007002 | Limitrin | –2.24 |
| 1370291_at | PDLIM3 | NM_053650 | PDZ and LIM domain 3 | –2.24 |
| 1367722_at | DPP7 | NM_031973 | Dipeptidyl-peptidase 7 | –2.23 |
| 1370026_at | CRYAB | NM_012935 | Crystallin, Alpha B | –2.23 |
| 1369724_at | F13A1 | NM_021698 | Coagulation factor XIII, A1 polypeptide | –2.22 |
| 1372301_at | AEBP1 | XM_223583 | AE binding protein 1 | –2.22 |
| 1368005_at | ITPR3 | NM_013138 | Inositol 1,4,5-triphosphate receptor 3 | –2.22 |
| 1385013_at | WNT1 | XM_235639 | Wingless-type MMTV integration site 1 | –2.22 |
| 1387588_at | EHD3 | NM_138890 | EH-domain containing 3 | –2.21 |
| 1398311_a_at | KIDINS220 | NM_053795 | Kinase D-interacting substance of 220 kDa | –2.21 |
| 1370182_at | PTPRN2 | NM_031600 | Protein tyrosine phosphatase receptor N2 | –2.21 |
| 1389423_at | DDR2 | NP_113952 | Discoidin domain receptor family 2 | –2.21 |
| 1397164_at | POLA2 | NM_053480 | Polymerase (DNA-directed), alpha 2 (70 kDa Subunit) | –2.20 |
| 1390233_at | GLI2 | NM_001107169 | Gli-kruppel family member GLI2 | –2.20 |
| 1390846_at | COL16A1 | XM_345584 | Collagen, type XVI, alpha 1 | –2.20 |
| 1383630_at | DOK3 | XM_225170 | Docking protein 3 | –2.20 |
| 1390882_at | HEYL | NM_001107977 | Hairy/enhancer-of-split related with VRPW motif-like | –2.20 |
| 1367859_at | TGFB3 | NM_013174 | Transforming growth factor beta 3 | –2.20 |
| 1387505_at | GNAI1 | NM_013145 | G-Protein, alpha inhibiting activity polypeptide 1 | –2.19 |
| 1389651_at | APLN | NM_031612 | Apelin, AGTRL1 ligand | –2.18 |
| 1374863_at | RBP7 | XM_575960 | Retinol binding protein 7 | –2.16 |
| 1367823_at | TIMP2 | NM_021989 | TIMP metalloproteinase inhibitor 2 | –2.16 |
| 1368292_at | DNM1 | NM_080689 | Dynamin 1 | –2.16 |
| 1375033_at | CPT1C | XM_218625 | Carnitine palmitoyltransferase 1C | –2.15 |
| 1374849_at | ADAMTS7 | XM_236471 | ADAM metallopeptidase with thrombospondin type 1 motif, 7 | –2.15 |
| 1370342_at | KCNK2 | NM_172041 | Potassium channel K2 | –2.15 |
| 1393245_at | PHYH | NM_053674 | Phytanoyl-CoA 2-hydroxylase | –2.13 |
| 1371237_a_at | MT1E | NM_138826 | Metallothionein 1E | –2.13 |
| 1369443_at | ANGPTL2 | NM_022926 | Angiopoietin-like 2 | –2.13 |
| 1389739_at | NEURL2 | XM_230848 | Neuralized homolog 2 | –2.11 |
| 1369640_at | GJA1 | NM_012567 | Gap junction protein, alpha 1, 43 kDa (Connexin 43) | –2.11 |
| 1377163_at | INHBB | XM_344130 | Inhibin, beta b (Activin AB beta polypeptide) | –2.10 |
| 1388070_a_at | AKAP1 | NM_053665 | A Kinase (PRKA) anchor protein 1 | –2.10 |
| 1381226_at | NAV1 | XM_222662 | Neuron navigator 1 | –2.10 |
| 1370713_at | CDC2L1 | NM_145766 | Cell division cycle 2-like 1 (PITSLRE proteins) | –2.10 |
| 1375871_at | SLC35B1 | NM_199081 | Solute carrier family 35, member B1 | –2.10 |
| 1378671_at | CREBBP | NM_133381 | CREB binding protein (Rubinstein-Taybi syndrome) | –2.10 |
| 1398295_at | SLC29A1 | NM_031684 | Solute carrier family 29 (nucleoside transporters), member 1 | –2.10 |
| 1369141_at | CSH1 | NM_017363 | Chorionic somatomammotropin hormone 1 | –2.08 |
| 1396831_at | MAML3 | XM_227165 | Mastermind-like 3 | –2.08 |
| 1375243_at | BTBD2 | XM_576181 | BTB (POZ) domain-containing 2 | –2.08 |
| 1382511_at | E2F1 | XM_230765 | E2F transcription factor 1 | –2.07 |
| 1368006_at | LAPTM5 | NM_053538 | Lysosomal-associated multispanning membrane protein 5 | –2.07 |
| 1373970_at | IL33 | NM_001014166 | Interleukin 33 | –2.07 |
| 1385587_at | MCOLN2 | NM_001039005 | Mucolipin 2 | –2.07 |
| 1369194_a_at | CDKN2A | NM_053434 | Cyclin-dependent kinase inhibitor 2A | –2.07 |
| 1379495_at | PLXDC2 | NM_001108422 | Plexin domain-containing 2 | –2.07 |
| 1377699_at | BACH1 | XM_221712 | BTB and CNC homology 1 | –2.06 |
| 1384667_x_at | GALR2 | NM_019172 | Galanin receptor 2 | –2.05 |
| 1370202_at | HRASLS3 | NM_017060 | HRAS-like suppressor 3 | –2.05 |
| 1387952_a_at | CD44 | NM_012924 | CD44 molecule | –2.04 |
| 1386953_at | HSD11B1 | NM_017080 | Hydroxysteroid (11-beta) dehydrogenase 1 | –2.04 |
| 1368254_a_at | SPHK1 | NM_133386 | Sphingosine kinase 1 | –2.04 |
| 1389115_at | EVPL | XM_221129 | Envoplakin | –2.04 |
| 1371441_at | PEA15 | NM_001013231 | Phosphoprotein-enriched in astrocytes 15 | –2.04 |
| 1369431_at | GALNT7 | NM_053648 | Polypeptide n-acetylgalactosaminyltransferase 7 | –2.03 |
| 1386160_at | TCHH | XM_227373 | Trichohyalin | –2.01 |
| 1389533_at | FBLN2 | XM_232197 | Fibulin 2 | –2.01 |
| 1367882_at | MAP1A | NM_030995 | Microtubule-associated protein 1A | –2.01 |
| 1387296_at | CYP2J2 | NM_023025 | Cytochrome P450 2J2 | –2.01 |
| 1394451_at | ANXA1 | NM_012904 | Annexin A1 | –2.01 |
| Affymetrix ID | Symbol | RefSeq | Upregulated gene name | Fold Δ |
|---|---|---|---|---|
| 1386980_at | APOM | NM_019373 | Apolipoprotein M | 2.00 |
| 1370259_a_at | PTHR1 | NM_020073 | Parathyroid hormone receptor 1 | 2.00 |
| 1369331_a_at | UNC13B | NM_031550 | unc-13 homolog B | 2.01 |
| 1391345_at | BMPER | NM_001135799 | BMP binding endothelial regulator | 2.02 |
| 1372690_at | RTN1 | NM_181377 | Reticulon 1 | 2.02 |
| 1398383_at | CYB561 | XM_221030 | Cytochrome B-561 | 2.02 |
| 1375726_at | LMO7 | NM_001001515 | LIM domain 7 | 2.02 |
| 1376799_a_at | CRLF1 | XM_214312 | Cytokine receptor-like factor 1 | 2.02 |
| 1370420_at | SRD5A1 | NM_017070 | Steroid-5 alpha-reductase | 2.02 |
| 1368785_a_at | PITX2 | NM_019334 | Paired-like homeodomain transcription factor 2 | 2.03 |
| 1369756_a_at | SLC4A4 | NM_053424 | Solute carrier family 4, sodium bicarbonate cotransporter 4 | 2.03 |
| 1386943_at | PLLP | NM_022533 | Transmembrane 4 SuperFamily, member 11 | 2.03 |
| 1385961_at | KLF5 | NM_053394 | Kruppel-like factor 5 | 2.04 |
| 1374273_at | CXADR | NM_053570 | Coxsackie virus and adenovirus receptor | 2.04 |
| 1377304_at | CDC26 | NM_001013240 | Cell division cycle 26 homolog | 2.05 |
| 1375849_at | RGMA | NM_001107524 | Rgm domain family, member a | 2.05 |
| 1368247_at | HSPA1B | NM_212504 | Heat shock 70 kDa protein 1B | 2.06 |
| 1389735_at | RPS6KA6 | XM_228473 | Ribosomal protein S6 kinase, 90 kDa, polypeptide 6 | 2.08 |
| 1387298_at | PGA5 | NM_021753 | Pepsinogen 5, group I (Pepsinogen a) | 2.12 |
| 1387232_at | BMP4 | NM_012827 | Bone morphogenetic protein 4 | 2.13 |
| 1392556_at | SHROOM3 | XM_223229 | Shroom family member 3 | 2.16 |
| 1383981_at | TRP53BP2 | XM_223012 | Tumor protein p53 binding protein 2 | 2.17 |
| 1394663_at | POLS | XM_225072 | Polymerase (DNA-directed) Sigma | 2.18 |
| 1392773_at | PCSK5 | XM_342032 | Proprotein convertase subtilisin/kexin type 5 | 2.20 |
| 1389276_at | L3MBTL3 | XM_001062689 | L(3)MBT-like 3 | 2.20 |
| 1383747_at | ECT2 | XM_342220 | Epithelial cell transforming sequence 2 oncogene | 2.20 |
| 1387574_at | CHRNA2 | NM_133420 | Nicotinic cholinergic receptor alpha 2 | 2.22 |
| 1389066_at | DSCR1L1 | NM_175578 | Down syndrome critical region gene 1-like 1 | 2.22 |
| 1375729_at | EPHA4 | XM_244186 | EPH receptor A4 | 2.25 |
| 1369727_at | APOA2 | NM_013112 | Apolipoprotein A-II | 2.29 |
| 1377379_at | IRF6 | XM_344194 | Interferon regulatory factor 6 | 2.33 |
| 1368339_at | S100 G | NM_012521 | S100 calcium binding protein G | 2.35 |
| 1386396_at | DUSP8 | XM_341963 | Dual specificity phosphatase 8 | 2.36 |
| 1367598_at | TTR | NM_012681 | Transthyretin (prealbumin, amyloidosis Type I) | 2.37 |
| 1371030_at | SPP2 | NM_053577 | Secreted phosphoprotein 2 | 2.39 |
| 1386873_at | TNNI1 | NM_017184 | Troponin I type 1 | 2.40 |
| 1393139_at | APOC2 | XM_214872 | Apolipoprotein C-II | 2.40 |
| 1389234_at | VWF | XM_342759 | Von Willebrand factor | 2.41 |
| 1371059_at | PRKAR2A | NM_019264 | CAMP-dependent protein kinase 2A | 2.44 |
| 1370463_x_at | HLA-F | NM_001008829 | Major histocompatibility complex, class I, F | 2.44 |
| 1389648_at | RIPK4 | XM_221619 | Receptor-interacting serine-threonine kinase 4 | 2.44 |
| 1368280_at | CTSC | NM_017097 | Cathepsin C | 2.46 |
| 1384747_at | GPR137B | XM_237907 | G Protein-coupled receptor 137B | 2.51 |
| 1369837_at | GULO | NM_022220 | Gulonolactone (L-) oxidase | 2.56 |
| 1387396_at | HAMP | NM_053469 | Hepcidin antimicrobial peptide | 2.59 |
| 1368278_at | LGALS2 | NM_133599 | Lectin, galactoside-binding, soluble, 2 (galectin 2) | 2.59 |
| 1367758_at | AFP | NM_012493 | Alpha-fetoprotein | 2.60 |
| 1386913_at | PDPN | NM_019358 | Podoplanin | 2.67 |
| 1382965_at | AMIGO3 | NM_178144 | Adhesion molecule with Ig-like domain 3 | 2.69 |
| 1367749_at | LUM | NM_031050 | Lumican | 2.77 |
| 1375183_at | ID4 | NM_175582 | DNA binding inhibitor 4, dominant negative helix-loop-helix prot. | 2.80 |
| 1368442_at | F2 | NM_022924 | Coagulation factor II (thrombin) | 2.85 |
| 1380621_at | FES | NM_001108488 | Feline sarcoma oncogene | 2.88 |
| 1379741_at | ATP6V0A4 | XM_231615 | ATPase, H+ transporting, lysosomal V0 subunit A4 | 2.92 |
| 1370086_at | FGG | NM_012559 | Fibrinogen, gamma chain | 2.92 |
| 1387414_at | DUOX2 | NM_024141 | Dual oxidase 2 | 2.94 |
| 1368316_at | AQP8 | NM_019158 | Aquaporin 8 | 2.97 |
| 1388190_at | APOB | NM_019287 | Apolipoprotein B | 2.98 |
| 1378866_at | ABLIM1 | XM_217645 | Actin binding LIM protein 1 | 3.11 |
| 1368527_at | PTGS2 | NM_017232 | Prostaglandin-endoperoxide synthase 2 | 3.16 |
| 1392703_at | TBX4 | NM_001107034 | T-box 4 | 3.18 |
| 1387565_at | TRPV6 | NM_053686 | Transient receptor potential cation channel V6 | 3.26 |
| 1370269_at | CYP1A1 | NM_012540 | Cytochrome P450 1A1 | 3.33 |
| 1369663_at | EPHX2 | NM_022936 | Epoxide hydrolase 2 | 4.10 |
| 1392948_at | CLIC6 | NM_176078 | Chloride intracellular channel 6 | 4.33 |
| 1393891_at | COL8A1 | XM_221536 | Collagen, type VIII, alpha 1 | 4.40 |
| 1370310_at | HMGCS2 | NM_173094 | 3-Hydroxy-3-methylglutaryl-coenzyme A synthase 2 | 4.65 |
| 1368335_at | APOA1 | NM_012738 | Apolipoprotein A-1 | 4.88 |
| 1380134_at | VTCN1 | NM_001024244 | V-set domain-containing T-cell activation inhibitor 1 | 5.08 |
| 1370594_at | IGSF1 | NM_175763 | Immunoglobulin SuperFamily, member 1 | 5.99 |
| 1370077_at | INS | NM_019130 | Insulin | 6.67 |
| 1370165_at | SMPX | NM_053395 | Small muscle protein, X-linked | 13.7 |
References
- Abel EL. An update on incidence of FAS: FAS is not an equal opportunity birth defect. Neurotoxicol. Teratol. 1995;17:437–443. doi: 10.1016/0892-0362(95)00005-c. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Cuadrado A, Navarro-Yubero C, Sonderegger P, Furley AJ, Bernal J, et al. Regulation of the L1 cell adhesion molecule by thyroid hormone in the developing brain. Mol. Cell. Neurosci. 2000;16:499–514. doi: 10.1006/mcne.2000.0879. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Iglesias T, Rodríguez-Peña A, Bernal J, Muñoz A. Expression of neurotrophins and the trk family of neurotrophin receptors in normal and hypothyroid rat brain. Brain Res. Mol. Brain. Res. 1994;27:249–257. doi: 10.1016/0169-328x(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Arber S, Caroni P. Thrombospondin-4, an extracellular matrix protein expressed in the developing and adult nervous system promotes neurite outgrowth. J. Cell Biol. 1996;131:1083–1094. doi: 10.1083/jcb.131.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T, Suzek TO, Troup DB, Wilhite SE, Ngau WC, Ledoux P, et al. NCBI GEO: mining millions of expression profiles–database and tools. (Database issue). Nucleic Acids Res. 2005;33:D562–D566. doi: 10.1093/nar/gki022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer CF. Markers to detect drinking during pregnancy. Alcohol Res. Health. 2001a;25:210–218. [PMC free article] [PubMed] [Google Scholar]
- Bearer CF. Mechanisms of brain injury: L1 cell adhesion molecule as a target for ethanol-induced prenatal brain injury. Semin. Pediatr. Neurol. 2001b;8:100–107. doi: 10.1053/spen.2001.25227. [DOI] [PubMed] [Google Scholar]
- Bearer CF, Gould S, Emerson R, Kinnunen P, Cook CS. Fetal alcohol syndrome and fatty acid ethyl esters. Pediatr. Res. 1992;31:492–495. doi: 10.1203/00006450-199205000-00017. [DOI] [PubMed] [Google Scholar]
- Bearer CF, Jacobson JL, Jacobson SW, Barr D, Croxford J, Molteno CD, et al. Validation of a new biomarker of fetal exposure to alcohol. J. Pediatr. 2003a;143:463–469. doi: 10.1067/S0022-3476(03)00442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer CF, Lee S, Salvator AE, Minnes S, Swick A, Yamashita T, et al. Ethyl linoleate in meconium: a biomarker for prenatal ethanol exposure. Alcohol. Clin. Exp. Res. 1999;23:487–493. [PMC free article] [PubMed] [Google Scholar]
- Bearer CF, Linsalata N, Yomtovian R, Walsh M, Singer L. Blood transfusions: a hidden source of lead exposure. Lancet. 2003b;362:332. doi: 10.1016/S0140-6736(03)13989-X. [DOI] [PubMed] [Google Scholar]
- Bearer CF, Santiago LM, O'Riordan MA, Buck K, Lee SC, Singer LT. Fatty acid ethyl esters: quantitative biomarkers for maternal alcohol consumption. J. Pediatr. 2005;146:824–830. doi: 10.1016/j.jpeds.2005.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995;57:289–300. [Google Scholar]
- Byrnes ML, Reynolds JN, Brien JF. Brain growth spurt-prenatal ethanol exposure and the guinea pig hippocampal glutamate signaling system. Neurotoxicol. Teratol. 2003;25:303–310. doi: 10.1016/s0892-0362(02)00354-9. [DOI] [PubMed] [Google Scholar]
- Chesnutt C, Burrus LW, Brown AM, Niswander L. Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev. Biol. 2004;274:334–347. doi: 10.1016/j.ydbio.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat. Rev. Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Smith DW. The fetal alcohol syndrome. N. Engl. J. Med. 1978;298:1063–1067. doi: 10.1056/NEJM197805112981906. [DOI] [PubMed] [Google Scholar]
- Conry J. Neuropsychological deficits in fetal alcohol syndrome and fetal alcohol effects. Alcohol. Clin. Exp. Res. 1990;14:650–655. doi: 10.1111/j.1530-0277.1990.tb01222.x. [DOI] [PubMed] [Google Scholar]
- Donato R. RAGE: a single receptor for several ligands and different cellular responses: the case of certain S100 proteins. Curr. Mol. Med. 2007;7:711–724. doi: 10.2174/156652407783220688. [DOI] [PubMed] [Google Scholar]
- Druse MJ, Kuo A, Tajuddin N. Effects of in utero ethanol exposure on the developing serotonergic system. Alcohol. Clin. Exp. Res. 1991;15:678–684. doi: 10.1111/j.1530-0277.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash AE. Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk J, Bechara A, Fiore R, Nawabi H, Zhou H, Hoyo-Becerra C, et al. Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron. 2005;48:63–75. doi: 10.1016/j.neuron.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with fetal alcohol syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behav. Brain Res. 2003;143:85–94. doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Hanson JW, Streissguth AP, Smith DW. The effects of moderate alcohol consumption during pregnancy on fetal growth and morphogenesis. J. Pediatr. 1978;92:457–460. doi: 10.1016/s0022-3476(78)80449-1. [DOI] [PubMed] [Google Scholar]
- Harris MA, Clark J, Ireland A, Lomax J, Ashburner M, Foulger R, et al. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 2004;32:D258–D261. doi: 10.1093/nar/gkh036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MC, Sangra M, French KL, Whittle IR, Paterson J, Mullins JJ, et al. 11beta-Hydroxysteroid dehydrogenase type 2 protects the neonatal cerebellum from deleterious effects of glucocorticoids. Neuroscience. 2006;137:865–873. doi: 10.1016/j.neuroscience.2005.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Sokol RJ, Ager JW., Jr. Relation of maternal age and pattern of pregnancy drinking to functionally significant cognitive deficit in infancy. Alcohol. Clin. Exp. Res. 1998;22:345–351. doi: 10.1111/j.1530-0277.1998.tb03659.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Chiodo LM, Corobana R. Maternal age, alcohol abuse history, and quality of parenting as moderators of the effects of prenatal alcohol exposure on 7.5-year intellectual function. Alcohol. Clin. Exp. Res. 2004;28:1732–1745. doi: 10.1097/01.alc.0000145691.81233.fa. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissguth P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1:1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Kulaga V, Caprara D, Iqbal U, Kapur B, Klein J, Reynolds J, et al. Fatty acid ethyl esters (FAEE); comparative accumulation in human and guinea pig hair as a biomarker for prenatal alcohol exposure. Alcohol Alcohol. 2006;41:534–539. doi: 10.1093/alcalc/agl048. [DOI] [PubMed] [Google Scholar]
- Lemoine P, Haronsseau H, Borteryu JP, Menuet JC. Les enfants de parents alcooliques: anomalies observees a propos de 127 cas. Ouest Med. 1968;25:476–482. [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lundquist F. The determination of ethyl alcohol in blood and tissues. In: Glick D, editor. Methods of Biochemical Analysis. Vol. 7. Inter-science Publishers, Inc.; New York, N.Y.: 1959. p. 217. 1959. [Google Scholar]
- May PA, Gossage JP, Marais AS, Adnams CM, Hoyme HE, Jones KL, et al. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. 2007;88:259–271. doi: 10.1016/j.drugalcdep.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Miller JH, Goodhart KA, Maestas OR, Buckley D, Trujillo PM, et al. Enhanced case management to prevent fetal alcohol spectrum disorders in Northern Plains communities. Matern. Child Health J. 2008;12:747–759. doi: 10.1007/s10995-007-0304-2. [DOI] [PubMed] [Google Scholar]
- McClintick JN, Edenberg HJ. Effects of filtering by present call on analysis of microarray experiments. BMC Bioinform. 2006;7:49. doi: 10.1186/1471-2105-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael AE, Thurston LM, Rae MT. Glucocorticoid metabolism and reproduction: a tale of two enzymes. Reproduction. 2003;126:425–441. doi: 10.1530/rep.0.1260425. [DOI] [PubMed] [Google Scholar]
- Michetti F, Gazzolo D. S100B protein in biological fluids: a tool for perinatal medicine. Clin. Chem. 2002;48:2097–2104. [PubMed] [Google Scholar]
- Millet C, Lemaire P, Orsetti B, Guglielmi P, François V. The human chordin gene encodes several differentially expressed spliced variants with distinct BMP opposing activities. Mech. Dev. 2001;106:85–96. doi: 10.1016/s0925-4773(01)00423-3. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Keller RW, Jr., Snyder-Keller A. Immediate-early gene expression in concurrent prenatal ethanol- and/or cocaine-exposed rat pups: intrauterine differences in cocaine levels and Fos expression. Brain Res. Dev. Brain Res. 2002;133:141–149. doi: 10.1016/s0165-3806(02)00282-1. [DOI] [PubMed] [Google Scholar]
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Fetal and maternal thyroid hormones. Horm. Res. 1987;26:12–27. doi: 10.1159/000180681. [DOI] [PubMed] [Google Scholar]
- Nakagawa O, McFadden DG, Nakagawa M, Yanagisawa H, Hu T, Srivastava D, et al. Members of the HRT family of basic helix-loop-helix proteins act as transcriptional repressors downstream of Notch signaling. Proc. Natl. Acad. Sci. U.S.A. 2001;97:13655–13660. doi: 10.1073/pnas.250485597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B, Gibbons HM, Mitchell MD, Glass M. Identification of the CB1 cannabinoid receptor and fatty acid amide hydrolase (FAAH) in the human placenta. Placenta. 2003;24:990–995. doi: 10.1016/s0143-4004(03)00165-6. [DOI] [PubMed] [Google Scholar]
- Pichini S, Pellegrini M, Gareri J, Koren G, Garcia-Algar O, Vall O, et al. Liquid chromatography-tandem mass spectrometry for fatty acid ethyl esters in meconium: assessment of prenatal exposure to alcohol in two European cohorts. J. Pharm. Biomed. Anal. 2008;48:927–933. doi: 10.1016/j.jpba.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Qiang M, Wang MW, Elberger AJ. Second trimester prenatal alcohol exposure alters development of rat corpus callosum. Neurotoxicol. Teratol. 2002;24:719–732. doi: 10.1016/s0892-0362(02)00267-2. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, et al. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS. Calcium-dependent and -independent interactions of the S100 protein family. Biochem. J. 2006;396:201–214. doi: 10.1042/BJ20060195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, et al. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol. Biol. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal “programming” of adult pathophysiology. Nat. Clin. Pract. Endocrinol. Metab. 2007;3:479–488. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Cohen DJ, Shaywitz BA. Behavior and learning difficulties in children of normal intelligence born to alcoholic mothers. J. Pediatr. 1980;96:978–982. doi: 10.1016/s0022-3476(80)80621-4. [DOI] [PubMed] [Google Scholar]
- Silva JE, Rudas P. Effects of congenital hypothyroidism on microtubule-associated protein-2 expression in the cerebellum of the rat. Endocrinology. 1990;126:1276–1282. doi: 10.1210/endo-126-2-1276. [DOI] [PubMed] [Google Scholar]
- Sood R, Zehnder JL, Druzin ML, Brown PO. Gene expression patterns in human placenta. Proc. Natl. Acad. Sci. U.S.A. 2006;103:5478–5483. doi: 10.1073/pnas.0508035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton KR, Howe CJ, Battaglia F, editors. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention and Treatment. National Academy Press; Washington D.C: 1996. [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD. Moderate prenatal alcohol exposure: effects on child IQ and learning problems at age 7 1/2 years. Alcohol. Clin. Exp. Res. 1990;14:662–669. doi: 10.1111/j.1530-0277.1990.tb01224.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Randels SP, Smith DF. A test-retest study of intelligence in patients with fetal alcohol syndrome: implications for care. J. Am. Acad. Child Adolesc. Psychiatry. 1991;30:584–587. doi: 10.1097/00004583-199107000-00009. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Sampson PD, Olson HC, Bookstein FL, Barr HM, Scott M, et al. Maternal drinking during pregnancy: attention and short-term memory in 14-year-old offspring–a longitudinal prospective study. Alcohol. Clin. Exp. Res. 1994;18:202–218. doi: 10.1111/j.1530-0277.1994.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Tio DL, Chiappelli F. Thymocyte development in male fetal alcohol-exposed rats. Alcohol. Clin. Exp. Res. 1999;23:465–470. [PubMed] [Google Scholar]
- Waddell BJ, Benediktsson R, Brown RW, Seckl JR. Tissue-specific messenger ribonucleic acid expression of 11beta-hydroxysteroid dehydrogenase types 1 and 2 and the glucocorticoid receptor within rat placenta suggests exquisite local control of glucocorticoid action. Endocrinology. 1998;139:1517–1523. doi: 10.1210/endo.139.4.5900. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Recent studies on the effects of fetal alcohol exposure on the endocrine and immune systems. Alcohol Alcohol. Suppl. 1994;2:401–409. [PubMed] [Google Scholar]
- Weinstock M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem. Res. 2007;32:1730–1740. doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11beta-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur. J. Neurosci. 2000;12:1047–1054. doi: 10.1046/j.1460-9568.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Schwartz S, Wagner L, Miller W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000;7:203–214. doi: 10.1089/10665270050081478. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Powrozek TA. Fetal alcohol exposure reduces serotonin innervation and compromises development of the forebrain along the serotonergic pathway. Alcohol. Clin. Exp. Res. 2005;29:141–149. doi: 10.1097/01.alc.0000150636.19677.6f. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Zhang JK, Goodlett CR, Li T. Prenatal alcohol exposure retards the migration and development of serotonin neurons in fetal C57BL mice. Brain Res. Dev. Brain Res. 2001;126:147–155. doi: 10.1016/s0165-3806(00)00144-9. [DOI] [PubMed] [Google Scholar]