Abstract
The PI-3 kinase (PI3K) pathway is critical for T-cell development and activation. Several negative regulators of this pathway have already been described and characterized: the lipid phosphatases SHIP, PHLPP and PTEN, the latter of which are tumor suppressors. PIK3IP1 is a recently described transmembrane protein that has the ability to bind the catalytic protein p110 and prevent its activation by the p85 family adaptor proteins. Thus far, nothing is known about the possible role of PIK3IP1 in the regulation of lymphocyte development or activation. Here, we show for the first time that PIK3IP1 is expressed in T cells. Ectopic expression of PIK3IP1 in Jurkat or D10 T cell lines inhibited activation of an NFAT/AP-1 transcriptional reporter. Conversely, siRNA-mediated silencing of PIK3IP1 in the same cell lines modestly augmented Akt phosphorylation, T-cell activation and production of IL-2. These results suggest that the novel PI3K regulator PIK3IP1 plays an inhibitory role in T-cell activation.
Keywords: T cells, activation, signal transduction
Introduction
The phosphatidylinositol 3-kinases (PI3Ks) are activated by numerous types of cell surface receptors, including the antigen receptors expressed on T and B cells [1]. These kinases mediate the phosphorylation of phosphatidylinositol precursors on the 3’ position of the inositol ring [2]. The resulting products act as second messengers that mediate the recruitment and activation of downstream kinases and other effectors, usually through binding to pleckstrin homology (PH) domains [2]. Class I PI3Ks consist of a catalytic subunit (p110α, β, δ), which is recruited to active signaling complexes by an adaptor subunit of 85 kD (p85α,β). The best characterized downstream effectors for PI3K are the Akt proteins [3], PH domain-containing serine/threonine kinases that regulate cellular survival, metabolism and activation [3].
The proper regulation of PI3K and its products is critical to normal cellular homeostasis. Activating mutations and amplification of p85, p110 and Akt have been implicated in various cancers [4, 5]. Conversely, at least two negative regulator of signaling downstream of PI3K are known to be tumor suppressors, i.e. the lipid phosphatases PTEN, which removes the phosphate from the 3’ position of the inositol ring of PIP3 [4], and INPP4B, which acts on the 4’ phosphate of PI(3,4)P2 [6]. Recently another type of negative regulator of PI3K has been described that acts more proximally to inhibit PI3K activity. PI3K interacting protein 1 (PIK3IP1) is a transmembrane protein, and contains an extracellular kringle domain. Its cytoplasmic domain contains a motif that is homologous to the inter-SH2, p110-binding, domain of p85 [7]. Interference with p110 activation, possibly through an allosteric mechanisms, is the proposed mechanism by which PIK3IP1 inhibits the PI3K pathway [7]. Recent data also suggest that PIK3IP1 can function as a tumor suppressor [8, 9]. Here we demonstrate that PIK3IP1 is expressed in T cells. Furthermore, while ectopic expression of PIK3IP1 inhibits signaling pathways associated with T-cell activation, decreasing the expression of this protein augments the same pathways. Thus, our data indicate that PIK3IP1 is a novel regulator of T-cell activation.
Results and Discussion
Expression of PIK3IP1 in T cells
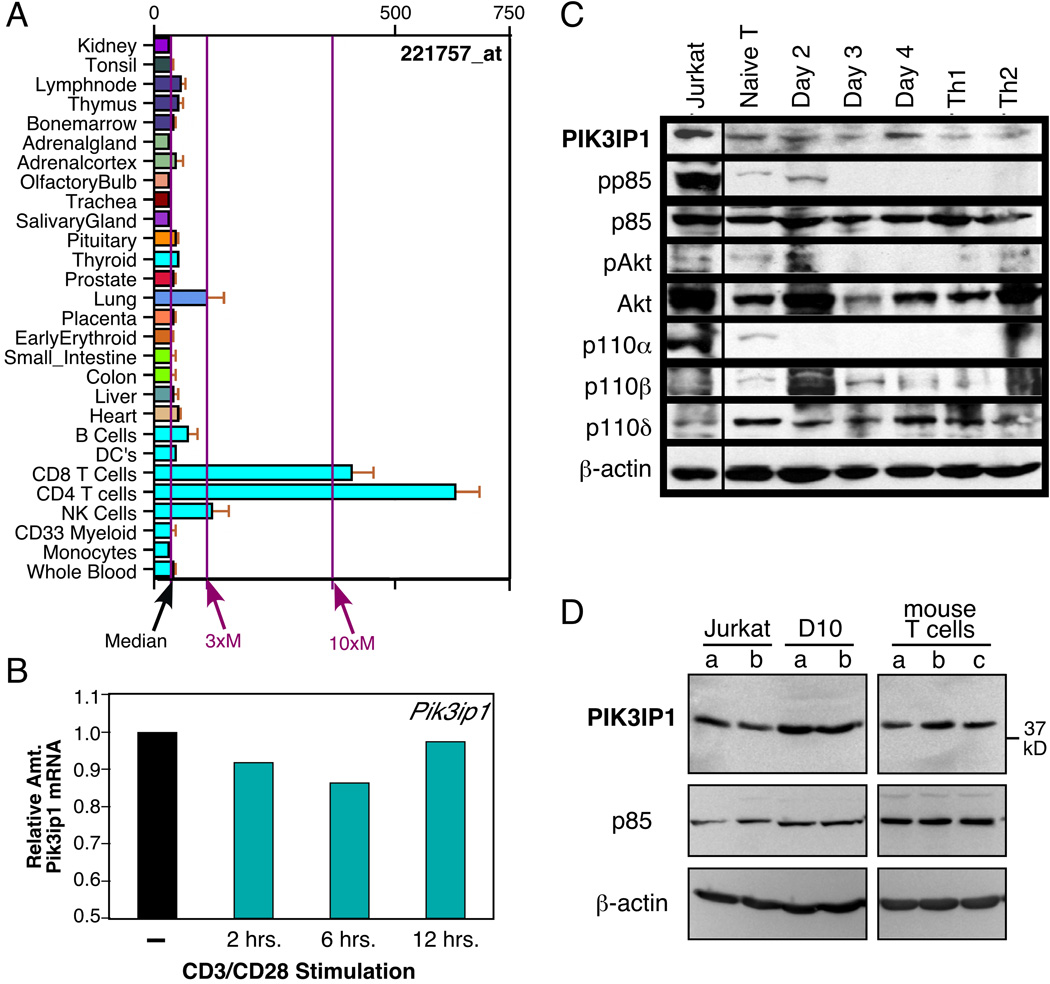
Recent studies indicated that PIK3IP1 is a negative regulator of PI3K, at least in certain cell types [7, 8]. Before exploring a possible function for PIK3IP1 in T cells, we determined whether it is expressed in T cells, both at the message and protein levels. To assess the former, we performed searches using two on-line gene expression databases. As shown in Figure 1A, analysis of gene expression in mouse tissues via the BioGPS portal (http://biogps.gnf.org) revealed relatively robust expression of Pik3ip1 in a number of hematopoietic lineages, including T cells. Analysis of expression data from the Immunological Genome Project (www.immgen.org) also revealed the presence of Pik3ip1 message in T cells and other immune cells (data not shown). We confirmed the expression of Pik3ip1 in T cells at the message level with the murine Th2 T cell clone D10.G41, using quantitative real-time RT-PCR. Thus, as shown in Figure 1B, Pik3ip1 message was detected in these cells, and stimulation with anti-CD3/CD28 antibodies led to a transient decrease in this mRNA, relative to the control (18S rRNA).
Figure 1.
Expression pattern of PIK3IP1. (A) Analysis of PIK3IP1 message expression in human tissues. Shown are partial results of a BioGPS search for human PIK3IP1. Some tissues have been omitted for clarity, all of which displayed levels of expression at or near the median. (B) Real-time PCR analysis of murine Pik3ip1 message in resting vs. stimulated D10 T cells, a murine Th2 T-cell clone, relative to the amount in resting cells. D10 T cells were stimulated for the indicated times with biotinylated anti-CD3 and anti-CD28 (1 µg/ml each), along with streptavidin (5 µg/ml). Results are representative of three independent experiments. (C) Western blot analysis of expression of PIK3IP1 and other members of the PI3K/Akt pathway in the Jurkat human T-cell line and primary murine T cells, either naïve or activated for the indicated number of days with anti-CD3/CD28 antibodies (1 and 5 µg/ml respectively), under neutral conditions. In addition, bulk cultures of cells stimulated under Th1 or Th2 conditions were also analyzed. PIK3IP1 expression was assessed with a previously described antibody [7]. Blots were also probed with an antibody to beta-actin as a loading control (bottom). Data shown are representative of two independent experiments. (D) Western blot analysis of PIK3IP1 protein in duplicate samples of Jurkat or D10 cells or triplicate samples of primary murine CD3+ T cells, with a commercial antibody (H-180, Santa Cruz Biotech.). p85 and beta-actin were also probed for as controls. Data shown are representative of three independent experiments.
We next sought to confirm that PIK3IP1 is also present at the protein level in T cells. Lysates from the Jurkat human T cell line, as well as primary murine T cells, both naïve and activated, were analyzed by western blotting for expression of PIK3IP1 and other members of the PI3K pathway, using a previously described antibody [7]. As shown in Figure 1C, PIK3IP1 protein was detected in all T cells, with particularly high levels in the human leukemic T cell line Jurkat. The latter is intriguing, since Jurkat cells were previously described as lacking expression two other regulators of the PI3K pathway, the lipid phosphatases PTEN and SHIP [10, 11]. We confirmed the expression of PIK3IP1 at the protein by western blotting with a different antibody (H-180, from Santa Cruz Biotech). Thus, as shown in Figure 1D, this antibody also detected PIK3IP1 in lysates of Jurkat T cells, as well as the mouse T cell clone D10 and naïve CD3+ T cells freshly isolated from mouse spleen and lymph node.
Inhibition of T-cell activation by ectopic PIK3IP1 expression
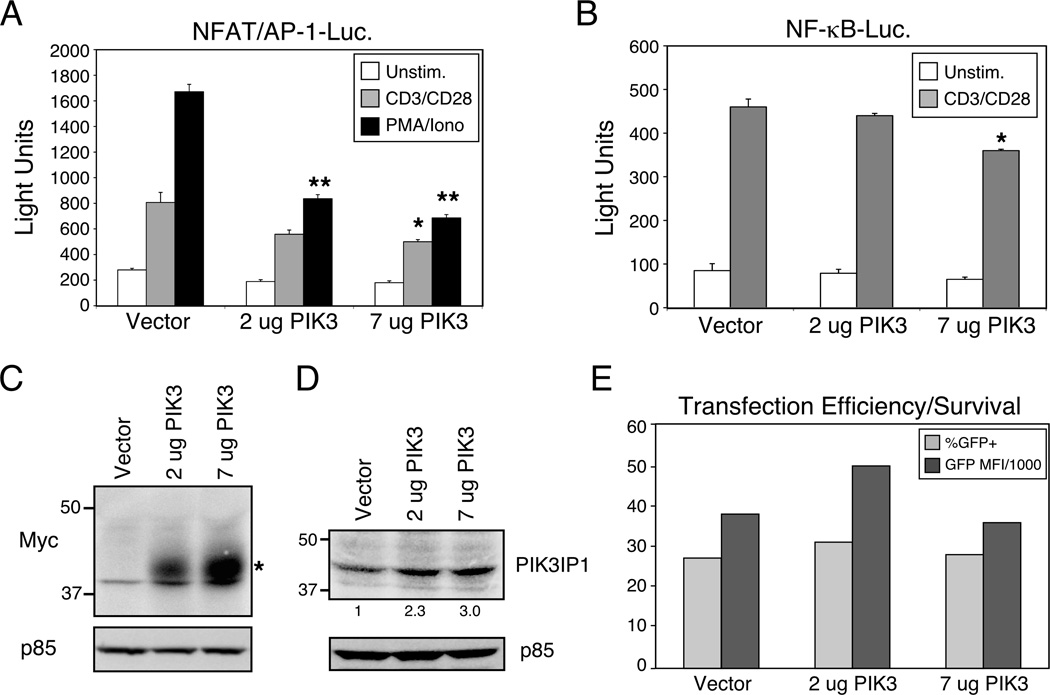
Since PIK3IP1 has been characterized as a negative regulator of the PI3K pathway in other cell types [7], we hypothesized that altered levels of PIK3IP1 expression might modulate signaling pathways that regulate T cell activation. We first investigated the effects of ectopic PIK3IP1 expression. T cell activation and effector function are critically regulated by the transcription factors NF-κB, NFAT and AP-1, the latter two of which often bind in tandem to composite elements in genes like that encoding IL-2. Thus, transfection of a myc-tagged PIK3IP1 construct into D10 T cells, a murine Th2 T cell line that expresses normal levels of both PTEN and SHIP [12], led to a dose-dependent decrease in the activation of an NFAT/AP-1 transcriptional reporter (Fig. 2A). This inhibition was evident in response to stimulation with anti-TCR/CD28 antibodies or the pharmacological agents PMA and ionomycin. We also examined the effects of ectopic PIK3IP1 expression on the NF-κB pathway, and although statistically significant inhibition was observed at the highest concentration of PIK3IP1 transfection, less dramatic results were observed with an NF-κB reporter (Fig. 2B). Transfected PIK3IP1 was detected with an antibody to the Myc epitope tag (Fig. 2C) or with an antibody to total PIK3IP1 (Fig. 2D). The latter revealed overexpression in the range of 2–3 fold over endogenous protein. Ectopic expression of PIK3IP1 had no apparent broad effects on transfection efficiency or viability, as determined by the expression of a constitutively expressed GFP reporter (Fig. 2E), which was co-transfected with the NFAT/AP-1 or NF-κB transcriptional reporters. These results are consistent with the possibility that PIK3IP1 can negatively regulate signaling from TCR/CD3 and CD28 to downstream transcription factors that are known to regulate T cell function.
Figure 2.
Suppression of CD3/CD28 signaling by ectopic expression of PIK3IP1. D10 T cells were transfected with either (A) a NFAT/AP-1 or (B) a NF-κB luciferase reporter, a constitutively expressed eGFP construct (as a transfection control) and the indicated plasmids. The next day, cells were stimulated with anti-CD3 and CD28 antibodies (1µg/ml each, plus streptavidin crosslinking (5 µg/ml) or with PMA (50ng/ml) plus ionomycin (1 μM) for six hours, followed by determination of luciferase activity. Data are shown as mean + SD of three replicates from a single experiment, representative of five (NFAT/AP-1) or three (NF-kB) experiments. Bars marked with * or ** indicate a significantly lower response than control vector (p<0.05 or p<0.01 respectively), as determined with the Student’s t test. (C) Expression of myc-tagged PIK3IP1 was confirmed by western blotting with an anti-myc antibody. (D) The degree of PIK3IP1 overexpression was estimated by blotting with a specific polyclonal antiserum that recognizes both endogenous and transfected PIK3IP1. Values under the PIK3IP1 blot indicate relative amounts of protein, compared with vector-transfected cells. (E) Transfection efficiency and survival of the transfected cells were confirmed by flow cytometric analysis of eGFP expression in the same cells. Results are presented as both the percent positive cells (over un-transfected control) and the percent positive x the mean fluorescence intensity (MFI) of the GFP-expressing cells, divided by 1000.
Enhancement of T cell activation by silencing of PIK3IP1
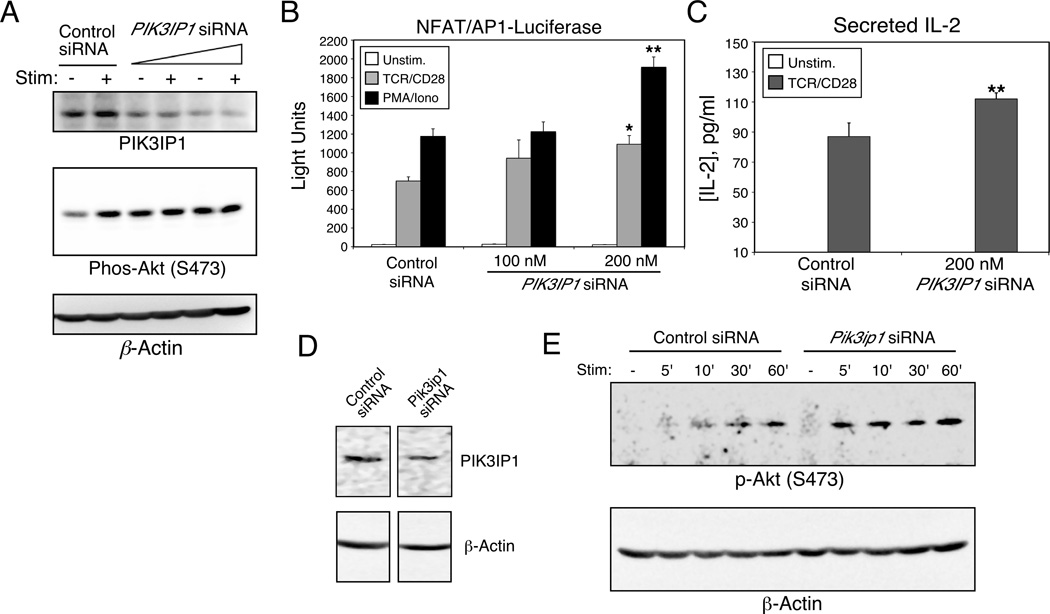
Because ectopic expression of signaling intermediates can sometimes result in misleading effects on downstream signaling pathways, we next performed siRNA-mediated knock-down of PIK3IP1. We first chose Jurkat T cells for these experiments since they express high levels of PIK3IP1 (Fig. 1B). Furthermore, we were intrigued by the fact that, although these cells lack expression of PTEN and SHIP, TCR and CD28 crosslinking can still lead to increased Akt activation [13, 14]. This suggests that while there is certainly some basal activity of this pathway in Jurkat T cells, it is not maximal, raising the possibility that one or more additional negative regulators of the PI3K pathway might be operational in these cells. Thus, Jurkat T cells were transfected with SmartPool siRNA oligos specific for human PIK3IP1. As shown in Figure 3A (upper panel), expression of PIK3IP1 protein was significantly reduced by 48 hours after transfection. We next examined the activation status of Akt in cells in which PIK3IP1 was knocked down. As shown in Figure 3A (lower panel), while anti-TCR/CD28 stimulation of Jurkat T cells before PIK3IP1 knock-down resulted in increased phosphorylation of Akt serine 473, after knock-down of PIK3IP1, basal phosphorylation of Akt was often increased, precluding further stimulation by TCR/CD28 antibodies. Consistent with these findings, when an NFAT/AP-1 transcriptional reporter was co-transfected with PIK3IP1-specific siRNA, a dose-dependent enhancement of reporter activity was observed (Fig. 3B). To determine whether these effects could also be seen at the level of an endogenous readout of T cell activation, we examined the effects of PIK3IP1 knock-down on IL-2 secretion. Thus, as shown in Figure 3C, transfection of PIK3IP1 siRNA also led to a modest increase in the secretion of endogenous IL-2 (by about 30%) by Jurkat cells, compared with cells transfected with a control siRNA. Consistent with this modest effect, we were unable to detect any differences in IL-2 mRNA (data not shown). We also knocked down PIK3IP1 expression in the murine D10 T cell line referred to above (Fig. 3D). Similar to the results obtained in Jurkat T cells, decreased PIK3IP1 expression in D10 T cells also led to heightened sensitivity of these cells to CD3/CD28-induced Akt phosphorylation (Fig. 3E and Supporting Information Figure 1). As in the Jurkat experiments, we sometimes observed increased basal phosphorylation of Akt (Supporting Information Figure 1). Importantly, in the D10 T cells, which appear to have otherwise normal PI3K signaling [12], we could detect an increase in endogenous cytokine message and protein after PIK3IP1 knockdown (Supporting Information Figure 2). These results are all consistent with a role for PIK3IP1 in negative regulation of the PI3K pathway and downstream signaling to cytokine production.
Figure 3.
Enhanced CD3/CD28 signaling by knock-down of PIK3IP1. Jurkat T cells were transfected with an NFAT/AP-1 luciferase reporter and the indicated amounts of PIK3IP1-specific or control siRNA. (A) At 42 hours after transfection, cells were split and either left untreated or stimulated for fifteen minutes with anti-TCR/CD28 antibodies (1 µg/ml and 2 µg/ml respectively). Cells were lysed and the phosphorylation of Akt at serine 473 was then determined by western blotting (middle panel). Cell lysates were also analyzed by western blotting for the expression of PIK3IP1 (upper panel). (B) At 42 hours after transfection, cells were stimulated as indicated for six hours, followed by measurement of luciferase activity. Data are shown as mean + SD of three replicates from a single experiment. Results in panels A and B are representative of three independent experiments. (C) Jurkat T cells were stimulated for 24 hours with anti-TCR/CD28 antibodies, 42 hours after transfection with PIK3IP1 siRNA. Cell-free supernatants were harvested and analyzed by ELISA for production of IL-2. Results are shown as the mean + SD of five experiments. (D–E) D10 T cells were transfected with 200 nM Pik3ip1-specific siRNA, and (D) analyzed 42 hours later by western blotting for PIK3IP1, or (E) stimulated for the indicated times with anti-CD3/CD28 antibodies (1 µg/ml each, plus streptavidin - 5 µg/ml) and analyzed for Akt phosphorylation. Results are representative of two separate experiments. Bars marked with * or ** indicate a significantly higher response than control siRNA (p<0.05 or p<0.01 respectively), as determined with the Student’s t test.
Here we have shown for the first time that the protein PIK3IP1 is expressed in T cells and acts as a negative regulator of TCR/CD28 signaling to the downstream transcription factors NFAT/AP-1 and NF-κB. Importantly, we demonstrated this negative regulatory activity not only in the leukemic Jurkat T cell line (which lacks PTEN and SHIP expression), but also in the mouse D10 T cell line, which expresses both PTEN and SHIP, and has apparently normal regulation of the PI3K pathway. At this point, we believe that at least a part of this activity of PIK3IP1 is due to its ability to dampen signaling through the PI3K pathway, since siRNA-mediated knock-down of PIK3IP1 resulted in enhanced phosphorylation of Akt. Also, this is consistent with a previous study that directly demonstrated inhibition of PI3K by PIK3IP1 [7].
Concluding Remarks
Unlike previously described negative regulators of the PI3K pathway, PIK3IP1 appears to function further upstream, at the level of PI3K activation itself. Further study will be necessary to determine more precisely the molecular mechanism behind this inhibition, including which isoforms of p110 are inhibited by PIK3IP1 in T cells. In addition, it will be of interest to understand the function of the PIK3IP1 extracellular kringle domain, which may mediate its association with other cell-surface proteins. Finally, our data indicate that further genetic analysis is warranted to more carefully tease out the role of PIK3IP1 in T-cell development and function in vivo.
Materials and Methods
Antibodies and Reagents
Anti-PIK3IP1 antibody and siRNA specific for human PIK3IP1 were described previously [7]. SmartPool siRNA oligos specific for murine Pik3ip1 were obtained from Dharmacon (Chicago, IL). The additional PIK3IP1 antibody H-180, and antibodies to p110α and p110β and the myc epitope tag were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-p110δ was from Abcam (Cambridge, MA). Anti–p85 (phospho and total) antibodies were obtained from Cell Signaling Technology (Danvers, MA). Polyclonal antibody specific for phospho (S473) Akt was obtained from Biosource/Invitrogen (Carlsbad, CA). Antibody to the Jurkat TCR was purified from the C305.2 hybridoma, which was obtained from ATCC (Manassas, VA). Biotinylated antibodies to human and mouse CD28 (10F3 and 37.51, respectively) and mouse CD3 (2C11), as well as streptavidin were from Invitrogen (Carlsbad, CA). Monoclonal antibody to b-actin was from Sigma (St. Louis, MO).
Real-Time PCR
mRNA from D10 T cells was isolated with the ArrayGrade mRNA purification kit (SA Biosciences, Frederick, MD). Total RNA was reverse transcribed using the RT2 first strand kit (C-03; SA Biosciences). 18s rRNA was chosen as the reference gene for normalization. Real-time PCR was performed with a StepOnePlus system (Applied Biosystems; Foster City, CA) using RT2 SYBR Green/ROX qPCR Master Mixes (SA Biosciences). PCR primers were from SA Biosciences. PCR products were analyzed by melt curve analysis and agarose gel electrophoresis to determine product size and to confirm that no by-products were formed.
PIK3IP1 Expression Vector
An I.M.A.G.E. clone (#4039129; accession BC055920) containing the cDNA encoding murine PIK3IP1 was obtained from Open Biosystems (Huntsville, AL). The coding sequence was amplified by PCR with Pfu proofreading polymerase, using primers containing BamH1 (forward primer: TCGGATTCGCCACCATGCTGTTGGCTTGGGTACAC) or XbaI (reverse primer: ATTCTAGAAGCTCCAGGGGTGCCAGCCTG) restriction sites. The resulting product was digested with BamHI and XbaI and ligated into the mammalian expression vector pEF1MycHisA (Invitrogen), resulting in the addition of C-terminal Myc and 6His tags to the PIK3IP1 sequence. The amplified sequence was verified by automated sequencing.
Database analysis
BioGPS (http://biogps.gnf.org) or the Immunological Genome Project (www.immgen.org) was searched using the keyword “pik3ip1.” Results from the former, shown in Figure 2, represent expression of human PIK3IP1 message across a wide range of tissues and cell types, while data from the latter (not shown) confirmed expression of murine Pik3ip1 in T cells.
Transfections
Jurkat and D10 T cells were transfected by electroporation. Cells in 400 µl total volume were pulsed at 250V (D10) or 260V (Jurkat), 950 µF, with exponential decay. For ectopic expression, cells were transfected with 15 µg luciferase reporter and the indicated concentrations of expression plasmids. Eighteen hours after transfection, cells were either lysed for western blot analysis or stimulated for six hours, followed by determination of luciferase activity. For siRNA knock-down, cells were transfected with 15 µg of luciferase reporter and the indicated amounts of siRNA. Forty-two hours after transfection, cells were stimulated for either fifteen minutes (for phospho-Akt analysis) or for six hours (for luciferase), as indicated. Microplate luciferase assays and western blotting were performed as described previously [15].
IL-2 ELISA
Jurkat T cells were transfected with siRNA specific for PIK3IP1. After 48 hours, cells were stimulated for 24 hours with anti-TCR/CD28 antibodies. Cell-free supernatants were analyzed by ELISA for human IL-2, using OptEIA matched antibodies (BD Bioscience; San Diego, CA).
Supplementary Material
Acknowledgements
We thank S. Gaffen and members of the Kane lab for helpful discussions and for critical reading of the manuscript. This work was supported by NIH grants GM080398 (to L.P.K.) and CA105242 (to M.C.D.).
Abbreviations
- PI3K
PI-3 Kinase
Footnotes
The authors declare no financial or commercial conflict of interest.
References
- 1.Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3:317–330. doi: 10.1038/nri1056. [DOI] [PubMed] [Google Scholar]
- 2.Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta. 2008;1784:159–185. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Fayard E, Xue G, Parcellier A, Bozulic L, Hemmings BA. Protein Kinase B (PKB/Akt), a Key Mediator of the PI3K Signaling Pathway. Curr Top Microbiol Immunol. 2010:31–56. doi: 10.1007/82_2010_58. [DOI] [PubMed] [Google Scholar]
- 4.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 5.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 6.Gewinner C, Wang ZC, Richardson A, Teruya-Feldstein J, Etemadmoghadam D, Bowtell D, Barretina J, et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009;16:115–125. doi: 10.1016/j.ccr.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu Z, He X, Johnson C, Stoops J, Eaker AE, Stoffer DS, Bell A, et al. PI3K is negatively regulated by PIK3IP1, a novel p110 interacting protein. Biochem Biophys Res Commun. 2007;358:66–72. doi: 10.1016/j.bbrc.2007.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He X, Zhu Z, Johnson C, Stoops J, Eaker AE, Bowen W, DeFrances MC. PIK3IP1, a negative regulator of PI3K, suppresses the development of hepatocellular carcinoma. Cancer Res. 2008;68:5591–5598. doi: 10.1158/0008-5472.CAN-08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert DC, McIntyre A, Summersgill B, Missiaglia E, Goddard NC, Chandler I, Huddart RA, Shipley J. Minimum regions of genomic imbalance in stage I testicular embryonal carcinoma and association of 22q loss with relapse. Genes Chromosomes Cancer. 2010;50:186–195. doi: 10.1002/gcc.20843. [DOI] [PubMed] [Google Scholar]
- 10.Freeburn RW, Wright KL, Burgess SJ, Astoul E, Cantrell DA, Ward SG. Evidence that SHIP-1 contributes to phosphatidylinositol 3,4,5-trisphosphate metabolism in T lymphocytes and can regulate novel phosphoinositide 3-kinase effectors. J Immunol. 2002;169:5441–5450. doi: 10.4049/jimmunol.169.10.5441. [DOI] [PubMed] [Google Scholar]
- 11.Shan X, Czar MJ, Bunnell SC, Liu P, Liu Y, Schwartzberg PL, Wange RL. Deficiency of PTEN in jurkat T cells causes constitutive localization of itk to the plasma membrane and hyperresponsiveness to CD3 stimulation. Mol Cell Biol. 2000;20:6945–6957. doi: 10.1128/mcb.20.18.6945-6957.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kane LP, Mollenauer MN, Weiss A. A proline-rich motif in the C terminus of Akt contributes to its localization in the immunological synapse. J Immunol. 2004;172:5441–5449. doi: 10.4049/jimmunol.172.9.5441. [DOI] [PubMed] [Google Scholar]
- 13.Genot E, Reif K, Beach S, Kramer I, Cantrell D. p21Ras initiates Rac-1 but not phosphatidyl inositol 3 kinase/PKB-mediated signaling pathways in T lymphocytes. Oncogene. 1998 doi: 10.1038/sj.onc.1202101. [DOI] [PubMed] [Google Scholar]
- 14.Parry RV, Reif K, Smith G, Sansom DM, Hemmings BA, Ward SG. Ligation of the T cell co-stimulatory receptor CD28 activates the serine-threonine protein kinase protein kinase B. Eur J Immunol. 1997;27:2495–2501. doi: 10.1002/eji.1830271006. [DOI] [PubMed] [Google Scholar]
- 15.Kane LP, Shapiro VSS, Stokoe D, Weiss A. Induction of NF-κB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.