Abstract
Background
BK virus (BKV) infection in kidney transplant recipients is associated with progressive graft dysfunction and graft loss. Cidofovir, an antiviral agent with known nephrotoxicity, has been used in low doses to treat BKV infections. However, the systemic exposure and disposition of the low-dose cidofovir regimen is unknown in kidney transplant recipients.
Methods
We investigated the pharmacokinetics of low-dose cidofovir both without and with oral probenecid in 9 transplant patients with persistent BK viremia without nephropathy in a crossover design.
Results
The mean estimated glomerular filtration rate (eGFR) of the study participants was 46.2 mL/min/1.73 m2 (range: 17 – 75 mL/min/1.73 m2). The contribution of active renal secretion to cidofovir total body clearance was assessed by evaluating the effect of probenecid on cidofovir pharmacokinetics. Maximum cidofovir plasma concentrations, which averaged approximately 1 μg/mL, were significantly below the 36 μg/mL 50% effective concentration in vitro for cidofovir against BKV. The plasma concentration of cidofovir declined with an overall disposition half-life of 5.1 ± 3.5 and 5.3 ± 2.9 h in the absence and in the presence of probenecid, respectively (P> 0.05).
Conclusions
Cidofovir clearance and eGFR were linearly related irrespective of probenecid administration (r2=0.8 without probenecid; r2=0.7 with probenecid). This relationship allows for the prediction of systemic cidofovir exposure in individual patients and may be utilized to evaluate exposure-response relationships in order to optimize the cidofovir dosing regimen for BKV infection.
Keywords: cidofovir, BK virus, pharmacokinetics, kidney transplantation
BK virus (BKV) is a kidney-tropic polyomavirus that is associated with premature graft failure in immunosuppressed renal transplant recipients (1-5). BKV carries a substantial disease burden, with an estimated incidence of viruria, viremia, and nephropathy of 35-40%, 11-13%, and 5-8%, respectively (6, 7). Optimal therapeutic interventions for BKV have yet to be elucidated and a firmly established antiviral therapy is lacking.
Cidofovir (Vistide®, Gilead Sciences, Inc., Foster City, California USA) is a nucleotide analog of deoxycytidine monophosphate with in vitro and in vivo activity against herpesviruses, adenoviruses, poxviruses, and polyomaviruses (8-11). A low-dose cidofovir regimen of 0.25–1.0 mg/kg weekly is used empirically for the management of BKV infections. However, the literature on the efficacy of cidofovir for BKV is conflicting, with some studies reporting apparent stabilization of renal function (12, 13), while others describe no discernible benefit (14, 15). These contradictory data may reflect the absence of a defined exposure-response relationship, which could be utilized to improve the dosing regimen. Our group has previously shown that the 50% effective concentration (EC50) in vitro for cidofovir against BKV is approximately 36 μg/mL (11), although cidofovir exposure following low-dose administration has not been evaluated in kidney transplant recipients.
The disposition of cidofovir has been investigated in human immunodeficiency virus (HIV)-infected patients with normal kidney function and with varying degrees of renal insufficiency (16-19). Tubular secretion plays a role in cidofovir clearance, as renal clearance is nearly 50% higher than baseline creatinine clearance. Further, in vitro studies have demonstrated that secretion of cidofovir involves human organic anion transporter 1 (OAT1)- mediated basolateral uptake into renal proximal tubule cells (20). However, the functional activity of OAT1 is unknown in kidney transplant recipients.
The prototypical OAT1 inhibitor probenecid blocks the tubular uptake of cidofovir and is used clinically to prevent cidofovir-associated nephrotoxicity during treatment of cytomegalovirus (CMV) retinitis (16). However, withholding probenecid when cidofovir is used for BKV may allow for increased cidofovir concentrations to be achieved within the proximal tubules, which is the site of viral replication. In HIV-infected patients, probenecid reduces the renal secretion of cidofovir to a level approaching the glomerular filtration rate (GFR) (19), consistent with near-complete inhibition of OAT1-mediated cidofovir secretion.
The objectives of the current study were to (i) characterize the pharmacokinetics of low- dose cidofovir in kidney transplant recipients with BKV infection in order to allow for future investigations into pharmacokinetic-pharmacodynamic relationships, and (ii) to assess the OAT1-dependent anionic renal secretion capacity in this population by evaluating the effect of probenecid on the systemic and renal clearance of cidofovir.
Materials and methods
PATIENTS
This study was performed in 9 adult renal transplant recipients who were diagnosed with BKV infection and received treatment with low-dose cidofovir after failing to respond to a period of reduced immunosuppression. The protocol was approved by the Institutional Review Board of the University of Pittsburgh (IRB# 08060393), and written informed consent was obtained from all patients before participation in this study. Exclusion criteria included (i) hypersensitivity to cidofovir or other nucleotide analogs; (ii) hypersensitivity to probenecid; (iii) currently receiving another drug known to affect renal anionic drug secretion, such as β-lactam antibiotics, nonsteroidal anti-inflammatory drugs, or diuretics (21); and (iv) pregnant or breastfeeding women.
STUDY DESIGN
Patients were studied on 2 separate occasions in a crossover design. In Part 1, the noncompartmental pharmacokinetic parameters of intravenous (IV) low-dose cidofovir were characterized. In Part 2, following a 1-week washout period, the study procedures were repeated in the same patients with the addition of concomitant oral probenecid. Probenecid (2 g) was given 1 h before cidofovir administration and again at 2 h and 8 h (1 g each) after the completion of the cidofovir infusion. On both occasions, patients received 1 L of 0.9% sodium chloride solution immediately before cidofovir administration. Cidofovir was diluted in 100 mL of 0.9% sodium chloride solution and infused over 1 h.
BLOOD AND URINE SAMPLING
Blood samples (7 mL) were collected in Vacutainers at 0, 0.5, 1, 1.5, 2, 4, 6, 8, and 12 h after starting the cidofovir infusion. Plasma was separated and frozen at – 80°C until analysis. Urine was collected in aliquots from 0-1, 1-2, 2-4, 4-8, and 8-12 h after the start of the cidofovir infusion and stored at – 80°C until analysis.
ANALYTICAL METHODOLOGY
The concentrations of cidofovir in plasma were determined by a previously reported validated liquid chromatographic-mass spectrometric method (22). Briefly, plasma samples were processed by an anion exchange solid phase extraction procedure and chromatography was performed using a Luna C8(2) analytical column, 5 μm, 150×3.0 mm, with isocratic elution. Cidofovir was detected by a triple quadrupole mass spectrometer in positive electron spray ionization mode using multiple reaction monitoring with 13C5-folic acid as the internal standard.
Cidofovir concentrations in urine samples were determined using high-performance liquid chromatography (HPLC) with ultraviolet (UV) detection at 274 nm. Chromatography was performed with a Waters 2695 separations module and Waters 2998 photodiode Array Detector set at 274 nm. The data acquisition was performed with Empower 3 Chromatography Data Software. Urine samples (100 μL) were mixed with 100 μL of mobile phase, consisting of 35% 1.5 mM of tetrabutylammonium dihydrogen phosphate and 3.5 mM of disodium hydrogenphosphate, 12% acetonitrile, and 53% water, and centrifuged at 14,000 rpm for 8 min. Fifty μL of the resulting supernatant was injected onto the HPLC. Chromatographic separation was achieved on a Symmetry 5 μm C18 column (250×4.6mm). The mobile phase was delivered isocratically at 1.2 mL/min and the retention time of cidofovir was 6.5 min.
The concentrations of probenecid in plasma were determined using HPLC with UV detection at 242 nm. Chromatography was performed with a Waters 2695 separations module and Waters 2998 photodiode Array Detector set at 242 nm. The data acquisition was performed with Empower 3 Chromatography Data Software. Plasma samples (200 μL) were mixed with 20 μL of HCl (3N) and vortexed with 300 μL methanol for 3 min. The mixture was centrifuged for 8 min at 14,000 rpm and 100 μL of the resulting supernatant was injected onto the HPLC system, where chromatographic separation was achieved on a Symmetry 5 μm C18 column (250 × 4.6mm). The mobile phase, consisting of 60% of 0.4% ammonium acetate (pH adjusted to 8) (solvent A) and 40% acetonitrile (solvent B), was delivered at a gradient at 1 mL/min. The retention time of probenecid was 5.8 min.
NONCOMPARTMENTAL PHARMACOKINETIC AND STATISTICAL ANALYSIS
Descriptive pharmacokinetic parameters for cidofovir were estimated by noncompartmental analysis (WinNonlin software, version 5; Pharsight Corp, Mountain View, California USA). The maximum concentration in plasma (Cmax) and the time to Cmax (tmax) were estimated by visual inspection of the concentration versus time profiles. The terminal disposition rate constant (λz) was obtained by linear regression of at least 3 data points in the terminal disposition phase, and half-life (t1/2) was calculated by dividing 0.693 by λz. The area under the plasma concentration-time profile (AUC) from the time of dosing until infinity was calculated by the log-linear trapezoidal method with extrapolation beyond the last measured concentration, according to:
Systemic clearance (CL) and the volume of distribution at steady state (Vss) were determined using the following equations:
Renal clearance (CLr) was calculated as Ae(0–12)/AUC0–12, where Ae is the amount of drug recovered in the urine in 12 h, and AUC0–12 is the area under the plasma concentration versus time curve from 0 to 12 h. The fraction eliminated unchanged in the urine (fe) was calculated as the amount of drug recovered in the urine over the entire collection interval divided by the dose.
Results are reported as mean ± standard deviation or standard error of mean. Statistical comparisons between cidofovir pharmacokinetic parameters without and with probenecid were performed by a Student’s paired t-test. The data were analyzed with GraphPad Prism 5.0 (GraphPad Software, San Diego, California, USA). The threshold of statistical significance was set at 5% (α= 0.05).
Results
PATIENT DEMOGRAPHICS
Patient characteristics are summarized in Table 1. On average, the study participants were 57.1 ± 11.7 years of age, weighed 85.6 ± 21.3 kg, and were 13.2 ± 16.7 months post kidney transplantation. Participants had received a median of 3 doses of cidofovir before enrollment in the study. All of the subjects had a reduced estimated GFR (eGFR), though none of the patients were on dialysis at the time of the study. A total of 8 subjects were Caucasian and 1 subject was African-American. All patients had BK viremia and viruria, although none had evidence of tubulointerstitial nephropathy. The median BK viral loads in plasma and urine were 3.5 (range: 2.7 – 6.1) log10 copies/mL and 6.2 (range: 4.7 – 9.3) log10 copies/mL, respectively. The majority of patients (8/9) were on a tacrolimus-based immunosuppressive regimen, and 1 patient was on a cyclosporine-based regimen. Antiviral and bacterial prophylactic regimens included valganciclovir and sulfamethoxazole-trimethoprim, respectively. No patients were taking any drugs known to inhibit OAT1.
Table 1.
Patient characteristics of kidney transplant patients with BK virus receiving cidofovir
| Pt. No. |
Gender | Age (years) |
Graft age (months) |
Body weight (kg) |
Serum creatinine (mg/dL) |
eGFRa (mL/min/1.73m 2) |
Cidofovir dose (mg) |
Cidofovir dose (mg/kg) |
No. of doses before enrollment |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 29 | 5.4 | 111.3 | 1.8 | 45 | 27 | 0.24 | 3 |
| 2 | M | 59 | 10.0 | 85.6 | 1.8 | 39 | 22 | 0.26 | 3 |
| 3 | M | 53 | 56.2 | 64.7 | 3.7 | 17 | 40 | 0.62 | 15 |
| 4 | F | 59 | 2.9 | 59.7 | 1.1 | 51 | 20 | 0.34 | 7 |
| 5 | M | 63 | 13.6 | 75.3 | 1.0 | 75 | 22 | 0.29 | 3 |
| 6 | M | 67 | 2.8 | 120.0 | 1.5 | 45 | 35 | 0.29 | 2 |
| 7 | M | 69 | 4.4 | 91.1 | 1.3 | 55 | 35 | 0.38 | 4 |
| 8 | F | 58 | 9.7 | 97 | 1.5 | 43 | 23 | 0.24 | 2 |
| 9 | F | 57 | 13.4 | 65.7 | 1.2 | 46 | 20 | 0.30 | 3 |
| Mean | 57.1 | 13.2 | 85.6 | 1.65 | 46.2 | 27.1 | 0.33 | 4.6 | |
| (SD) | (11.7) | (16.7) | (21.3) | (0.8) | (15.2) | (7.6) | (0.12) | (4.2) |
Estimated glomerular filtration rate (eGFR), calculated by the 4-variable Modification of Diet in Renal Disease (MDRD) Study equation.
M, male; F, female; SD, standard deviation.
NON-COMPARTMENTAL PHARMACOKINETICS OF CIDOFOVIR
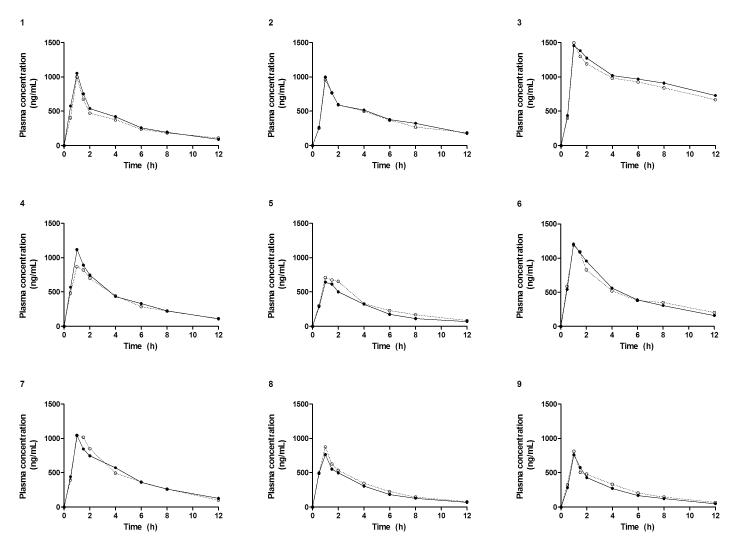
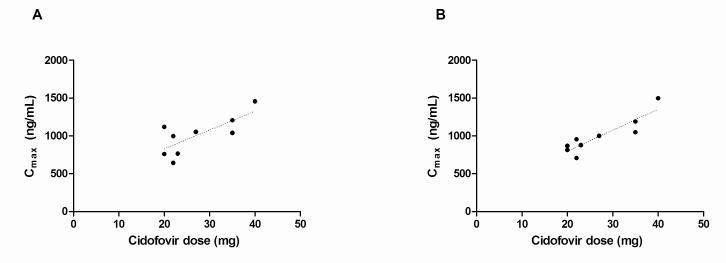
Linear plots of cidofovir plasma concentration versus time profiles without and with concomitant oral probenecid in each patient are displayed in Figure 1. The concentration-time curves were nearly superimposable, suggesting probenecid-insensitive cidofovir elimination. A positive linear relationship was observed between cidofovir dose and the Cmax both without probenecid (r2=0.6) and with probenecid (r2=0.8) (Fig. 2). The fraction of the cidofovir dose eliminated unchanged in the urine (fe) from 0 to 12 h was not significantly different without (0.64 ± 0.17) and with (0.60 ± 0.18) probenecid administration.
Fig. 1.
Cidofovir plasma concentration versus time profiles following intravenous administration with a 1 h infusion without (•) and with (○) concomitant oral probenecid in nine individual renal transplant recipients.
Fig. 2.
Relationship between cidofovir dose and the maximum plasma concentration without (A) and with (B) concomitant probenecid.
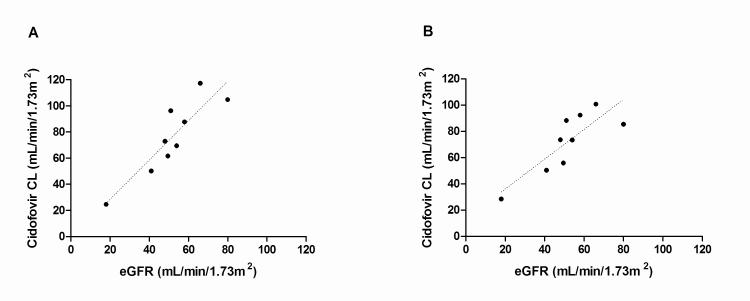
Cidofovir clearance and eGFR were linearly related irrespective of probenecid administration (r2=0.8 without probenecid; r2=0.7 with probenecid). Linear regression of systemic cidofovir clearance versus eGFR is presented in Figure 3.
Fig. 3.
Linear regression of systemic cidofovir clearance and estimated glomerular filtration (eGFR) rate without (A) and with (B) concomitant probenecid.
A summary of the model-independent pharmacokinetic parameters for IV low-dose cidofovir is presented in Table 2. Overall, pharmacokinetic parameters obtained from plasma concentration data (Cmax, tmax, λz, AUC0∞, Vss, CL) and urine concentration data (ClR, fe) were not significantly different when probenecid was simultaneously administered (p > 0.05).
Table 2.
Non-compartmental pharmacokinetic parameters of low-dose cidofovir in kidney transplant recipients without and with concomitant oral probenecid (n=9)
| Cidofovir | Cidofovir + probenecid | P-value * | |
|---|---|---|---|
| Plasma | |||
| Cmax (ng/mL/mg) | 37.9 ± 8.4 | 37.4 ± 4.7 | 0.73 |
| Tmax (hr) | 1.0 ± 0 | 1.0 ± 0 | - |
| AUC (ng*hr/mL/mg) | 241.2 ± 164.6 | 239.1 ± 132.5 | 0.82 |
| λz (1/hr) | 0.16 ± 0.05 | 0.15 ± 0.05 | 0.32 |
| t ½ β (hr) | 5.1 ± 3.5 | 5.3 ± 2.9 | 0.53 |
| Vss (L) | 29. 6 ± 4.5 | 30.6 ± 5.2 | 0.46 |
| Vss/BW (L/kg) | 0.36 ± 0.08 | 0.37 ± 0.06 | 0.68 |
| CL (mL/min) | 86.0 ± 30.7 | 81.5 ± 25.6 | 0.21 |
| CL/BW (mL/min/kg) | 1.03 ± 0.42 | 0.97 ± 0.33 | 0.65 |
| Urine | |||
| CLR (mL/min) | 44.2 ± 12.7 | 41.4 ± 13.1 | 0.68 |
| CLR/BW (mL/min/kg) | 0.52 ± 0.13 | 0.64 ± 0.18 | 0.58 |
| fe | 0.64 ± 0.17 | 0.60 ± 0.18 | 0.63 |
Calculated from 2-tailed Student’s paired t-test.
Cmax, maximum concentration in plasma
Tmax
time to Cmax
AUC, area under the curve
λz, terminal disposition rate constant
t 1/2 , half-life
Vss, volume of distribution at steady state
BW, body weight; CL, systemic clearance
CLR, renal clearance
fe, fraction eliminated unchanged.
Considerable systemic probenecid exposure was observed in all subjects following oral dosing. The average AUC0-14 for probenecid was 1109 ± 171.3 μg*hr/mL. One subject was incorrectly administered 4 gm of probenecid at time 0 rather than the correctly spaced regimen. Blood and urine sampling were not performed during the probenecid elimination phase and thus a full pharmacokinetic profile is not reported.
SAFETY AND TOLERABILITY
Low-dose cidofovir and probenecid were well tolerated. No acute changes were observed in biochemical indices of kidney or liver function after administration of cidofovir and probenecid. One patient experienced transient nausea and vomiting, which was successfully treated with IV antiemetics. This incident was attributed to incorrect probenecid administration by the nursing staff.
Discussion
The current study describes the pharmacokinetics of low-dose cidofovir in kidney transplant recipients with BK viremia, and assesses the active secretion capacity of this patient population by evaluating the impact of concomitant probenecid administration on cidofovir clearance. Cidofovir is a suitable probe drug to assess anionic secretion because it is not significantly metabolized (18, 23), exhibits negligible binding to plasma proteins (< 0.5%) (19), is transported by renal OAT1 (20), and is cleared via renal filtration and secretion with no evidence of reabsorption (18). The results demonstrate that renal filtration is the primary clearance mechanism, and that active secretion does not appreciably contribute to the elimination of low-dose cidofovir in kidney transplant patients with BKV infection.
Low doses of cidofovir are used in kidney transplant patients to avoid nephrotoxicity. In the present study, cidofovir was dosed at 4.8%–12.4% of the 5 mg/kg dose used for the treatment of CMV retinitis in individuals with HIV (19). Cmax, which averaged approximately 1 μg/mL, was significantly below the 36 μg/mL EC50 in vitro for cidofovir against BKV (11). Nonetheless, in rabbits, cidofovir achieves concentrations 10-fold higher in the kidney than in plasma (24). The high affinity of cidofovir for the kidney may partially explain the virologic response observed in some patients despite low systemic exposure.
Probenecid was dosed according to the U.S. Food and Drug Administration-approved use of cidofovir for CMV retinitis in individuals with HIV (25). Probenecid plasma concentrations were determined to ensure sufficient absorption from the gastrointestinal tract to a degree that would be expected to inhibit renal secretion. Significant systemic exposure of probenecid was noted, indicating that the drug was adequately absorbed. Further, previous studies have described a competitive inhibition constant of probenecid of 15.86 μg/mL for the inhibition of tubular secretion of diprophylline (26). In this study, average probenecid plasma concentrations were 4-fold higher than this value within 2 h of oral dosing.
A potential explanation for the lack of cidofovir secretion may be related to the expression and activity of anionic renal drug transporters in this population. It has been established that inflammatory cytokines can downregulate drug metabolizing enzymes and transporters (27). Transplant patients are susceptible to a high degree of inflammation (28), and BKV infection in the kidney is likely to further increase the concentrations of inflammatory mediators, including circulating cytokines (1). In addition, uremic toxins may play a role. The protein expression of renal OAT1 is significantly reduced in rats with chronic renal failure as compared to control rats, and this effect is reproduced in vitro when human kidney-2 cells are incubated with uremic serum (29). The majority of the patients in the current study had chronic kidney disease stage 3, and the concentrations of uremic toxins, including indoxyl sulfate, may be increased in this group (30). This raises the possibility that renal OAT1 expression is reduced in kidney transplant patients with BKV infection, potentially as a result of inflammation and/or uremia, leading to reduced basolateral uptake of organic anion substrates and, ultimately, decreased renal secretion.
The current study has a few limitations. First, the majority of the study participants were Caucasian, and genotyping of OAT1 was not performed. This raises the possibility that the results may not be dependably extrapolated to other ethnic groups. However, only 6 nonsynonymous OAT1 variants have been identified and none are associated with loss of function. Further, these variants were identified in < 1% of a large population with diverse ethnicities (31). Thus, polymorphisms in OAT1 do not appear to contribute to inter-individual variation in drug disposition, and the ethnic homogeneity of the study population and the lack of OAT1 genotyping do not represent major pitfalls. Another potential limitation is the relatively small sample size (n=9), though variability in the pharmacokinetic parameters could be accounted for by clinical estimates of renal filtration. Lastly, intracellular concentrations of cidofovir in the kidney were not measured because of logistical considerations, although this data would allow for an assessment of drug exposure at the site of action.
A linear relationship was observed between cidofovir clearance and eGFR both in the absence and presence of probenecid. This relationship allows for the prediction of systemic cidofovir exposure in individual patients and can be utilized to evaluate the pharmacokinetic-pharmacodynamic relationship between cidofovir exposure and BK virologic response. This association can potentially be used to improve the dosage regimen of cidofovir for kidney transplant patients with BKV. Considering that the Cmax of cidofovir in this study averaged only 3% of the half maximal effective concentration previously observed in vitro, the currently used low-dose cidofovir regimen is unlikely to result in sustained inhibition of BKV replication.
In the present study, the probenecid-insensitive elimination of cidofovir potentially suggests impaired renal secretion of organic anions via this mechanism. This finding may have implications for drug therapy in renal transplant patients with BKV. Several commonly used medications are substrates for OAT1, including angiotensin-converting enzyme inhibitors (captopril, quinapril), angiotensin II receptor blockers (olmesartan), diuretics (bumetadine, furosemide), antibiotics (ceftibuten, ceftizoxime, cephaloride, tetracycline), antivirals (adefovir, ganciclovir, acyclovir), antineoplasics (methotrexate), histamine receptor 2 blockers (cimetidine, ranitidine), and non-steroidal anti-inflammatory agents (ibuprofen, indomethacin) (32). Therefore, transplant recipients with BK viremia receiving these drugs may be subject to increased exposure and potential toxicities. It is unknown if the impaired secretion observed in this study is unique to renal transplant recipients with BK viremia, or if the finding applies to kidney transplant patients in general.
Acknowledgements
Thanks: The authors thank Stephenie Dermont, Laurie Hope, Leslie Mitrik, Sheila Fedorek, and the nurses and staff of the Clinical and Translational Research Center at the University of Pittsburgh Medical Center.
Support: This work was supported in part by the NIH grant R01-AI063360-05 and NIH/NCRR/CTSA Grant UL1 RR024153.
Footnotes
Disclosure: The authors of this manuscript have no conflicts of interest to disclose.
Author contributions: J.D.M.: Concept/design, data analysis/interpretation, drafting article, critical revision of article, approval of article, statistics, and data collection. Y.Z.: Data analysis/interpretation, critical revision of article, data collection. R.S.: Concept/design, data analysis/interpretation, critical revision of article. K.S.S. Concept/design, data collection. G.Y.: Statistics, data analysis/interpretation. P.S.R.: Concept/design, critical revision of article. R.V.: Concept/design, data analysis/interpretation, critical revision of article, approval of article, statistics.
References
- 1.Nickeleit V, Hirsch HH, Zeiler M, et al. BK-virus nephropathy in renal transplantstubular necrosis, MHC-class II expression and rejection in a puzzling game. Nephrol Dial Transplant. 2000;15(3):324–332. doi: 10.1093/ndt/15.3.324. [DOI] [PubMed] [Google Scholar]
- 2.Purighalla R, Shapiro R, McCauley J, Randhawa P. BK virus infection in a kidney allograft diagnosed by needle biopsy. Am J Kidney Dis. 1995;26(4):671–673. doi: 10.1016/0272-6386(95)90608-8. [DOI] [PubMed] [Google Scholar]
- 3.Ramos E, Drachenberg CB, Papadimitriou JC, et al. Clinical course of polyoma virus nephropathy in 67 renal transplant patients. J Am Soc Nephrol. 2002;13(8):2145–2151. doi: 10.1097/01.asn.0000023435.07320.81. [DOI] [PubMed] [Google Scholar]
- 4.Randhawa PS, Finkelstein S, Scantlebury V, et al. Human polyoma virus-associated interstitial nephritis in the allograft kidney. Transplantation. 1999;67(1):103–109. doi: 10.1097/00007890-199901150-00018. [DOI] [PubMed] [Google Scholar]
- 5.Randhawa P, Brennan DC. BK virus infection in transplant recipients: an overview and update. Am J Transplant. 2006;6(9):2000–2005. doi: 10.1111/j.1600-6143.2006.01403.x. [DOI] [PubMed] [Google Scholar]
- 6.Brennan DC, Agha I, Bohl DL, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant. 2005;5(3):582–594. doi: 10.1111/j.1600-6143.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347(7):488–496. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 8.Bronson JJ, Ferrara LM, Hitchcock MJ, et al. (S)-1-(3-hydroxy-2-(phosphonylmethoxy) propyl)cytosine (HPMPC): a potent antiherpesvirus agent. Adv Exp Med Biol. 1990;278:277–283. doi: 10.1007/978-1-4684-5853-4_28. [DOI] [PubMed] [Google Scholar]
- 9.de Oliveira CB, Stevenson D, LaBree L, McDonnell PJ. Trousdale MD Evaluation of Cidofovir (HPMPC, GS-504) against adenovirus type 5 infection in vitro and in a New Zealand rabbit ocular model. Antiviral Res. 1996;31(3):165–172. doi: 10.1016/0166-3542(95)00962-0. [DOI] [PubMed] [Google Scholar]
- 10.Bernhoff E, Gutteberg TJ, Sandvik K, Hirsch HH, Rinaldo CH. Cidofovir inhibits polyomavirus BK replication in human renal tubular cells downstream of viral early gene expression. Am J Transplant. 2008;8(7):1413–1422. doi: 10.1111/j.1600-6143.2008.02269.x. [DOI] [PubMed] [Google Scholar]
- 11.Farasati NA, Shapiro R, Vats A, Randhawa P. Effect of leflunomide and cidofovir on replication of BK virus in an in vitro culture system. Transplantation. 2005;79(1):116–118. doi: 10.1097/01.tp.0000149338.97084.5f. [DOI] [PubMed] [Google Scholar]
- 12.Araya CE, Lew JF, Fennell RS, Neiberger RE, Dharnidharka VR. Intermediate dose cidofovir does not cause additive nephrotoxicity in BK virus allograft nephropathy. Pediatr Transplant. 2008;12(7):790–795. doi: 10.1111/j.1399-3046.2008.00937.x. [DOI] [PubMed] [Google Scholar]
- 13.Vats A, Shapiro R, Singh Randhawa P, et al. Quantitative viral load monitoring and cidofovir therapy for the management of BK virus-associated nephropathy in children and adults. Transplantation. 2003;75(1):105–112. doi: 10.1097/00007890-200301150-00020. [DOI] [PubMed] [Google Scholar]
- 14.Wadei HM, Rule AD, Lewin M, et al. Kidney transplant function and histological clearance of virus following diagnosis of polyomavirus-associated nephropathy (PVAN) Am J Transplant. 2006;6(5 Pt 1):1025–1032. doi: 10.1111/j.1600-6143.2006.01296.x. [DOI] [PubMed] [Google Scholar]
- 15.Wu SW, Chang HR, Lian JD. The effect of low-dose cidofovir on the long-term outcome of polyomavirus-associated nephropathy in renal transplant recipients. Nephrol Dial Transplant. 2009;24(3):1034–1038. doi: 10.1093/ndt/gfn675. [DOI] [PubMed] [Google Scholar]
- 16.Cundy KC. Clinical pharmacokinetics of the antiviral nucleotide analogues cidofovir and adefovir. Clin Pharmacokinet. 1999;36(2):127–143. doi: 10.2165/00003088-199936020-00004. [DOI] [PubMed] [Google Scholar]
- 17.Wachsman M, Petty BG, Cundy KC, et al. Pharmacokinetics, safety and bioavailability of HPMPC (cidofovir) in human immunodeficiency virus-infected subjects. Antiviral Res. 1996;29(2-3):153–161. doi: 10.1016/0166-3542(95)00829-2. [DOI] [PubMed] [Google Scholar]
- 18.Brody SR, Humphreys MH, Gambertoglio JG, Schoenfeld P, Cundy KC, Aweeka FT. Pharmacokinetics of cidofovir in renal insufficiency and in continuous ambulatory peritoneal dialysis or high-flux hemodialysis. Clin Pharmacol Ther. 1999;65(1):21–28. doi: 10.1016/S0009-9236(99)70118-9. [DOI] [PubMed] [Google Scholar]
- 19.Cundy KC, Petty BG, Flaherty J, et al. Clinical pharmacokinetics of cidofovir in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 1995;39(6):1247–1252. doi: 10.1128/aac.39.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cihlar T, Lin DC, Pritchard JB, Fuller MD, Mendel DB, Sweet DH. The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol Pharmacol. 1999;56(3):570–580. doi: 10.1124/mol.56.3.570. [DOI] [PubMed] [Google Scholar]
- 21.Lee W, Kim RB. Transporters and renal drug elimination. Annu Rev Pharmacol Toxicol. 2004;44:137–166. doi: 10.1146/annurev.pharmtox.44.101802.121856. [DOI] [PubMed] [Google Scholar]
- 22.Momper JD, Zhang S, Randhawa PS, Shapiro R, Schonder KS, Venkataramanan R. Determination of cidofovir in human plasma after low dose drug administration using high-performance liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2010;53(4):1015–1021. doi: 10.1016/j.jpba.2010.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilead Sciences . Cidofovir Package Insert. Gilead Sciences; Foster City, CA: 2000. [Google Scholar]
- 24.Cundy KC, Li ZH, Lee WA. Effect of probenecid on the distribution, metabolism, and excretion of cidofovir in rabbits. Drug Metab Dispos. 1996;24(3):315–321. [PubMed] [Google Scholar]
- 25.Wolf DL, Rodriguez CA, Mucci M, Ingrosso A, Duncan BA, Nickens DJ. Pharmacokinetics and renal effects of cidofovir with a reduced dose of probenecid in HIV-infected patients with cytomegalovirus retinitis. J Clin Pharmacol. 2003;43(1):43–51. doi: 10.1177/0091270002239705. [DOI] [PubMed] [Google Scholar]
- 26.Nadai M, Apichartpichean R, Hasegawa T, Nabeshima T. Pharmacokinetics and the effect of probenecid on the renal excretion mechanism of diprophylline. J Pharm Sci. 1992;81(10):1024–1027. doi: 10.1002/jps.2600811014. [DOI] [PubMed] [Google Scholar]
- 27.Morgan ET, Goralski KB, Piquette-Miller M, et al. Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab Dispos. 2008;36(2):205–216. doi: 10.1124/dmd.107.018747. [DOI] [PubMed] [Google Scholar]
- 28.Abedini S, Holme I, Marz W, et al. Inflammation in renal transplantation. Clin J Am Soc Nephrol. 2009;4(7):1246–1254. doi: 10.2215/CJN.00930209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naud J, Michaud J, Beauchemin S, et al. Effects of chronic renal failure on kidney drug transporters and cytochrome P450 in rats. Drug Metab Dispos. 2011;39(8):1363–1369. doi: 10.1124/dmd.111.039115. [DOI] [PubMed] [Google Scholar]
- 30.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4(10):1551–1558. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita T, Brown C, Carlson EJ, et al. Functional analysis of polymorphisms in the organic anion transporter, SLC22A6 (OAT1) Pharmacogenet Genomics. 2005;15(4):201–209. doi: 10.1097/01213011-200504000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Burckhardt G, Burckhardt BC. In vitro and in vivo evidence of the importance of organic anion transporters (OATs) in drug therapy. Handb Exp Pharmacol. 2011;(201):29–104. doi: 10.1007/978-3-642-14541-4_2. [DOI] [PubMed] [Google Scholar]