Abstract
A sensitive and specific CYP cocktail assay for simultaneous measurement of the activities of major human cytochrome P450 enzymes (CYP1A2 (phenacetin), CYP3A4/5 (midazolam), CYP2C9 (diclofenac), CYP2C19 (S-mephenytoin) and CYP2D6 (dextromethorphan) in primary cultures of human hepatocytes, was developed and validated using liquid chromatography tandem mass spectrometry (LC-MS/MS). Hepatocyte incubation medium was processed by a solid phase extraction (SPE) using Oasis SPE extraction cartridges prior to chromatography. The metabolites derived from each of the substrates was simultaneously quantitated using the corresponding stable isotope-labeled internal standards by a positive electrospray ionization mode using multiple reactions monitoring with a single eight minute run. The mean accuracy was in the range of 98–114%. The interday and intraday precision over the concentration ranges evaluated for all the analytes were lower than 15%, and 14%, respectively. All the generated metabolites were stable under the conditions used for sample analysis. Additionally, the interaction of a cocktail substrate on other CYP substrates was also analyzed. Due to substantial inter-substrate interaction, chlorzoxazone (CYP2E1) and bupropion (CYP2B6) were removed from the initial seven probes CYP cocktail assay. Therefore, the final CYP cocktail assay consisting of five probes provides a robust method to simultaneously measure activities of CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A4/5 in primary cultures of human hepatocytes.
Keywords: CYP cocktail assay, Primary cultures of human hepatocytes, LC-MS/MS
1. Introduction
Cytochrome P450 enzymes (CYP450) are commonly involved in clinically important drug-drug interactions (DDI). Among the various human CYP450 enzymes, CYP3A4/5, CYP2C9, CYP2C19, CYP2D6 and CYP1A2 isoforms account for the metabolism of approximately 90% of drugs [1]. In order to avoid unwanted DDI and associated toxicities in human, several in vitro DDI studies are routinely performed in human liver microsomes (HLM) and primary hepatocytes for the prediction of in vivo DDI [2]. While HLM can only be used for short term CYP inhibitory studies, the primary cultures of human hepatocytes have an added advantage that they can be useful for long term CYP induction and short term/long term CYP inhibition studies.
In a conventional DDI study, the CYP activities are measured individually for the assessment of CYP isoform susceptible for inhibition/induction by drugs [3]. Because of the limited availability of human livers [4] and the extensive time [5] required to perform individual CYP enzyme activity, there is a need to minimize the amount of time and human liver microsomes or hepatocytes needed to perform in vitro DDI studies. Hence, the CYP cocktail approach was adopted by many researchers to simultaneously assess various CYP activities [6–8]. In a CYP cocktail assay, the preferred and acceptable probe substrates for individual CYP isoform will be mixed together as a cocktail and then incubated with suitable in vitro system (HLM or hepatocytes) for the simultaneous measurement of different CYP enzymes activities.
Although several CYP cocktail assays were developed for HLM [9] or microsomes obtained from primary cultures of human hepatocytes [10], very limited number of CYP cocktail assays have been reported for the simultaneous assessment of CYP activities directly in the primary cultures of human hepatocytes [11–14]. In addition, the use of stable isotope labeled internal standards for each of CYP probe substrate was very limited in a CYP cocktail assay that was performed in primary cultures of human hepatocytes. Therefore the objective of this study was to develop and validate a sensitive CYP cocktail assay for the simultaneous measurement of major human cytochrome P450 enzymes (CYP3A4/5, CYP2C9, CYP2C19, CYP2D6 and CYP1A2) activities in primary cultures of human hepatocytes using stable isotope labeled internal standards using liquid chromatography tandem mass spectrometry (LC-MS/MS). Additionally, the CYP cocktail assay was cross validated by comparing the individual and simultaneous incubation of CYP substrates in primary cultures of human hepatocytes to identify any potential interactions among the CYP substrates used in the CYP cocktail assay.
2. Materials and methods
2.1 Chemicals and materials
Phenacetin, dextromethorphan hydrobromide monohydrate, bupropion, chlorzoxazone and acetaminophen were obtained from Sigma-Aldrich (St. Louis, MO, USA). Diclofenac sodium salt and dextrorphan-D-Tartrate were purchased from MP Biomedical Inc. (Solon, OH, USA). S-mephenytoin, midazolam, hydroxy bupropion, 4′-Hydroxy Diclofenac, (S)-4-Hydroxy Mephenytoin, 1′-Hydroxy Midazolam and deuterated internal standards such as acetaminophen-D4, (±)-4-Hydroxy Mephenytoin-d3, dextrorphan-d3, Tartrate salt, midazolam-d5, 4′-Hydroxy Diclofenac-D4 and hydroxyl bupropion-d6 were procured from Toronto research chemicals (Ontario, Canada). Oasis HLB 1 mL (30 mg) extraction cartridges were purchased from Waters Corporation (Milford, MA, USA). Luna C8(2) column (150 mm × 3.0 mm, 5 μm, 100 A°) and C8 Security Guard cartridge (4.0 mm × 2.0 mm) were procured from Phenomenex (Torrance, CA, USA). Hepatocyte maintenance medium (HMM) was obtained from Lonza Inc (Allendale, NJ, USA). Bovine Serum Albumin and Bio-Rad Protein Assay Dye Reagent concentrate were obtained from Bio-Rad (Hercules, CA, USA). All the solvents were of MS grade and were obtained from Fisher Scientific (Pittsburgh, PA, USA).
2.2 Preparation of standards and quality control samples
Stock solutions of acetaminophen, 1′-Hydroxy midazolam, (S)-4-Hydroxy mephenytoin, dextrorphan, 4′-Hydroxy diclofenac were prepared independently at 1 mg/mL in methanol and used for a maximum of 6 months, while being stored at −20°C in the dark. On assay days, the stock solutions were diluted in 50% methanol to produce the following working solutions 1, acetaminophen (5 μg/mL), 1′-Hydroxy midazolam (5 μg/mL), (S)-4′-Hydroxy mephenytoin (5 μg/mL), hydroxy diclofenac (50 μg/mL) and dextrorphan (2.5 μg/mL). Seven calibration standards were prepared from working solutions 1 by spiking in serum and supplements free hepatocyte maintenance medium (HMM). The concentrations of calibrators used in the assay are as follows: acetaminophen (5, 10, 20, 40, 60, 80 and 100 μg/mL), 1′-Hydroxy midazolam (5, 10, 20, 40, 60, 80 and 100 μg/mL), (S)-4′-Hydroxy mephenytoin (10, 20, 40, 60, 80, 100 and 120 μg/mL), hydroxy diclofenac (50,100, 200, 400, 600, 800 and 1000 μg/mL) and dextrorphan (1, 2, 5, 10, 20, 50 and 100 μg/mL).
Quality control (QC) working solutions (working solution 2) were prepared separately from the stock solutions and stored at −20°C in the dark. The concentrations of working solutions 2 were similar to that of working solutions 1. These solutions were diluted in serum and supplements free HMM to produce three QC samples (QC low (QC-L), QC medium (QC-M) and QC high (QC-H). The concentrations of QC samples are given in Table 2.
Table 2.
Calibration ranges, regression and the concentrations of quality controls (QC-L, QC-M and QC-H) for CYP metabolites
| Enzyme | Probe Substrates | Metabolites | Metabolites Calibration range (ng/mL) | Regression | R2 |
|---|---|---|---|---|---|
| CYP1A2 | Phenacetin | Acetaminophen | 5–100 | y=0.0177×+0.0412 | 0.996 |
| CYP2C9 | Diclofenac | 4′-Hydroxy diclofenac | 50–1000 | y=0.1221×−3.0899 | 0.991 |
| CYP2C19 | S-mephenytoin | 4-Hydroxy mephenytoin | 10–120 | y=0.0140×−0.1380 | 0.989 |
| CYP2D6 | Dextromethorphan | Dextrorphan | 1–100 | y=0.2925×−0.0042 | 0.994 |
| CYP3A4 | Midazolam | 1′-Hydroxy midazolam | 5–100 | y=0.0156×+0.0151 | 0.992 |
| Enzyme | Metabolites | Conc of QC (ng/mL)
|
Internal Standards | ||
|---|---|---|---|---|---|
| QC-L | QC-M | QC-H | |||
| CYP1A2 | Acetaminophen | 10 | 40 | 80 | Acetaminophen-D4 |
| CYP2C9 | 4′-Hydroxy diclofenac | 100 | 400 | 800 | 4′-Hydroxy Diclofenac-D4 |
| CYP2C19 | 4-Hydroxy mephenytoin | 20 | 60 | 100 | Mephenytoin-d3 |
| CYP2D6 | Dextrorphan | 2 | 10 | 50 | Dextrorphan-d3 |
| CYP3A4 | 1′-Hydroxy midazolam | 10 | 40 | 80 | Midazolam-d5 |
The stock solutions of deuterated internal standards were prepared independently at the following concentrations, acetaminophen-D4 (10 mg/mL), (±)-4-Hydroxy Mephenytoin-d3 (2.5 mg/mL), dextrorphan-d3, Tartrate salt (2.5 mg/mL), midazolam-d5 (1 mg/mL) and 4′-Hydroxy Diclofenac-D4 (1 mg/mL) in methanol and stored at −20°C in the dark. On the day of the assay, the internal standard working solutions (IS working solutions) were prepared independently in 50% methanol at 2 μg/mL concentration except (±)-4-Hydroxy Mephenytoin-d3 and dextrorphan-d3, which were prepared at 5 μg/mL concentration. Finally, the IS working solutions were diluted in 50% methanol to achieve a final concentration of 50 ng/mL.
2.3. Sample preparation
Routine daily calibration standards, quality controls, and hepatocyte medium samples were thawed at room temperature. Exactly 200 μL of medium, 20 μL of IS and 500 μL of water were mixed together in a micro centrifuge tube and then passed through Oasis HLB 1 mL (30 mg) extraction cartridges, previously conditioned with 1mL methanol and 1 mL water. After washing with 1 mL of 5% methanol, the analytes were eluted with 1 mL of methanol and the eluent was evaporated to dryness under air at room temperature. The residue was reconstituted in 100 μL of 50% methanol and 20 μL of the solution was injected into the LC–MS/MS system.
2.4. Chromatographic and mass spectrometer conditions
The HPLC system used for the CYP cocktail assay was a Waters 2759 model (Waters Corporation, MA, USA). Separation was performed with a Luna C8(2) column (150 mm×3.0 mm, 5 μm, 100 Å) with a C8 Security Guard cartridge (4.0 mm×2.0 mm). The temperature of the column was maintained at 40°C and the auto sampler temperature was set at 4°C. A gradient mobile phase system was used consisting of solvent A (95% water + 5% methanol + 0.1% formic acid + 2 mM ammonium acetate) and solvent B (Methanol + 0.1% formic acid + 2 mM ammonium acetate) with a gradient starting from 100% solvent A to 0% solvent A over 0.5 min, held till 5.5 min, followed by returning to the initial condition of 100% solvent A, held till 8 min, to achieve the baseline. The total run time was 8 min at a flow rate of 0.4 mL/min. Analysis was performed on a Micromass Quattro triple quadrupole mass spectrometer (Waters Corporation, MA, USA) with positive electrospray ionization mode using multiple reaction monitoring (MRM). For the analytes and internal standard, MRM settings used were as follows: capillary voltage 3.2 kV; source temperature 130°C; desolvation temperature 300°C; cone gas flow 25 L/h; desolvation gas flow 300 L/h; argon pressure 20±10 psig; nitrogen pressure 100±20 psig. The MS conditions for the metabolites and internal standards are presented in Table 1. The LC–MS system was controlled by the Masslynx® software version 4.1, and data were collected with the same software.
Table 1.
MS conditions for the CYP metabolites and internal standards
| Enzyme | Metabolites | Precursor ion mass (m/z) | Product ion mass (m/z) | Ionisation Mode | Cone voltage (V) | Collision energy (eV) | Retention time (min.) |
|---|---|---|---|---|---|---|---|
| CYP1A2 | Acetaminophen | 152.03 | 110.03 | ESI + | 25 | 15 | 4.26 |
| CYP2C9 | 4′-Hydroxy diclofenac | 311.82 | 229.97 | ESI + | 25 | 32 | 5.24 |
| CYP2C19 | 4-Hydroxy mephenytoin | 234.91 | 132.77 | ESI + | 30 | 20 | 4.64 |
| CYP2D6 | Dextrorphan | 257.98 | 156.96 | ESI + | 30 | 30 | 4.29 |
| CYP3A4 | 1′-Hydroxy midazolam | 341.91 | 323.85 | ESI + | 20 | 25 | 5.09 |
| Enzyme | Internal Standards | Precursor ion mass (m/z) | Product ion mass (m/z) | Ionisation mode | Cone voltage (V) | Collision energy (eV) | Retention time (min.) |
|---|---|---|---|---|---|---|---|
| CYP1A2 | Acetaminophen-D4 | 155.71 | 113.86 | ESI + | 25 | 15 | 4.26 |
| CYP2C9 | 4′-Hydroxy Diclofenac-D4 | 315.82 | 234.00 | ESI + | 25 | 32 | 5.24 |
| CYP2C19 | Mephenytoin-d3 | 237.88 | 150.03 | ESI + | 30 | 20 | 4.64 |
| CYP2D6 | Dextrorphan-d3 | 261.00 | 244.00 | ESI + | 30 | 30 | 4.29 |
| CYP3A4 | Midazolam-d5 | 330.76 | 295.77 | ESI + | 20 | 25 | 4.79 |
2.5. Validation procedures
2.5.1. Calibration curve and lower limit of quantitation (LLOQ)
Decreasing concentrations of analytes in hepatocyte medium, prepared as previously described, were injected into the analytical system to achieve a signal-to-noise ratio of at least 5:1. Calibration standards, blank, and zero samples were analyzed in triplicate to establish the calibration range with acceptable accuracy and precision. The response for each sample was calculated by dividing the area of the analyte peak by the area of the corresponding internal standard peak. Standard curves of analytes were constructed by plotting the analyte-to-internal standard response ratio versus the nominal concentration of analyte in each sample. Standard curves were fit by linear regression with weighting by 1/χ2, without forcing the line through the origin, followed by the back calculation of concentrations. The deviations of these back-calculated concentrations from the nominal concentrations, expressed as percentage of the nominal concentration, reflected the assay performance over the concentration range.
2.5.2. Accuracy and precision
The accuracy and precision of the developed method were determined by analyzing hepatocyte medium samples with a cocktail of CYP substrates include acetaminophen, hydroxy midazolam, hydroxy mephenytoin, hydroxy diclofenac and dextrorphan at the QC-L, QC-M, and QC-H concentrations in a minimum of five replicates in 3 analytical runs together with an independently prepared, triplicate calibration curve. Accuracy was calculated at each test concentration as:
The precision of the assay was expressed using % coefficient of variation (CV). Intra-assay and inter-assay precision were assessed by replicate analysis of specimen aliquots on a single day or successive days, respectively.
2.5.3. Selectivity and specificity
To investigate whether endogenous matrix constituents interfered with the assay, six individual batches of serum, drug and other supplements free hepatocyte medium were processed and analyzed according to the described procedures. Responses of analytes at the LLOQ concentration were compared with the responses in the blank samples.
2.5.4. Extraction recovery and ion suppression
The extraction recovery of analytes from hepatocyte medium was determined by comparing the absolute response of an extract of control hepatocyte medium to which a cocktail of analytes had been added after extraction with the absolute response of an extract of hepatocyte medium to which the same amount of a cocktail of analytes had been added before extraction. The matrix effect of hepatocyte medium on various analytes was defined as the effect on the signal when comparing the absolute response of an extract of hepatocyte medium to which a cocktail of analytes had been added after the extraction with the absolute response of reconstitution solvent to which the same amount of a cocktail of analytes had been added. Experiments were performed at the QC-L, QC-M, and QC-H concentrations in four replicates.
2.5.5. Stability
The stability of CYP cocktail substrates in hepatocyte medium was evaluated at the QC-L, QC-M and QC-H concentrations in triplicate under different conditions. The control medium samples were stored for either 4 h or 24 h at room temperature (RT) or post-preparation storage of 24 h at 4°C. Additionally, three freeze–thaw cycles of control medium samples prior to extraction were assessed. The reference concentration was calculated from freshly spiked hepatocyte medium samples injected immediately post-extraction. Stability was expressed in terms of percentage of nominal concentration.
2.6 Human hepatocytes
The human liver samples (n=9) were obtained from Hepatocytes Transplantation Laboratory, University of Pittsburgh. Hepatocytes were isolated by collagenase perfusion method as reported before [15]. The viability of hepatocytes was determined using trypan blue cell exclusion assay and was >90% for all the experiments. The hepatocytes were seeded at a density of 1.5 × 106 cells per well of six well plates that were previously coated with rat tail collagen. The cells were maintained at 37 °C in carbon dioxide incubator. After 2 h of seeding, the medium was replaced with fresh medium to remove non-adherent cells. All the experiments were carried out after 48 h of seeding the hepatocytes into the plates.
2.7 Human hepatocyte incubation of CYP cocktail substrates
To demonstrate the applicability of the method, human hepatocytes were incubated with either individual CYP substrate or cocktail of CYP substrates. The specific CYP substrates used in the initial cocktail assay included phenacetin (CYP1A2; 100 μM), bupropion (CYP2B6; 50 μM), diclofenac (CYP2C9; 90 μM), S-mephenytoin (CYP2C19; 50 μM), dextromethorphan (CYP2D6; 20 μM), chlorzoxazone (CYP2E1; 90 μM) and midazolam (CYP3A4/5; 5 μM). The cocktail substrates were incubated with hepatocytes for 30 min. Since the CYP2C19 activity is relatively low in hepatocytes, an extended incubation (120 min) of CYP2C19 substrate and cocktail of substrates with hepatocytes was also evaluated. The culture medium supernatant was collected and stored at −80°C until analyzed using the procedure described above. Additionally, the hepatocyte pellets were collected in phosphate buffered saline and then centrifuged at 10000 g at 4 °C. Subsequently, the pellets were lysed using 125 μL of the cell lysis buffer (50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 150 mM NaCl, 1.5 mM MgCl2, 1 mM ethylene glycol tetraacetic acid (EGTA), 10% glycerol, 1% Triton X-100, and complete, Mini protease inhibitor cocktail tablet (Roche Diagnostics, Mannheim, Germany; one tablet per 10 ml of cell lysis buffer) and then protein was estimated using Bradford assay. Finally, the CYP activity was corrected for protein and expressed as the ratio of the activity after CYP cocktail substrates incubation to the activity after individual CYP substrate incubation with 95% confidence intervals. In case of any inter-substrates interactions, the major interacting substrate was removed and the similar procedure was followed. Each CYP cocktail assay was performed in hepatocytes isolated from three independent human livers.
3. Results and Discussion
Because of the characteristic resemblance to human liver, the primary cultures of human hepatocytes are routinely used for in vivo prediction of DDI [16]. The objective of this study was to develop a sensitive and specific CYP cocktail assay for assessing the activities of major CYP450 enzymes involved for the metabolism based DDI.
The selection of CYP cocktail substrates was based on the recent FDA guidance over the preferred and acceptable CYP substrates (http://www.fda.gov/drugs/developmentapproval-process/developmentresources/druginteractionslabeling/ucm093664.htm) for evaluating the CYP activities in vitro. Typically dimethyl sulfoxide (DMSO) is used to dissolve CYP probe substrates. Earlier studies have revealed that the solvent concentration of DMSO greater than or equal to 1% v/v can affect the CYP450 enzymes activities in vitro [17, 18]. Therefore, all the CYP probe substrates were dissolved in DMSO and then diluted in hepatocyte medium to achieve a final concentration of 0.5% v/v for all the hepatocytes CYP cocktail studies.
Preliminary mass spectrometric analysis was performed using both positive and negative electro spray ionization modes. Because of the enhanced sensitivity with positive ion mode, all the CYP metabolites were analyzed using positive ion mode. The mass-to-charge transitions from parent ions to daughter ions were observed for each CYP probe metabolite and are represented in Table 1.
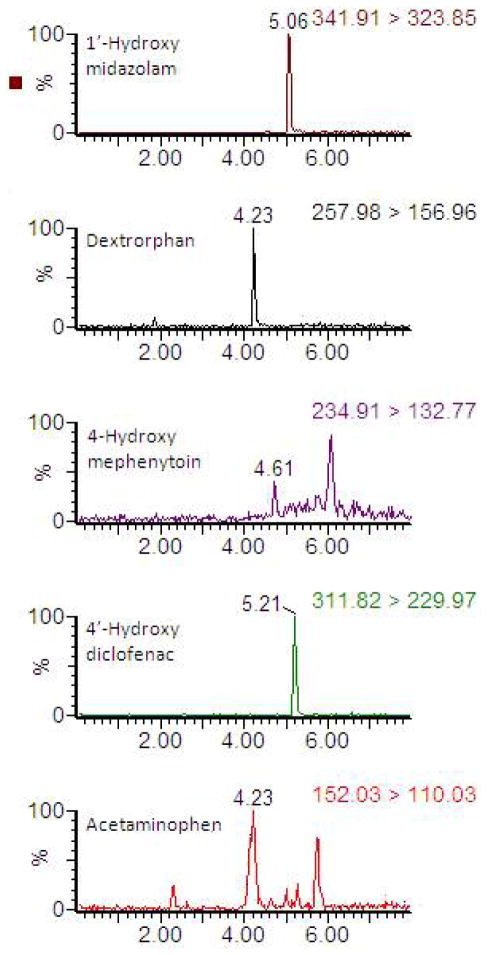
Prior to chromatography, several sample extraction procedures were tested which include liquid-liquid extraction using either methanol or ethyl acetate and solid phase extraction using Oasis® HLB extraction cartridge. Finally, a solid phase extraction process was selected for obtaining the cleaner extracts of all CYP metabolites together. Because of the heterogeneous physicochemical properties of CYP cocktail metabolites, the universal chromatographic conditions are required for the separation of analytes. Several mobile phase systems and chromatographic separation columns were tested. Finally, the best resolution of analytes was achieved using mobile phase compositions (Solvent A: 95% water + 5% methanol + 0.1% formic acid + 2 mM ammonium acetate; Solvent B: 100% methanol + 0.1% formic acid + 2 mM ammonium acetate) and Luna C8(2) analytical column. The chromatographic separation of CYP probe metabolites were achieved with a single 8 min LC-MS/MS run. The retention times of acetaminophen, hydroxy midazolam, hydroxy mephenytoin, hydroxy diclofenac and dextrorphan are shown in Table 1. Typical chromatograms of hepatocyte medium spiked with a cocktail of CYP metabolites at LLOQ are represented in Fig. 1. The LLOQ of each analyte was selected with a signal-to-noise ratio of >10.
Figure 1.
Representative chromatogram for CYP cocktail metabolites at LLOQ in human hepatocytes maintenance medium.
The bioanalytical method validation of this assay followed the partial validation guidelines of FDA [19]. The calibration curves in hepatocyte medium were analyzed on four sequential days. The ratio of peak area of CYP probe metabolites to the corresponding internal standards were linearly related to the concentration of CYP probe metabolites. The calibration range, quality control concentrations, type of regression of each analyte in the selected concentration range are represented in Table 2.
To test for interference, six different batches of hepatocyte medium were analyzed as blanks and after addition of CYP probe metabolites at their LLOQ such as acetaminophen (5 ng/mL), hydroxy midazolam (5 ng/mL), hydroxy mephenytoin (10 ng/mL), hydroxy diclofenac (50 ng/mL) and dextrorphan (1 ng/mL). The responses in blank hepatocyte medium were always less than 5% of the signal at the LLOQ. Table 3 represents the effect of matrix on accuracy and precision of CYP probe metabolites estimation at LLOQ. These results demonstrate that there was no influence of matrix on the detection of CYP cocktail metabolites at LLOQ. This suggests that the assay was specific to detect the CYP metabolites in hepatocyte medium.
Table 3.
Influence of matrix on accuracy and precision of estimation of CYP metabolites at LLOQ in six different batches of hepatocytes maintenance medium
| Metabolites | LLOQ (ng/mL) | Accuracy (%) | CV (%) |
|---|---|---|---|
| Acetaminophen | 5.0 | 99.3 | 3.0 |
| 4′-Hydroxy diclofenac | 50.0 | 96.9 | 2.1 |
| 4-Hydroxy mephenytoin | 10.0 | 99.2 | 7.5 |
| Dextrorphan | 1.0 | 95.0 | 5.7 |
| 1′-Hydroxy midazolam | 5.0 | 98.0 | 4.3 |
The accuracies for all tested concentrations of analytes should be within ± 15% deviation, except for the LLOQ, in which case these parameters should not exceed 20% deviation. The intra-day and inter-day coefficient of variation should be within the 15% acceptable limits. The accuracies and intra- and inter-assay precisions for the tested concentrations (QC-L, QC-M, QC-H) were all with in these pre-defined acceptance criteria (Table 5).
Table 5.
Intra-assay and inter-assay precision and accuracy
| Metabolites | Nominal Concentrations (ng/mL) | Accuracy (%) | Intra-assay precision (CV%) | Inter-assay precision (CV%) |
|---|---|---|---|---|
| Acetaminophen | 10 | 98.0 | 3.2 | 9.9 |
| 40 | 101.5 | 2.3 | 5.4 | |
| 80 | 107.7 | 3.1 | 6.2 | |
| 4′-Hydroxy diclofenac | 100 | 99.4 | 3.7 | 4.7 |
| 400 | 113.7 | 7.2 | 3.6 | |
| 800 | 98.3 | 7.6 | 4.7 | |
| 4-Hydroxy mephenytoin | 20 | 103.1 | 6.4 | 7.0 |
| 60 | 109.4 | 14.2 | 3.8 | |
| 100 | 100.4 | 8.1 | 15.0 | |
| Dextrorphan | 2 | 107.5 | 6.8 | 6.0 |
| 10 | 105.5 | 2.8 | 7.6 | |
| 50 | 97.7 | 1.1 | 11.3 | |
| 1′-Hydroxy midazolam | 10 | 106.3 | 3.5 | 2.5 |
| 40 | 111.2 | 3.0 | 6.0 | |
| 80 | 98.3 | 4.5 | 6.2 |
The extraction recovery and ion suppression effects of CYP metabolites are represented in Table 4. The total extraction recoveries for CYP metabolites were in the range of 57–93%. The ion suppression recovery for most of the CYP metabolites were in the range of 95–106% except 1′-Hydroxy midazolam (CYP3A4/5), which exerted an average ion suppression of ~26%. However, the precision and accuracy of estimation of 1′-Hydroxy midazolam was not affected by the ion suppression observed in this assay.
Table 4.
Recovery and ion-suppression
| Metabolites | Nominal Concentrations (ng/mL) | Extraction recovery | Ion suppression recovery |
|---|---|---|---|
|
| |||
| Mean ± S.D. (%) | Mean ± S.D. (%) | ||
| Acetaminophen | 10 | 70.6 ± 2.2 | 100.1 ± 5.5 |
| 40 | 72.3 ± 1.7 | 94.8 ± 2.5 | |
| 80 | 74.2 ± 3.4 | 97.2 ± 4.6 | |
| 4′-Hydroxy diclofenac | 100 | 57.4 ± 2.6 | 99.5 ± 6.6 |
| 400 | 66.1 ± 7.2 | 97.8 ± 9.6 | |
| 800 | 65.1 ± 2.2 | 103.3 ± 10.2 | |
| 4-Hydroxy mephenytoin | 20 | 83.9 ± 8.1 | 96.4 ± 11.6 |
| 60 | 90.3 ± 5.9 | 106.0 ± 8.0 | |
| 100 | 85.4 ± 9.8 | 102.6 ± 11.0 | |
| Dextrorphan | 2 | 75.6 ± 3.4 | 102.9 ± 6.5 |
| 10 | 82.9 ± 1.4 | 98.4 ± 6.9 | |
| 50 | 82.1 ± 2.7 | 102.1 ± 5.9 | |
| 1′-Hydroxy midazolam | 10 | 90.0 ± 6.2 | 72.5 ± 5.2 |
| 40 | 93.3 ± 2.1 | 73.7 ± 2.4 | |
| 80 | 88.4 ± 2.9 | 74.9 ± 2.8 | |
The stability of analytes that were maintained under different storage conditions are represented in Table 6. The analytes were stable in hepatocyte medium after three freeze thaw cycles, with an accuracy of 94–107% and a CV <15%. All the analytes were stable in hepatocyte medium for 4 hr at RT with an accuracy of 97–111% and a CV <13%. All analytes except 4′-Hydroxy diclofenac, were stable in hepatocyte medium for 24 h at RT with an accuracy of 95–107% and a CV <12%. Additionally, the post-preparation storage of extract of CYP metabolites at 4°C for 24 h, did not affect the stability of the CYP metabolites. Under the later condition, analytes were estimated with an accuracy of 94–109% and a CV<14%.
Table 6.
Stability of CYP metabolites in hepatocyte maintenance medium after three freeze-thaw cycles, 24 h at room temperature (RT) and post-preparation storage at 4° C for 24 h.
| Metabolites | Nominal Concentrations (ng/mL) | Three freeze-thaw cycles in medium (n=3) | 4 h in medium at RT (n=3) | 24 h in medium at RT (n=3) | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Stability (%) | CV (%) | Stability (%) | CV (%) | Stability (%) | CV (%) | ||
| Acetaminophen | 10 | 106.9 | 5.4 | 103.8 | 1.1 | 107.3 | 4.5 |
| 40 | 100.2 | 15.3 | 104.3 | 2.7 | 99.1 | 0.5 | |
| 80 | 99.9 | 3.8 | 102.1 | 4.5 | 94.8 | 1.0 | |
| 4′-Hydroxy diclofenac | 100 | 103.4 | 1.4 | 106.0 | 3.1 | 50.3 | 1.3 |
| 400 | 94.1 | 8.5 | 99.0 | 0.1 | 55.7 | 3.7 | |
| 800 | 104.4 | 6.3 | 101.3 | 3.0 | 58.1 | 4.2 | |
| 4-Hydroxy mephenytoin | 20 | 100.5 | 13.3 | 106.0 | 11.6 | 106.9 | 11.7 |
| 60 | 100.1 | 8.3 | 100.8 | 7.4 | 100.2 | 9.0 | |
| 100 | 102.9 | 10.8 | 97.9 | 9.8 | 98.1 | 2.1 | |
| Dextrorphan | 2 | 104.8 | 4.6 | 96.7 | 7.5 | 103.2 | 7.1 |
| 10 | 98.4 | 4.7 | 99.3 | 7.1 | 101.6 | 5.9 | |
| 50 | 103.8 | 7.8 | 104.0 | 2.8 | 100.4 | 2.6 | |
| 1′-Hydroxy midazolam | 10 | 96.2 | 6.6 | 99.1 | 12.5 | 100.6 | 2.5 |
| 40 | 98.9 | 5.4 | 103.5 | 3.3 | 98.9 | 0.5 | |
| 80 | 100.3 | 4.5 | 106.1 | 3.4 | 98.8 | 1.0 | |
The developed method was applied for the simultaneous assessment of human CYP activities (CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A4/5) in human hepatocyte samples. The substrate interactions are a major concern when multiple CYP probe substrates are concurrently used in a CYP cocktail assay. The activities of CYP enzymes will be compromised as a result of potential substrate interactions. Therefore, it is essential to demonstrate a minimal/no substrate interaction in a CYP cocktail assay by individual and simultaneous incubation of CYP cocktail substrates with primary cultures of human hepatocytes. Fig. 2 represents the ratio of the activities of various CYP enzymes after simultaneous and individual incubation of seven substrates (the above five different CYP enzymes plus CYP2B6 and CYP2E1), six substrates (the removal of CYP2E1 probe from seven probes CYP cocktail) and five substrates (the removal of CYP2B6 probe from six probes CYP cocktail) CYP cocktail assays in human hepatocytes. The initial CYP cocktail assay was designed to evaluate seven different CYP enzymes activities (Phenacetin (CYP1A2), Diclofenac (CYP2C9), S-mephenytoin (CYP2C19), Dextromethorphan (CYP2D6), Midazolam (CYP3A4/5), Bupropion (CYP2B6) and Chlorzoxazone (CYP2E1)) in human hepatocytes. However, the substantial inhibition of CYP3A4/5 (78 ± 5 %), CYP2B6 (40 ± 14 %) and CYP2D6 (54 ± 24 %) activities was observed in seven probes cocktail assay as compared to a single probe assay. Additionally, the activities of CYP1A2, CYP2C19 and CYP2E1 were below the detection limit. Earlier evidence based on in vivo CYP cocktail studies suggest that chlorzoxazone can significantly inhibit midazolam hydroxylation (CYP3A activity) in healthy human subjects [20]. In addition, chlorzoxazone was also reported to interact with CYP1A2 substrates [20, 21], which may be responsible for the slower rate of metabolism of phenacetin (CYP1A2) in this study. Although several of the previous in vitro CYP cocktail assays had both chlorzoxazone and midazolam as substrates [14, 22], they did not report this potential interaction. In order to avoid potential inter-substrate interaction, the CYP2E1 probe, chlorzoxazone was excluded from the initial CYP cocktail substrate mixtures. Indeed, the slower rate of metabolism of S-mephenytoin (CYP2C19) or the inter-substrate inhibition of CYP2C19 activity may be responsible for the reduced activity of CYP2C19 in human hepatocytes. Therefore, the duration of incubation of S-mephenytoin was prolonged to 2 hr for further studies.
Figure 2.
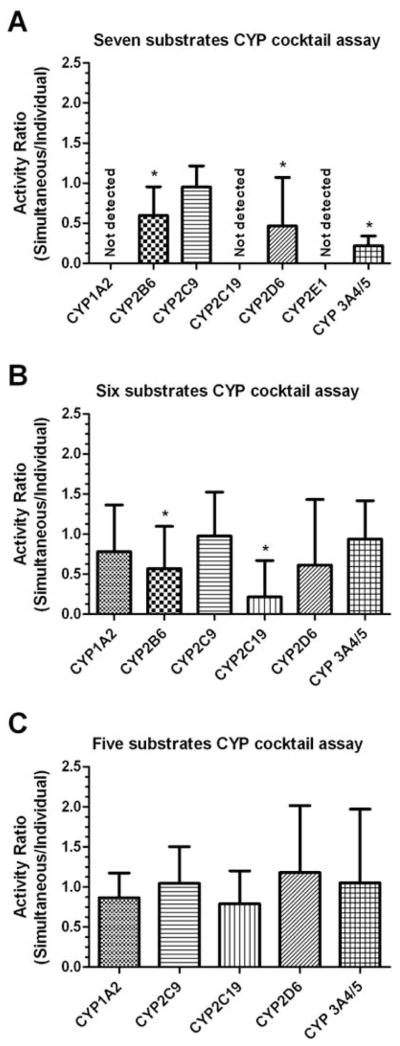
CYP activity in human hepatocytes using seven probes (A), six probes (B) and five probes (C) CYP cocktails was expressed as the ratio of the activity after CYP cocktail substrates incubation to the activity after individual CYP substrate incubation. Data represents the mean with 95% confidence intervals (n=3 independent hepatocyte cultures). In the seven probes CYP cocktail assay, the activities of CYP3A4/5, CYP2B6 and CYP2D6 were significantly inhibited whereas CYP1A2, CYP2C19 and CYP2E1 activities were below the detection limit. In the six probes CYP cocktail assay, the activity of CYP2C19 was significantly inhibited whereas both CYP2B6 (43%) and CYP2D6 (39%) activities were moderately inhibited. In the five probes CYP cocktail assay, minimal (<21%) or no substrate interaction was observed. * indicates p < 0.05; One sided paired t test was used for the comparison of the CYP activities after simultaneous and individual CYP substrates incubation.
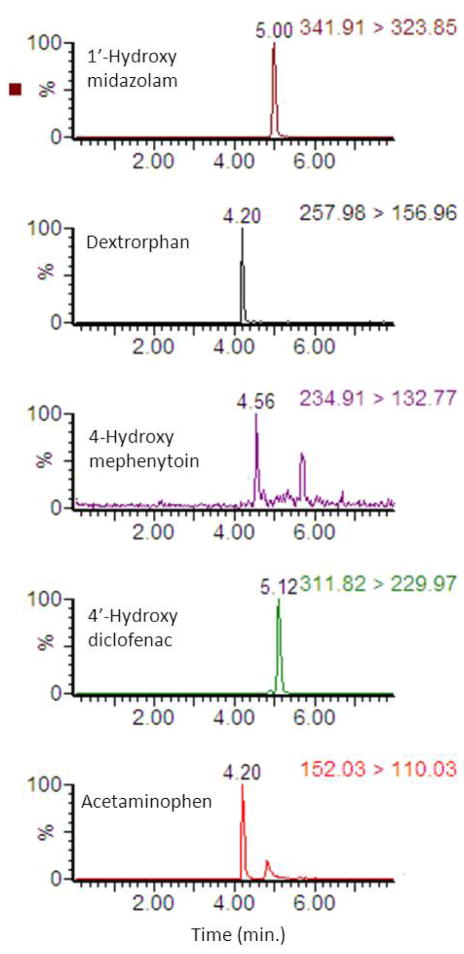
Although the removal of chlorzoxazone from the initial CYP cocktail substrate mixtures completely restores the CYP3A activity, the profound inhibition of CYP2C19 (78 ± 18 %), CYP2B6 (43 ± 21 %) and CYP2D6 (34 ± 41 %) activities was still observed in a six probes cocktail assay as compared to a single probe assay. Previous reports suggest that bupropion can inhibit dextromethorphan O-demethylation (CYP2D6 activity) in human liver microsomes [23] and healthy human subjects [24] and S-mephenytoin 4-hydroxylation (CYP2C19 activity) in human liver microsomes [9]. The results from this study have further confirmed the above major interactions and necessitated exclusion of bupropion probe from the six probes CYP cocktail substrate mixtures. Subsequently, the five probes CYP cocktail assay was performed, which did not have any significant inter-substrate interactions. A representative chromatogram from a final five substrates CYP cocktail assay performed in human hepatocytes is displayed in Fig. 3. Therefore, the final five probes CYP cocktail assay was chosen to simultaneously assess human CYP3A4/5, CYP2C9, CYP2C19, CYP2D6 and CYP1A2 activities in primary cultures of human hepatocytes.
Figure 3.
Representative chromatogram for CYP cocktail metabolites from a five substrates CYP cocktail assay performed in human hepatocytes.
4. Conclusions
This CYP cocktail assay consisting of five probe drugs provides a sensitive, specific and robust method to measure drug-drug interactions involving CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4/5 in primary cultures of human hepatocytes. This assay can be used to assess the CYP activities in human fetal hepatocytes or human liver microsomes.
Highlights.
Developed a sensitive and specific CYP cocktail assay in primary cultures of human hepatocytes
Partial validation of the assay with respect to FDA guidelines on analytical method validation.
Simultaneously measure activities of CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A4/5
Cross validated the assay for inter-substrate interaction
Acknowledgments
This study was partially supported by NICHD, OPRU network grant 5U10 HD047905.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lynch T, Price A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician. 2007;76:3996. [PubMed] [Google Scholar]
- 2.Bjornsson TD, Callaghan JT, Einolf HJ, Fischer V, Gan L, Grimm S, Kao J, King SP, Miwa G, Ni L, Kumar G, McLeod J, Obach RS, Roberts S, Roe A, Shah A, Snikeris F, Sullivan JT, Tweedie D, Vega JM, Walsh J, Wrighton SA. The conduct of in vitro and in vivo drug-drug interaction studies: a Pharmaceutical Research and Manufacturers of America (PhRMA) perspective. Drug Metab Dispos. 2003;31:815–832. doi: 10.1124/dmd.31.7.815. [DOI] [PubMed] [Google Scholar]
- 3.Takusagawa S, Miyashita A, Iwatsubo T, Usui T. In vitro inhibition and induction of human cytochrome P450 enzymes by mirabegron, a potent and selective beta3-adrenoceptor agonist. Xenobiotica. 2012 doi: 10.3109/00498254.2012.700140. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Lechon MJ, Donato MT, Castell JV, Jover R. Human hepatocytes as a tool for studying toxicity and drug metabolism. Curr Drug Metab. 2003;4:292–312. doi: 10.2174/1389200033489424. [DOI] [PubMed] [Google Scholar]
- 5.Zientek M, Miller H, Smith D, Dunklee MB, Heinle L, Thurston A, Lee C, Hyland R, Fahmi O, Burdette D. Development of an in vitro drug-drug interaction assay to simultaneously monitor five cytochrome P450 isoforms and performance assessment using drug library compounds. J Pharmacol Toxicol Methods. 2008;58:206–214. doi: 10.1016/j.vascn.2008.05.131. [DOI] [PubMed] [Google Scholar]
- 6.Kozakai K, Yamada Y, Oshikata M, Kawase T, Suzuki E, Haramaki Y, Taniguchi H. Reliable High-Throughput Method for Inhibition Assay of 8 Cytochrome P450 Isoforms Using Cocktail of Probe Substrates and Stable Isotope-Labeled Internal Standards. Drug Metab Pharmacokinet. 2012 doi: 10.2133/dmpk.dmpk-12-rg-014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Oh KS, Park SJ, Shinde DD, Shin JG, Kim DH. High-sensitivity liquid chromatography-tandem mass spectrometry for the simultaneous determination of five drugs and their cytochrome P450-specific probe metabolites in human plasma. J Chromatogr B: Analyt Technol Biomed Life Sci. 2012;895–896:56–64. doi: 10.1016/j.jchromb.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Dixit V, Hariparsad N, Desai P, Unadkat JD. In vitro LC-MS cocktail assays to simultaneously determine human cytochrome P450 activities. Biopharm Drug Dispos. 2007;28:257–262. doi: 10.1002/bdd.552. [DOI] [PubMed] [Google Scholar]
- 9.Lee KS, Kim SK. Direct and metabolism-dependent cytochrome P450 inhibition assays for evaluating drug-drug interactions. J Appl Toxicol. 2011 doi: 10.1002/jat.1720. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Mugundu GM, Kirby BJ, Samineni D, Desai PB, Unadkat JD. Quantification of human hepatocyte cytochrome P450 enzymes and transporters induced by HIV protease inhibitors using newly validated LC-MS/MS cocktail assays and RT-PCR. Biopharm Drug Dispos. 2012;33:207–217. doi: 10.1002/bdd.1788. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Lechon MJ, Lahoz A, Castell JV, Donato MT. Evaluation of cytochrome P450 activities in human hepatocytes in vitro. Methods Mol Biol. 2012;806:87–97. doi: 10.1007/978-1-61779-367-7_7. [DOI] [PubMed] [Google Scholar]
- 12.Rhodes SP, Otten JN, Hingorani GP, Hartley DP, Franklin RB. Simultaneous assessment of cytochrome P450 activity in cultured human hepatocytes for compound-mediated induction of CYP3A4, CYP2B6, and CYP1A2. J Pharmacol Toxicol Methods. 2011;63:223–226. doi: 10.1016/j.vascn.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Feidt DM, Klein K, Hofmann U, Riedmaier S, Knobeloch D, Thasler WE, Weiss TS, Schwab M, Zanger UM. Profiling induction of cytochrome p450 enzyme activity by statins using a new liquid chromatography-tandem mass spectrometry cocktail assay in human hepatocytes. Drug Metab Dispos. 2010;38:1589–1597. doi: 10.1124/dmd.110.033886. [DOI] [PubMed] [Google Scholar]
- 14.Lahoz A, Donato MT, Montero S, Castell JV, Gomez-Lechon MJ. A new in vitro approach for the simultaneous determination of phase I and phase II enzymatic activities of human hepatocyte preparations. Rapid Commun Mass Spectrom. 2008;22:240–244. doi: 10.1002/rcm.3359. [DOI] [PubMed] [Google Scholar]
- 15.Gramignoli R, Green ML, Tahan V, Dorko K, Skvorak KJ, Marongiu F, Zao W, Venkataramanan R, Ellis EC, Geller D, Breite AG, Dwulet FE, McCarthy RC, Strom SC. Development and application of purified tissue dissociation enzyme mixtures for human hepatocyte isolation. Cell Transplant. 2011 doi: 10.3727/096368911X600939. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Brandon EF, Raap CD, Meijerman I, Beijnen JH, Schellens JH. An update on in vitro test methods in human hepatic drug biotransformation research: pros and cons. Toxicol Appl Pharmacol. 2003;189:233–246. doi: 10.1016/s0041-008x(03)00128-5. [DOI] [PubMed] [Google Scholar]
- 17.Hickman D, Wang JP, Wang Y, Unadkat JD. Evaluation of the selectivity of In vitro probes and suitability of organic solvents for the measurement of human cytochrome P450 monooxygenase activities. Drug Metab Dispos. 1998;26:207–215. [PubMed] [Google Scholar]
- 18.Chauret N, Gauthier A, Nicoll-Griffith DA. Effect of common organic solvents on in vitro cytochrome P450-mediated metabolic activities in human liver microsomes. Drug Metab Dispos. 1998;26:1–4. [PubMed] [Google Scholar]
- 19.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), and Center for Veterinary Medicine (CVM) Guidance for Industry-Bioanalytical Method Validation. 2001 http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf.
- 20.Palmer JL, Scott RJ, Gibson A, Dickins M, Pleasance S. An interaction between the cytochrome P450 probe substrates chlorzoxazone (CYP2E1) and midazolam (CYP3A) Br J Clin Pharmacol. 2001;52:555–561. doi: 10.1046/j.0306-5251.2001.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ono S, Hatanaka T, Hotta H, Tsutsui M, Satoh T, Gonzalez FJ. Chlorzoxazone is metabolized by human CYP1A2 as well as by human CYP2E1. Pharmacogenetics. 1995;5:143–150. doi: 10.1097/00008571-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 22.De Bock L, Boussery K, Colin P, De Smet J, T’Jollyn H, Van Bocxlaer J. Development and validation of a fast and sensitive UPLC-MS/MS method for the quantification of six probe metabolites for the in vitro determination of cytochrome P450 activity. Talanta. 2012;89:209–216. doi: 10.1016/j.talanta.2011.11.083. [DOI] [PubMed] [Google Scholar]
- 23.Hesse LM, Venkatakrishnan K, Court MH, von Moltke LL, Duan SX, Shader RI, Greenblatt DJ. CYP2B6 mediates the in vitro hydroxylation of bupropion: potential drug interactions with other antidepressants. Drug Metab Dispos. 2000;28:1176–1183. [PubMed] [Google Scholar]
- 24.Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206–1227. doi: 10.1016/s0149-2918(08)80047-1. [DOI] [PubMed] [Google Scholar]