Abstract
Background
Lower limb lymphedema (LLL) is a common complication of cancer treatment. The disease is chronic and progressive with no cure. Although a common and significant source of morbidity, the impact of this condition on health-related quality of life (HRQOL) has only recently been addressed. In effort to identify valid treatment strategies for LLL, we performed a systematic review, identifying studies describing HRQOL outcomes in patients with LLL secondary to cancer.
Methods and Results
Seven medical databases were searched to identify reports using validated Patient Reported Outcome (PRO) instruments on patients with cancer-related LLL. Studies were classified by levels of evidence set by the Agency for Healthcare Research and Quality (AHRQ) and evaluated using the Efficace criteria. 25 studies were identified, 6 met inclusion criteria. Levels of evidence included: no level I studies, level II (n=3), level III (n=1), and level 4 (n=2). 50% of studies were compliant with the Efficace criteria. 5 PRO HRQOL instruments were used, but only 1 was specific to cancer-related lymphedema. Treatment strategies assessed included complete decongestive physiotherapy (CDP), exercise, and compression bandaging. CDP yielded significant enhancements in HRQOL.
Conclusions
There is a deficit in high quality studies for HRQOL in patients with LLL secondary to cancer. Furthermore, of the studies present, most did not conform to guidelines set for assessment of HRQOL, nor did they use lymphedema condition specific PRO instruments. New measures specific to assessing LLL are necessary to gain more accurate evaluation of how this debilitating disorder affects HRQOL.
Introduction
Lymphedema is a chronic, debilitating disorder characterized by abnormal tissue swelling, adipose deposition, tissue fibrosis, and edema resulting from disruption, blockage, or genetic abnormalities of the lymphatic system. In the United States and Western countries, the most common cause of lymphedema is cancer treatment and is termed secondary lymphedema. Although lymphatic injury in these cases occurs most commonly as a result of lymph node removal, massive skin/subcutaneous tissue resections, particularly in combination with radiation therapy, can also result in significant lymphedema, even when lymph nodes are not disturbed. In fact, a recent meta analysis estimated that 1 in 6 patients who undergo cancer surgery for a variety of solid tumors including genitourinary cancers, melanoma, and sarcoma develop lymphedema.1
Breast cancer treatment has historically been the most common cause of secondary lymphedema due to the high incidence of these tumors, and the fact that a large proportion of patients undergo lymph node sampling or complete lymph node dissection. As a result, the vast majority of studies published to date have focused on diagnosis, treatment, and outcomes in patients with breast cancer related lymphedema.
However, lower extremity lymphedema also occurs commonly after treatment for a variety of tumors. In addition, the treatment of lower extremity lymphedema is arguably even more complicated than breast cancer-related lymphedema due to the dependent position of the leg, the larger volume of the lower extremity, and the effects of ambulation. Yet, despite the fact that lower extremity lymphedema is common and seemingly more morbid than upper extremity lymphedema, there are only a few studies that have analyzed diagnosis, treatment, and outcomes in this patient population.2–8 Therefore, the goal of the current study was to perform a systematic review of health-related quality of life (HRQOL) studies in patients with lower extremity lymphedema in order to summarize the known literature on the effects of lymphedema. In addition, we aimed to use validated standards including the Consensus-based Standards for the Selection of Health Measurement Instruments (COSMIN)9 and check lists such the Efficace criteria10 (Table 1) to assess the validity of these studies, identify potential biases, and define high quality measures. This study is an important first step in designing high quality, validated HRQOL studies designed to identify the true morbidity of lower extremity lymphedema.
Table 1.
Efficace Criteria Checklist for Evaluating HRQOL10
| HRQOL Topic | Answer | ||
|---|---|---|---|
| Conceptual | |||
| A priori hypothesis stated | Yes | No | N/A* |
| Rational for instrument reported | Yes | No | |
| Measurement | |||
| Psychometric properties reported | Yes | No | N/A† |
| Cultural validity verified | Yes | No | |
| Adequacy of domains covered | Yes | No | |
| Methodology | |||
| Instrument administration reported | Yes | No | |
| Baseline compliance reported | Yes | No | |
| Timing of assessments documented | Yes | No | |
| Missing data documented | Yes | No | |
| Interpretation | |||
| Clinical significance addressed | Yes | No | |
| Presentation of results in general | Yes | No | |
If a study explicitly states an exploratory HRQOL evaluation; †If the HRQOL measure is validated in the same population as the one of the trial.
Materials and Methods
Search strategy
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement11 as a guide to ensure that current standards for systematic review methodology were met. Systematic literature searches were performed in seven databases: PubMed (from 1966 to February, 2011); PsycINFO (Psychological Abstracts), (1966 to February, 2011); Web of Science (from 1966 to February, 2011); HAPI (Health and Psychosocial Instruments), from 1985 to February, 2011); Cochrane (from 1966 to February, 2011); CINAHL (Cumulative Index to Nursing and Allied Health Literature), (from 1981 to February, 2011); and EMBASE from (1966 to February, 2011).
We searched for articles of all languages. For the PubMed, EMBASE, CINAHL, PsycINFO, and Cochrane databases, both controlled vocabulary (Medical Subject Headings (MeSH) in PubMed and Cochrane; EMTREE in EMBASE; CINAHL Headings in CINAHL; and Subject Headings in PsycINFO), and keyword searches were carried out. For the Web of Science and HAPI databases, only keyword searches were conducted because neither database has a controlled vocabulary. Four categories of terms were searched: 1. Cancer; 2. Lymphedema; 3. Lower limbs; 4. Quality of life, including Emotions, Pain, Social Behavior, Self-Concept, and Outcome Assessment.
Relevant studies after application of eligibility criteria were selected by review of title and abstracts from a database using the search strategy described above and outlined in the PRISMA flow diagram (Fig. 1). Eligibility criteria included studies with validated patient reported outcome questionnaires measuring the quality of life of patients with cancer-related lower limb lymphedema (LLL) and articles in English. Studies presented in only abstract form or poster presentations were excluded. Each article that was selected for full-text review was hand searched for a review of references in order to find additional relevant articles (one additional article was found).
FIG. 1.
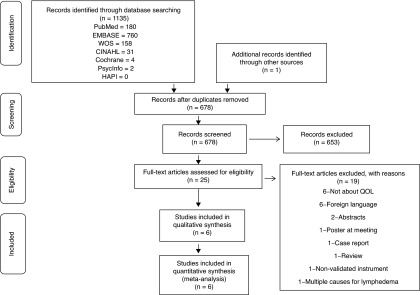
Flow diagram for systematic review methodology in line with PRISMA guidelines.11
Validity of HRQOL methodology
Patient Reported Outcome (PRO) measure questionnaires used in all articles were scrutinized with respect to the adequacy of their development and validation. Those with no evidence of formal development or validation were deemed ad hoc questionnaires and excluded from further analysis.
PRO measure development, validation, and psychometrics were evaluated using the COSMIN criteria (9). The Efficace criteria (Table 1) was used to assess the methodological quality of each study for its robustness. It includes 11 key HRQOL topics that are categorized into 4 areas; conceptual, measurement, methodology, and interpretation. In order for studies to yield robust outcomes, the authors state that there must be inclusion of 8 of the 11 topics, 3 of which must include the key topics of psychometrics analyzed, baseline compliance, and missing data reported (10).
Data extraction
Two independent authors (YC and CA) were blinded to each other whilst assessing the studies for inclusion. Studies that met selection criteria were evaluated and the data extracted onto a data extraction sheet we developed specific to this systematic review. The sheet was pilot-tested on 5 articles randomly selected and was adjusted as necessary. Information was grouped by reference/author, year, study design and evidence level, population, sample size, timings of assessment, HRQOL instrument, Efficace Criteria compliancy, use of psychometric analysis, and findings. Evidence levels were assigned as guided by the Agency for Healthcare Research and Quality (AHRQ) (Table 2).12 Selected articles were then compared and reviewed. Any discrepancies that arose were then reviewed and resolved by a third senior member of the research team (AP).
Table 2.
Description of Scientific Levels of Evidence and Corresponding Studies as Outlined by the AHRQ12
| Level of evidence | Description |
|---|---|
| Level 1 | Randomized controlled trials with adequate follow-up |
| Meta analysis of multiple randomized control trials | |
| Level 2 | Non-randomized, controlled prospective trial |
| Prospective cohort studies | |
| Level 3 | Well designed observational studies (e.g., comparative studies, correlation study, case control study) |
| Level 4 | Retrospective observational studies without controls |
| Case-series | |
| Level 5 | Expert opinions or committee recommendations |
Results
The search identified 1135 articles. Based on our exclusion criteria (non-English articles, studies using nonvalidated PRO measures, abstracts, poster presentations, case reports, and studies not including HRQOL), 25 were assessed for eligibility after which 1 additional study was identified. Six met inclusion criteria (studies with validated PRO measures assessing HRQOL in patients with cancer-related LLL and articles in English) and were assessed in detail to evaluate methodological quality, PRO measures, and fulfilment of COSMIN criteria via psychometric analysis and study findings (Table 3). One article that included patients with lower extremity lymphedema secondary to cancer but also ulceration was included, and we also included one study with both LLL and upper extremity lymphedema patients.
Table 3.
Assessing the Methodology of Studies Looking at QOL in LLL Patients
| Author | Year | Study design & Evidence levels | Population | Sample Size | Timings of assessments (after cancer diagnosis/treatment | HRQOL Instrument | Efficace Criteria compliant | Psychometric Analysis | Findings |
|---|---|---|---|---|---|---|---|---|---|
| Katz et al.2 | 2010 | Cohort (non-controlled) Level II |
Patients with LLL secondary to cancer treatment | 10 | Range - Not stated Mean - 13.05 years |
SF-36 | Yes | No | Weight lifting did not improve QOL in patients with LLL |
| Kim and Park13 | 2008 | Cohort II | Patients with LLL secondary to gynecological cancer within 5 years of treatment | 57 | Range - 2.4 years Mean - 0.3–4.8 years |
SF-36 | Yes | No | QOL increased significantly in patients with LLL after CDP within 1 month |
| De Vries et al.14 | 2009 | Cohort II | Patients having inguinal or axillary SLNB after stage I/II melanoma | 116 | Range - 4–94 months Median - 56.6 months |
EORTC-QLQ-C30 | No | No | No significant difference in QOL in melanoma patients with or without lymphedema after SLNB, but generally a better QOL compared to a normal population. |
| Franks et al.15 | 2005 | Prospective Observational III | Patients with LLL (15.2% with previous cancer, 30.4% with leg ulceration) | 164 | Range - Not given Mean - Not given |
SF-36 MOS-Short Form McGill pain questionnaire |
Yes | Yes | QOL improved significantly after 24 weeks in patients having treatment with compression bandaging, but improvement was greater in patients with leg ulceration then cancer patients |
| Carmelli & Bartoletti16 | 2011 | Retrospective cohort IV | Patients with melanoma and LLL (men and women) | 12 | Range - 1–4 years post diagnosis Mean - Not given |
IDI-ILA part II | No | Yes | Patients with active lifestyles reported the highest QOL outcomes and those with a BMI>26.5 had a significantly lower QOL after CDP. |
| Brouns et al.17 | 2008 | Retrospective IV | Patients having ilio-inguinal lymphadenectomy secondary to cancer | 62 | Range - 6–194 months Mean - 34 months |
EORTC-QLQ-C30 + WHO-ICF |
No | No | Patients having ilio-inguinal lymphadenectomy report a good QOL postoperatively. Lymphedema present or not has little or no impact on disability level or performing daily activities. |
CDP, complete decongestive physiotherapy; EORTC-QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core questionnaire version; IDI-ILA, Instituto Dermopatico Dell'Immacolata - Italian Lymphedema Association; LLL, lower limb lymphedema; QOL, quality of life; SF-36, Medical Outcome Study - Short Form, 3.0; SLNB, sentinel lymph node biopsy; WHO-ICF, World Health Organisation International Classification of Functioning disability and health).
Study quality
No level I studies were identified. Of the 6 studies assessed, 3 were level II,2,13,14 1 was a level III,15 and 2 were level IV studies.16,17 50% of studies were compliant with the Efficace criteria showing use of robust methodology; 2 level II,2,13 and 1 level III.15 Sample sizes ranged from 10–164 patients. One study was performed in 2011,16 1 in 2010,2 1 in 2009,14 2 in 2008,13,17 and 1 in 2005.15
PRO measure quality
Five PRO HRQOL measures were identified in the 6 studies; 3 were generic measures and 2 were oncology-specific measures. The generic measures included the MOS-Short Form McGill pain questionnaire, the World Health Organisation International Classification of Functioning disability and health (WHO-ICF) and the Medical Outcome Study- Short Form (SF-36). The SF-36 was used in 3 studies.2,13,15
The oncology-specific measures included the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core questionnaire version 3.0 (EORTC-QLQ-30) used in 2 studies,14,17 and the Instituto Dermopatico Dell'Immacolata–Italian Lymphedema Association part II (IDI-ILA part II) used in 1 study.16 Only 2 of the 6 studies (33%) used psychometric analysis.15,16 Most of the measures were not specific to lymphedema patients; therefore their efficacy in assessing HRQOL in these patients is limited.
Only 1 study was found to use a PRO measure that was specifically developed to measure HRQOL in patients with lymphedema secondary to melanoma, the IDI-ILA part II.16 The IDI-ILA was developed following review of the International Society of Lymphology Consensus document and was aimed directly for patients with lymphedema. The developers consisted of a mixture of 5 lymphedema or oncologist experts with backgrounds in lymphedema secondary to melanoma. It has two parts: part I consists of demographic and medical information and general health/medical status. Part II focuses on quality of life including 5 domains: physical ability, pain, fatigue, anxiety, and life satisfaction. The questionnaire was tested by specialists in melanoma and lymphedema and qualitatively assessed by three melanoma patients. It did undergo psychometric analysis that showed it had good sensitivity to change and reliability as validated on a sample of patients with cancer; however the article does not detail any of the psychometric analysis or findings.16
HRQOL outcomes measured
Four of the 6 studies (66%) focused on evaluating the impact of different treatment strategies on HRQOL in patients with LLL. Complete Decongestive Physiotherapy (CDP) was used twice, 1 level II study13 and 1 level IV study.16 One study used exercise (level II)2 and one used treatment with compression bandaging (level III).15
In both studies using CDP, patients had significant improvement in HRQOL and specifically, patients reporting a more active lifestyle were found to have higher QOL in all domains measured in the IDI-ILA part II, specifying less pain, fatigue, and anxiety. These patients were also found to have lower degrees of lymphedema present. Patients with BMI's more than 26.5 kg/m2 reported significantly decreased quality of life.16 Kim et al. found that at 1 month after CDP therapy, QOL improved significantly in domains of physical and social functioning, mental and general health, and the role physical domain compared to baseline.13
The exercise study found that weight lifting did not improve HRQOL with no significant changes in any of the domains measured by the SF-36.2
Usage of compression bandaging significantly improved HRQOL but this was greater in patients with lymphedema secondary to ulcers when compared to those whose lymphedema was secondary to cancer. Improvements were significant in the physical functioning, role physical, bodily pain, social functioning, role emotional and mental health domains of the SF-36. They also found that patients having active treatment had the most improvements but also, patients having no treatment also had considerable improvements in role physical and pain scores. They explained this as, perhaps those not having treatment had less severe forms of lymphedema, but the reasons remained ambiguous.15
One study looked at LLL development in patients who underwent inguinal lymph node dissection (ILND; level IV).17 Brouns reports that patients undergoing ILND presenting postoperatively with or without lymphedema had no significant differences in performing daily activities or with their disability level. Another study assessed the quality of life of patients undergoing axillary or inguinal sentinel lymph node biopsy (SLNB) (level II).14 No statistical significant differences were found with respect to HRQOL, pain, and daily activities between patients with lymphedema or those without lymphedema who underwent SLNB. They report findings that patients surviving melanoma after axillary or inguinal SLNB with or without completion lymph node dissection had better QOL then a normal population as measured by the EORTC-QLQ-C30. An explanation for this is that they included an exclusive cohort of melanoma patients with favorable prognosis, and also suggest could be due to a shift response, where the patients shift in how they appreciate and hence estimated their health status secondary to survival.14
Conclusions
The aim of this systematic review was to summarize the findings of published studies analyzing QOL outcomes of patients with cancer-related LLL. For each study we evaluated the quality of the publication with respect to its HRQOL methodology and PRO measurement. To our knowledge, this is the first comprehensive systematic review of QOL outcomes in this patient group.
We identified only 6 studies satisfying our inclusion criteria (validated PRO questionnaires measuring the HRQOL of patients with cancer-related LLL and articles in English). This finding is surprising since LLL is a significant and common source of morbidity in cancer survivors. Although the true incidence of LLL in this patient population remains unknown, it is likely that a significant number of patients are affected, since some reports have reported lymphedema in as many as 50% of patients. Moreover, it is likely that the incidence of LLL is significantly underestimated since the diagnosis is often missed or delayed. In other cases, diagnosis is complicated by simultaneous development of other disorders such as deep venous thrombosis that may independently cause lower limb swelling. The lack of recognition of LLL influences the psychosocial impact this condition has on patients. Patients have voiced recurrent frustrations with respect to the lack of treatment resources and lymphedema research and are eager for research to be undertaken in order to find and try new treatments.18
The studies we identified were composed mainly of evidence level II. Five of 6 studies (83%) were performed from 2008 onwards, showing an increase in awareness of this patient population in recent times. Only 3 of the 6 publications (50%)2,13,15 were found to have robust methodology as compliant by the Efficace criteria, only 1 of which used psychometric analysis.15 Our review therefore highlights the urgent need for additional future studies using established standards to analyze HRQOL of patients with LLL. This analysis would serve as an important step in analyzing and designing effective treatment strategies in this patient population.
Three studies reported an improvement in HRQOL after a treatment intervention (2 using CDP,13,16 1 using compression bandaging15) with most improvements in the domains of pain and physical functioning. Two studies reported no differences in HRQOL after SLNB14 or ILND,17 regardless of development of lymphedema. This finding is interesting since SLNB has been shown to be associated with significantly decreased rates of lymphedema as compared to ILND,4,19 suggesting that lymph node surgery alone has significant detrimental effects on postoperative HRQOL. Interestingly, we did not identify any studies evaluating the effect of surgical approaches for patients with lower limb lymphedema (e.g., lymph node transfer, lymphovenous bypass, liposuction) on HRQOL or patient-reported outcomes. Given that utilization of these techniques is increasing,20 it is imperative that the therapeutic efficacy is tested using validated patient reported outcome measures.
We also aimed to evaluate the quality of the PRO measures used in these studies. Five PRO measures were used. Most studies utilized well-established generic or cancer-specific PRO measures and did not include a condition-specific PRO measure for lymphedema. Only 1 PRO measure used was found to be specifically directed for the cancer-related lower limb lymphedema patient. Whilst the study reports that this measure is valid and reliable, it did not detail the psychometric analytical findings, therefore making acceptance of its validity and reliability difficult to interpret.
Due to the paucity of literature focusing on this topic, we included one study involving patients with LLL secondary to cancer but also with lymphedema secondary to ulceration, and one study that included patients with either upper or lower extremity lymphedema. Our review suggests that there is a deficit in high quality studies for HRQOL in patients with LLL secondary to cancer. This is both surprising and worrisome since the lower limb is the most commonly affected area in patients developing lymphedema worldwide. Furthermore, of the studies present, most did not conform to guidelines set for the assessment of HRQOL. We recommend that high level studies with large population sizes should be performed to bridge such a gap in our understanding of this disease. New measures specific to assessing this detrimental condition should be developed as set out by guidelines. With the information obtained from such studies, development of condition-specific measures would help in gaining more accurate evaluation of how LLL affects HRQOL. This would help to enhance patient education, therapeutic approaches, and professionals managing such patients, and also in the allocation of resources to improve the quality of life in these patients.
Disclosure Statement
Drs. Cemal, Albomoz, Pusic, Mehrara and Ms Jewell have no conflicts of interest or financial ties to disclose.
References
- 1.Cormier JN. Askew RL. Mungovan KS, et al. Lymphedema beyond breast cancer: A systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer. 2010;116:5138–5149. doi: 10.1002/cncr.25458. [DOI] [PubMed] [Google Scholar]
- 2.Katz E. Dugan NL. Cohn JC, et al. Weight lifting in patients with lower-extremity lymphedema secondary to cancer: A pilot and feasibility study. Arch Phys Med Rehabil. 2010;91:1070–1076. doi: 10.1016/j.apmr.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabel MS. Griffith KA. Arora A, et al. Inguinal node dissection for melanoma in the era of sentinel lymph node biopsy. Surgery. 2007;141:728–735. doi: 10.1016/j.surg.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Williams AF. Franks PJ. Moffatt CJ. Lymphoedema: Estimating the size of the problem. Palliat Med. 2005;19:300–313. doi: 10.1191/0269216305pm1020oa. [DOI] [PubMed] [Google Scholar]
- 5.Beesley V. Janda M. Eakin E, et al. Lymphedema after gynecological cancer treatment : Prevalence, correlates, and supportive care needs. Cancer. 2007;109:2607–2614. doi: 10.1002/cncr.22684. [DOI] [PubMed] [Google Scholar]
- 6.Lockwood-Rayermann S. Lymphedema in gynecologic cancer survivors: An area for exploration? Cancer Nurs. 2007;30:E11–18. doi: 10.1097/01.NCC.0000281734.48479.33. [DOI] [PubMed] [Google Scholar]
- 7.Lawenda BD. Mondry TE. Johnstone PA. Lymphedema: A primer on the identification and management of a chronic condition in oncologic treatment. CA Cancer J Clin. 2009;59:8–24. doi: 10.3322/caac.20001. [DOI] [PubMed] [Google Scholar]
- 8.Ward LC. Dylke E. Isenring E, et al. Reference ranges for assessment of unilateral lymphedema in legs by bioelectrical impedence spectroscopy. Lymphat Res Biol. 2011;9:43–6. doi: 10.1089/lrb.2010.0024. [DOI] [PubMed] [Google Scholar]
- 9.Mokkink LB. Terwee CB. Patrick DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: An international Delphi study. Qual Life Res. 2010;19:539–549. doi: 10.1007/s11136-010-9606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Efficace F. Bottomley A. Osoba D, et al. Beyond the development of health-related quality-of-life (HRQOL) measures: A checklist for evaluating HRQOL outcomes in cancer clinical trials—Does HRQOL evaluation in prostate cancer research inform clinical decision making? J Clin Oncol. 2003;21:3502–3511. doi: 10.1200/JCO.2003.12.121. [DOI] [PubMed] [Google Scholar]
- 11.Liberati A. Altman DG. Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Agency for Healthcare Research and Quality. www.ahrq.gov. 2011. www.ahrq.gov [DOI] [PubMed]
- 13.Kim SJ. Park YD. Effects of complex decongestive physiotherapy on the oedema and the quality of life of lower unilateral lymphoedema following treatment for gynecological cancer. Eur J Cancer Care (Engl) 2008;17:463–468. doi: 10.1111/j.1365-2354.2007.00877.x. [DOI] [PubMed] [Google Scholar]
- 14.de Vries M. Hoekstra HJ. Hoekstra-Weebers JE. Quality of life after axillary or groin sentinel lymph node biopsy, with or without completion lymph node dissection, in patients with cutaneous melanoma. Ann Surg Oncol. 2009;16:2840–2847. doi: 10.1245/s10434-009-0602-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franks PJ. Moffatt CJ. Doherty DC, et al. Assessment of health-related quality of life in patients with lymphedema of the lower limb. Wound Repair Regen. 2006;14:110–118. doi: 10.1111/j.1743-6109.2006.00099.x. [DOI] [PubMed] [Google Scholar]
- 16.Carmeli E. Bartoletti R. Retrospective trial of complete decongestive physical therapy for lower extremity secondary lymphedema in melanoma patients. Support Care Cancer. 2011;19:141–147. doi: 10.1007/s00520-009-0803-3. [DOI] [PubMed] [Google Scholar]
- 17.Brouns F. Donceel P. Stas M. Quality of life and disability after ilio-inguinal lymphadenectomy. Acta Chirurgica Belgica. 2008;108:685–690. doi: 10.1080/00015458.2008.11680316. [DOI] [PubMed] [Google Scholar]
- 18.Ridner SH. The psycho-social impact of lymphedema. Lymphat Res Biol. 2009;7:109–112. doi: 10.1089/lrb.2009.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James JH. Lymphoedema following ilio-inguinal lymph node dissection. Scand J Plastic Reconst Surg. 1982;16:167–171. doi: 10.3109/02844318209006586. [DOI] [PubMed] [Google Scholar]
- 20.Mehrara BJ. Zampell JC. Suami H, et al. Surgical management of lymphedema: Past, present and future. Lymphat Res Biol. 2011;9:159–167. doi: 10.1089/lrb.2011.0011. [DOI] [PubMed] [Google Scholar]


