Abstract
Objective
Identification of unanswered research questions about the management of gestational diabetes mellitus (GDM) is necessary to focus future research endeavors. We developed a process for elucidating the highest priority research questions on GDM.
Methods
Using a systematic review on GDM as a starting point, we developed an eight-step process: (1) identification of research gaps, (2) feedback from the review's authors, (3) translation of gaps into researchable questions using population, intervention, comparators, outcomes, setting (PICOS) framework, (4) local institutions' stakeholders' refinement of research questions, (5) national stakeholders' use of Delphi method to develop consensus on the importance of research questions, (6) prioritization of outcomes, (7) conceptual framework, and (8) evaluation.
Results
We identified 15 high priority research questions for GDM. The research questions focused on medication management of GDM (e.g., various oral agents vs. insulin), delivery management for women with GDM (e.g., induction vs. expectant management), and identification of risk factors for, prevention of, and screening for type 2 diabetes in women with prior GDM. Stakeholders rated the development of chronic diseases in offspring, cesarean delivery, and birth trauma as high priority outcomes to measure in future studies.
Conclusions
We developed an eight-step process using a multidisciplinary group of stakeholders to identify 15 research questions of high clinical importance. Researchers, policymakers, and funders can use this list to direct research efforts and resources to the highest priority areas to improve care for women with GDM.
Introduction
Gestational diabetes mellitus (GDM) is a common pregnancy complication, affecting about 7% of pregnancies in the United States, and its prevalence has been increasing.1,2 GDM is associated with both perinatal and longer-term maternal and offspring risks, such as cesarean delivery,3,4 fetal macrosomia,3,5 development of type 2 diabetes in the mother,6 and obesity in the offspring.7 Because of these risks and the potential implications of treatment,8,9 GDM is an important, emerging area for clinical, epidemiologic, and basic research. Notably, between 2001 and 2010, MEDLINE included >3000 citations indexed as “gestational diabetes mellitus” compared with <1700 citations in the prior decade. In addition, the majority of clinical trials on GDM have been published in the last 10 years.
In 2008, we completed an Agency for Healthcare Research and Quality (AHRQ)-funded systematic review on specific aspects of management of GDM.10 The review addressed 4 questions proposed by the American College of Obstetricians and Gynecologists (ACOG) because of their high clinical relevance: (I) What are the risks and benefits of an oral diabetes agent (e.g., glyburide), as compared with all types of insulin, for GDM? (II) What is the evidence that elective labor induction, cesarean delivery, or timing of induction is associated with benefits or harm to the mother and neonate? (III) What risk factors are associated with the development of type 2 diabetes after a pregnancy with GDM? (IV) What are the performance characteristics of diagnostic tests for type 2 diabetes in women with prior GDM?10 We identified 11,400 unique citations, independently reviewed titles, abstracts, and full articles and included 45 articles, which included 9 randomized controlled trials (RCT) that applied to review questions I and II.10 We graded the evidence as either insufficient or low strength for addressing the 4 review questions, suggesting widespread deficiencies in the field and the need for higher-quality studies to address the gaps.10 Although the review synthesized and graded the existing evidence, the next step of identifying and prioritizing research gaps was descriptive and not systematic, as few frameworks currently exist to inform this final process.11 AHRQ recognized that relying on systematic reviews to identify and report research needs was not sufficient and, thus, has funded various pilot studies, including the one reported here, to develop standard methods.
The primary objective of this study was to identify clinically important research questions for the management GDM using a process that involved stakeholder input and the 2008 systematic review's findings as a starting point. The secondary objectives were to prioritize outcomes to measure in future trials and to highlight feasibility and study design challenges related to the identified research questions. Ultimately, the goal was to guide future research endeavors on GDM management.
Materials and Methods
We developed and completed an eight-step process to identify research needs for GDM. We describe the process that began with the 2008 systematic review's 4 original questions, followed by the identification of research gaps and 17 research questions, and ending with 15 final questions, which multidisciplinary stakeholders deemed to have the highest clinical impact and potential benefit. We also described the methods in more detail in the final report prepared for AHRQ12 and in an article focused on the methods.13
Steps 1, 2, and 3: Identification of research gaps from review and formulation of 17 research questions
In step 1, two investigators independently abstracted statements about research gaps from the published AHRQ evidence report10 and five articles based on findings from the review.14–18 The two investigators compared and combined the lists using a consensus process. In step 2, we sought feedback from the eight authors of the 2008 systematic review via electronic communication. The authors provided free-text feedback about existing gaps identified within the review and suggested additional deficiencies, including challenges in the design of future studies. Readers are referred to the final report for a tabulation of all identified gaps.12 For step 3, our research team organized the list of gaps into the population, intervention, comparators, outcomes, setting (PICOS) framework. We then translated these gaps into 17 new research questions.
Step 4: Refinement of the 17 research questions by institutionally based local stakeholders
For step 4, we invited six stakeholders from our own institution (local stakeholders) with expertise in GDM research or patient care to provide feedback on the 17 research questions. A list of the local stakeholders is available in the final AHRQ report.12 This multidisciplinary group included two academic obstetricians, one dietitian whose clinical practice is focused on GDM, one epidemiologist whose research has focused on diabetes, and two members with insight into the patient perspective (a social worker and the director of a Medicaid case management program for women with complicated pregnancies), but no patients. Stakeholders first completed an online questionnaire in which we listed the 17 draft research questions and asked them to comment on (1) the clarity of the questions (or suggest alternate wording), (2) the clinical benefit and importance of addressing the questions, using a 9-point Likert scale, where 9 indicated highly clinically important/high clinical benefit and 1 indicated unlikely to have clinical importance or benefit, and (3) the feasibility for researchers to conduct a study that would address the research questions, using a 9-point scale, where 9 indicated highly feasible and 1 indicated unlikely to be feasible. We refined these 17 research questions based on the online feedback. The online questionnaire was developed using SurveyGizmo™ (Widgix LLC, Boulder, CO).
We invited the six local stakeholders to an interactive in-person meeting to solicit further feedback about missed research gaps and develop informal consensus on the wording, content of the research questions, and missed gaps. Finally, we asked the local stakeholders to consider study design needs and challenges for each of the research questions. Following step 4, we refined each question and added questions suggested by the stakeholders to yield 19 research questions.
Step 5: Consensus development by national stakeholders about research questions' clinical benefit/importance, leading to 15 final questions
We identified national leaders and experts in GDM and invited nine of them to be stakeholders for this project (national stakeholders). They represented the areas of obstetrics and gynecology, nutrition, epidemiology, research funders, and consumers. A list of the national stakeholders is available in the final AHRQ report.12 We used the Delphi method for consensus development using an online form. The Delphi method involves iterative rounds of responses by group members, providing aggregated feedback about other members' responses until consensus is reached.19 A priori we determined the maximum number of rounds to be three, because we believed, based on prior experience with the Delphi method, that consensus would be reached by then and to minimize stakeholder burden. For each round, we used an online instrument (SurveyGizmo) to gain feedback on the list of the research questions. As with the local stakeholders, we asked the national stakeholders to comment on the clarity of each question (or to suggest alternate wording) and the clinical benefit and importance of addressing each question, using the same 9-point Likert scale. We categorized clinical benefit/importance scores into high (between 7 and 9), medium (between 4 and 6), and low (between 1 and 3) clinical benefit/importance. We defined achievement of consensus for each research question as ≥75% (i.e., 7 of 9) of external stakeholders' ratings within the same single category. We refined all research questions based on comments. The research questions without consensus were retained and entered into the next round. In Delphi rounds 2 and 3, we also provided stakeholders with information about how the other stakeholders had scored the questions in the prior Delphi round (the mean and range of the clinical benefit/importance scores) as well as a brief synopsis of comments and changes we made to the questions. Following completion of step 5, we eliminated 4 of the 19 research questions, as 3 had failed to achieve consensus and 1 had achieved consensus but as having medium clinical benefit/importance.12
During Delphi round 1, national stakeholders additionally commented on the study needs, challenges, and feasibility issues that had been identified from the local stakeholders' meeting. Comments on study needs, challenges, and feasibility were collated with the feedback from the local stakeholder meeting and organized into the PICOS framework. We did not require consensus on these topics.
Step 6: Prioritization by national stakeholders of outcomes for the two questions on medication and delivery management
During Delphi round 3, national stakeholders completed step 6 of the process by prioritizing the maternal and fetal outcomes related to the research questions on medication and delivery management (which corresponded to the original review's questions I and II). We focused on these research questions because they had high potential for clinical benefit, were the most amenable to the clinical trial design, and had a long list of outcomes necessitating priority ranking. From a list of >20 possible outcomes that had been suggested in steps 1 through 4, each stakeholder ranked their top three outcomes that would be most important to include in a clinical trial that assessed medication and delivery management.
Steps 7 and 8: Refinement of 15 questions and evaluation of process by all participants
Step 7 involved the final refinement of the questions after Delphi round 3 and the development of a conceptual framework to display the results of the process, which included high priority questions and outcomes.
In step 8, we developed an online evaluation tool (using SurveyGizmo) and invited all systematic review authors (except the three who were involved with this project) and local and national stakeholders to evaluate the process. We asked them to comment on whether they had adequate information to participate effectively, which mode of participation they would have preferred (i.e., web-based survey, phone, in-person), whether they believed that we had accomplished our objective, whether the representation of the local and national stakeholder groups was sufficiently comprehensive, and whether we needed each of the eight steps in the process to accomplish our aim.
Results
Identification of 15 research questions with high clinical importance and benefit
We developed an eight-step process, using a systematic review on GDM10 as a starting point and incorporating feedback from multidisciplinary stakeholders to identify 15 high priority research questions on GDM. Table 1 displays the 15 questions and the mean and range of the clinical importance/benefit scores from the Delphi round where each achieved consensus. We organized the research questions by the 2008 review's questions' topics. Based on feedback from the local stakeholders, we added questions to address the prevention of type 2 diabetes in women with GDM and adherence to recommendations on postpartum screening for type 2 diabetes.
Table 1.
Final 15 Research Questions on Gestational Diabetes Mellitus with High Clinical Importance/Benefit
| |
Clinical importance/benefit scorea |
|
|---|---|---|
| Research questions | Mean | Range |
| Medication management of GDM | ||
| What are the comparative effectiveness and safety of | ||
| Sulfonylureas compared with any insulin | 8.2 | 7–9 |
| Metformin compared with any insulin | 7.9 | 6–9 |
| Various insulin regimens in terms of type/duration, dosing, and frequency of administration | 7.3 | 6–9 |
| Other drug classes (e.g., thiazolidinediones, DPP-4 inhibitors) compared with any insulin or oral agent | 6.9 | 4–9 |
| Delivery management for women with GDM | ||
| What are the comparative effectiveness and safety of | ||
| Elective labor induction at 40 weeks compared with expectant management | 7.8 | 6–9 |
| Elective cesarean delivery at 40 weeks compared with expectant management | 7.3 | 4–9 |
| Risk factors for type 2 diabetes | ||
| What is the evidence that each of the following factors is associated with the development of glucose intolerance and diabetes following a pregnancy with GDM? | ||
| Maternal health behaviors (e.g., breastfeeding, physical activity, diet) | 8.1 | 7–9 |
| Comorbid conditions (e.g., advanced age, obesity, hypertension) | 7.4 | 6–9 |
| Maternal metabolic measures (e.g., fasting insulin) | 7.3 | 6–8 |
| Family history, gene mutations, gene-environment interactions | 7.4 | 3–9 |
| Prevention of type 2 diabetes | ||
| What is the comparative effectiveness of the following in preventing type 2 diabetes, glucose intolerance, and obesity? | ||
| Lifestyle interventions (e.g., exercise, diet) | 7.7 | 6–9 |
| Educational and behavioral interventions (patient education on diabetes risk, lactation support) | 7.3 | 2–9 |
| Screening for type 2 diabetes | ||
| What are the performance characteristics of the following tests and what is the optimal screening interval? | ||
| Single fasting blood glucose compared with 2-hour oral glucose tolerance test | 6.7 | 1–9 |
| HbA1c testing compared with 2-hour oral glucose tolerance test | 7.9 | 6–9 |
| What is the comparative effectiveness of strategies to improve patient and clinician adherence with postpartum screening recommendations? | 7.8 | 5–9 |
The 4 research questions excluded and reason in parentheses:
1. What is the evidence that the interconception interval is associated with the risk of developing type 2 diabetes or glucose intolerance/impaired fasting glucose following GDM? (Consensus of medium level of importance)
2. What is the evidence that maternal psychosocial factors (e.g., mood disorders, substance use disorders, eating disorders, stress) are associated with the risk of developing type 2 diabetes or glucose intolerance/impaired fasting glucose following GDM? (No consensus achieved)
3. What is the evidence that contraceptive method (e.g., progestin-only) is associated with the risk of developing type 2 diabetes or glucose intolerance/impaired fasting glucose following GDM? (No consensus achieved)
4. What is the comparative effectiveness of health information technology interventions to track postpartum screening for the development of type 2 diabetes and glucose intolerance/impaired fasting glucose in women with a history of GDM? (No consensus achieved)
Score was on a scale of 1–9 where 1=lowest clinical importance or benefit and 9=highest clinical benefit or importance. Mean and range of scores are provided for the Delphi round in which the listed question achieved consensus.
DPP-4, Dipeptidyl-peptidase-4; HbA1c, hemoglobin A1c.
Regarding medication management of GDM, both local and national stakeholders agreed that the effectiveness and safety of oral hypoglycemic agents in pregnancy have not clearly been established, even though they are commonly used in clinical practice. Stakeholders rated comparisons of sulfonylureas with insulin as having the highest clinical benefit/importance, with a mean score of 8.2 on a scale of 1 (lowest) to 9 (highest). National stakeholders were also interested in examining the long-term effects of treatment on offspring, particularly metformin as an insulin sensitizer (Table 1).
National stakeholders reached consensus that identifying risk factors for type 2 diabetes in women with GDM was of high clinical importance and benefit, particularly to guide future preventive interventions. Research that addresses maternal health behaviors (e.g., breastfeeding, physical activity, diet) received the second highest clinical benefit/importance score out of the 15 questions (mean score 8.1). In Delphi round 1, national stakeholders also highlighted that future research on genetics, including the gene-environment interaction, would have high potential benefit. Although the local stakeholders with input into the patient perspective had suggested examining psychosocial factors (e.g., anxiety and postpartum depression) as risk factors for the development of type 2 diabetes, national stakeholders rated these questions with low clinical importance/benefit; thus, they were excluded from the final list (Table 1).
The two research questions addressing delivery management for women with GDM achieved consensus as having high clinical benefit and importance because of a dearth of evidence in this clinically important area. Both local and national stakeholders emphasized the importance of including patient-oriented outcomes for these questions, such as satisfaction with delivery care.
Finally, stakeholders generally agreed that patient and provider adherence to postpartum testing recommendations has higher clinical importance than assessment of the performance characteristics of the various screening tests.
Prioritization of outcomes for future studies of medication or delivery management
Table 2 lists the highest priority maternal and offspring outcomes, defined as appearing in the top 3 list of two or more of the nine national stakeholders. These outcomes were identified as being high priority for future studies assessing the impact of medication or delivery management on GDM. When assessing the impact of medication management, four of nine stakeholders ranked the long-term offspring outcome of chronic diseases (e.g., obesity, type 2 diabetes) as one of their top 3, making it the highest rated outcome. The next most highly rated outcomes were the short-term maternal outcomes of hypertensive disorders of pregnancy (e.g., gestational hypertension, preeclampsia) and medication adherence and the neonatal outcomes of large for gestational age and macrosomia.
Table 2.
Highest Priority Outcomes to Assess Impact of Medication or Labor Management in Gestational Diabetes Mellitus
| Outcome type | No. of stakeholders who ranked outcome among top 3 |
|---|---|
| Medication management of GDM | |
| Short-term outcomes | |
| Hypertensive disorders of pregnancy (e.g., gestational hypertension, preeclampsia) | 3 |
| Medication adherence | 3 |
| Large for gestational age and macrosomia | 3 |
| Gestational weight gain | 2 |
| Neonatal hypoglycemia | 2 |
| Neonatal intensive care unit admission | 2 |
| Long-term outcomes | |
| Chronic disease incidence in offspring (e.g., obesity, type 2 diabetes) | 4 |
| Postpartum incident type 2 diabetes mellitus or glucose intolerance/impaired fasting glucose | 2 |
| Delivery management for women with GDM | |
| Short-term outcomes | |
| Cesarean delivery (including primary cesarean and repeat cesarean) and indication for cesarean deliverya | 6 |
| Birth trauma (e.g., bone fractures, brachial plexus palsy) | 4 |
| Neonatal intensive care unit admission | 3 |
| Patient-reported outcomes (e.g., patient preference, quality of life) | 2 |
| Complications of cesarean delivery (e.g., wound infection, wound dehiscence) | 2 |
| Vaginal delivery (spontaneous, operative) | 2 |
| Hypoxia/anoxia | 2 |
| Respiratory distress syndrome | 2 |
Defined as≥two of nine national stakeholders ranked it among top 3 outcomes to measure in future studies.
The outcomes that were not ranked in the top 3 by≥two of nine stakeholders were:
Medication management of GDM—glycemic control, patient-reported outcomes (e.g., patient treatment preference, quality of life), cesarean delivery and indication, complications of cesarean delivery (e.g., wound infection), other obstetric complications (e.g., birth trauma, shoulder dystocia, perineal lacerations, postpartum hemorrhage), neonatal complications (e.g., hypoxia/anoxia, hypoglycemia, respiratory distress syndrome, hyperbilirubinemia), peripartum mortality, birth weight, postpartum weight retention, longer-term infant, and child growth.
Delivery management of GDM—resource utilization (e.g., cost of care, length of stay), peripartum mortality, birth weight (and large or small for gestational age, macrosomia), other obstetric complications (e.g., perineal lacerations, postpartum hemorrhage, pulmonary embolism), other neonatal complications (e.g., neonatal hypoglycemia, perinatal mortality).
When assessing the impact of delivery management, the highest priority outcome, for which six of nine stakeholders ranked as one of the top 3 outcomes, was cesarean delivery, including primary and repeat cesarean delivery as well as the indications for cesarean delivery (e.g., suspected macrosomia, birth weight). Cesarean delivery was followed by the neonatal outcomes of birth trauma, highly ranked by four of nine stakeholders, and neonatal intensive care unit admission, highly ranked by three of nine stakeholders.
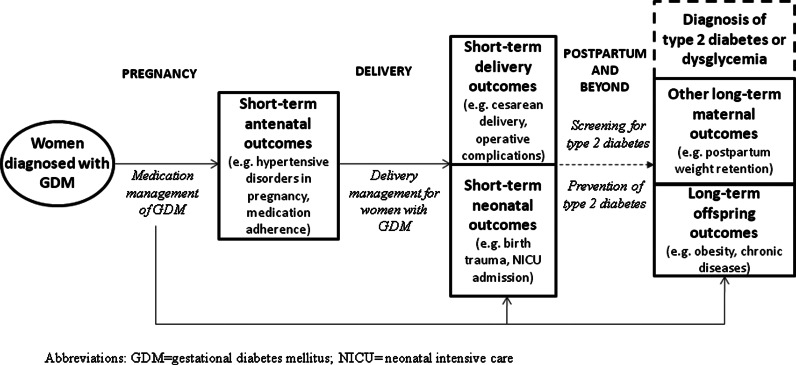
Conceptual framework
Figure 1 displays the conceptual framework to illustrate the results of the process. The framework displays the highly clinically important research questions addressing the management of GDM during pregnancy, delivery, and the postpartum period, as well as the high priority outcomes.
FIG. 1.
Conceptual framework displaying high priority research needs (in italics) addressing pregnancy, delivery, and postpartum management of gestational diabetes mellitus (GDM), with examples of high priority outcomes. NICU, neonatal intensive care unit.
Feasibility and study design challenges
At each step of the process, report authors, local stakeholders, and national stakeholders commented on the feasibility of addressing the research gaps, study design needs, and potential challenges. Where possible, we organized and presented these comments in the PICOS framework. Overall, stakeholders concurred that it would be feasible to design studies to address the research gaps in GDM. In terms of selecting a population, stakeholders noted the need for racial diversity and applying standard methods for the diagnosis of GDM. In terms of interventions, stakeholders noted a need for pharmacologic and pharmacokinetic studies, including the development of animal models, to examine the effects of oral medications on the fetus and neonate. When considering comparators of interest, stakeholders noted challenges with designing an RCT to address the comparative effectiveness of delivery management strategies. They discussed the potential risk of high crossover between arms because of patient and provider preference for delivery mode (Table 1). To address medication comparisons, they suggested a need for large, well-designed prospective observational studies in large medical centers (or collaborating centers), with stratification by GDM therapies (e.g., diet vs. insulin). Such studies could be completed in a shorter period of time than a trial, thus mitigating the impact of changing practice patterns, and could lead to long-term prospective cohorts to examine both high priority maternal and offspring outcomes (Table 2). Finally, stakeholders highlighted the need for future studies to apply standard outcome definitions, such as for hypoglycemia, and to aim for complete, consistent outcome ascertainment to improve the ability to make comparisons between studies.
Evaluation of the eight-step process
All 20 contributors, including 5 authors of the original systematic review, 6 local stakeholders and 9 national stakeholders, completed evaluations on the eight-step process and their involvement. Saldanha et al.13 included a complete description of the evaluation results. After review of the final list of 15 research questions and a summary of the eight-step process, evaluators responded that they had “adequate information to effectively participate” in the process and that our research team “had accomplished the objective of identifying important research questions for GDM.”
Discussion
Using a systematic review on GDM as a starting point, we developed an eight-step process, which included stakeholder feedback to identify and prioritize 15 clinically important research questions in GDM. The research questions focused on medication management of GDM, delivery management for patients with GDM, identification of risk factors for type 2 diabetes in women with prior GDM, prevention of type 2 diabetes and other chronic diseases in women with prior GDM, and postpartum screening for type 2 diabetes. Stakeholders expressed high levels of interest in examining medication comparisons, particularly sulfonylureas vs. insulin, and the influence of maternal lifestyle factors in the subsequent development of type 2 diabetes. In addition, stakeholders ranked long-term offspring chronic disease, cesarean delivery, and birth trauma as high priority outcomes to include when designing clinical trials and proposed key study design elements to increase research yield.
To our knowledge, ours is the first study to develop a method for identification of high priority research questions in the field of GDM. Prior research on identification of research needs in other disease areas has focused on the prioritization20 or the presentation of research needs.21 Recently, members of our team (K.A.R., I.J.S.) developed other frameworks to identify research needs using evidence-based clinical practice guidelines22 and systematic reviews11 but did not involve stakeholders. Our process for identifying high priority research questions has implications for a broader application beyond the field of GDM by using systematic reviews to identify future research needs and formulate researchable questions with stakeholder input. We invited committed, multidisciplinary stakeholders to comment on and refine the questions to ensure a balanced and broad perspective on research needs in GDM. Stakeholders were able to efficiently use the Delphi method to share comments and classify the clinical benefit of the questions to come to consensus.
We believe that these 15 high priority research questions will be useful to GDM researchers, funders, and policymakers. Based on the low and insufficient quality of evidence in the published systematic review,10 substantial gaps in the evidence exist. GDM continues to be a very active area of research, with several major treatment trials published,8,23,24 as well as ongoing innovative trials on diabetes prevention in women with recent GDM.25 In addition, policymakers are particularly interested in boosting funding in GDM. In 2010, the U.S. House of Representatives passed the Gestational Diabetes Act (GEDI Act) to increase funding to the Centers for Disease Control and Prevention (CDC) for research in GDM.26 The 15 questions we identified can provide a tool for researchers and funders planning high impact grants to target areas with existing gaps and where results are likely to be quickly translated into clinical practice. Of particular interest, with high potential for clinical benefit, are trials comparing medications for treatment of GDM, with attention to outcomes that are not traditionally measured, such as medication adherence, comorbid hypertensive disorders in pregnancy, and longer-term offspring outcomes. Studies examining the role of maternal lifestyle factors in the development of type 2 diabetes will also have high clinical impact, if funded.
Several limitations to our study deserve mention. First, we invited nonresearch-oriented clinicians who work closely with patients, but their feedback may have been limited by the complexity of the research in the project and amount of information presented from the 2008 review. Although we did not include patients in the process we reported here, developing methods that involve patients in identifying and prioritizing research gaps is extremely important. The local stakeholders' group proposed questions about psychosocial factors, which would have provided insight from the patients' perspectives. Unfortunately, these questions were not deemed of high clinical importance in subsequent Delphi rounds, highlighting the necessity for a systematic and effective way to elicit and incorporate patients' perspectives without risking their input being superseded by other stakeholders. Second, the eight steps were resource intense and may not be feasible or practical to conduct following all systematic reviews. Third, there may be additional useful steps to refine these research needs and questions, as well as to identify possible study designs for future research. For example, additional steps may include an update of the literature search in the 2008 systematic review, a search for ongoing studies, or more systematic discussion about appropriate study designs and the feasibility of addressing the 15 questions with involved stakeholders. Fourth, because we used the 2008 systematic review as a starting point, our project had a similar scope and, thus, did not assess research needs related to screening or diagnostic criteria for GDM, which are important, actively debated topics and will be addressed in an upcoming AHRQ evidence report and NIH consensus panel in the fall of 2012.27 We believe our findings to be useful and significant, however, as the topics addressed in the 2008 review were suggested by the ACOG because of their high clinical relevance, and our process engaged multiple stakeholders beyond the results of the 2008 review.
Conclusions
Using a comprehensive systematic review on GDM as a starting point, we developed an eight-step process and identified 15 research questions on GDM management considered of high clinical importance by a multidisciplinary group of stakeholders. We prioritized outcomes to be examined in future studies on medication and delivery management. We anticipate that our process could be used as is or modified as necessary as a model for taking the results of other systematic reviews to the next stage, toward identification of researchable questions in areas of highest clinical importance. These 15 questions can be used by researchers and funders to target areas of highest need for future research in GDM to make a significant clinical impact in this rapidly growing field.
Acknowledgments
This project was funded under Contract No. HHSA 290-2007-10061-I from the Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services. The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by the Agency for Healthcare Research and Quality or the U.S. Department of Health and Human Services.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Ferrara A. Kahn HS. Quesenberry CP. Riley C. Hedderson MM. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991–2000. Obstet Gynecol. 2004;103:526–533. doi: 10.1097/01.AOG.0000113623.18286.20. [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D. Snell-Bergeon JK. Hartsfield CL, et al. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM screening program. Diabetes Care. 2005;28:579–584. doi: 10.2337/diacare.28.3.579. [DOI] [PubMed] [Google Scholar]
- 3.Xiong X. Saunders LD. Wang FL. Demianczuk NN. Gestational diabetes mellitus: Prevalence, risk factors, maternal and infant outcomes. Int J Gynaecol Obstet. 2001;75:221–228. doi: 10.1016/s0020-7292(01)00496-9. [DOI] [PubMed] [Google Scholar]
- 4.Casey BM. Lucas MJ. Mcintire DD. Leveno KJ. Pregnancy outcomes in women with gestational diabetes compared with the general obstetric population. Obstet Gynecol. 1997;90:869–873. doi: 10.1016/s0029-7844(97)00542-5. [DOI] [PubMed] [Google Scholar]
- 5.Pettitt DJ. Knowler WC. Baird HR. Bennett PH. Gestational diabetes: Infant and maternal complications of pregnancy in relation to third-trimester glucose tolerance in the Pima Indians. Diabetes Care. 1980;3:458–464. doi: 10.2337/diacare.3.3.458. [DOI] [PubMed] [Google Scholar]
- 6.Kim C. Newton KM. Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 7.Gillman MW. Rifas-Shiman S. Berkey CS. Field AE. Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics. 2003;111:e221–226. doi: 10.1542/peds.111.3.e221. [DOI] [PubMed] [Google Scholar]
- 8.Crowther CA. Hiller JE. Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 9.HAPO Study Cooperative Research Group. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Int J Gynaecol Obstet. 2002;78:69–77. doi: 10.1016/s0020-7292(02)00092-9. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson WK. Wilson LM. Witkop CT, et al. Therapeutic management, delivery, and postpartum risk assessment and screening in gestational diabetes. Evid Rep Technol Assess. 2008;162:1–96. [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson KA. Saldanha IJ. McKoy NA. Development of a framework to identify research gaps from systematic reviews. J Clin Epidemiol. 2011;64:1325–1330. doi: 10.1016/j.jclinepi.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Bennett WL. Nicholson WK. Saldanha IJ. Wilson LM. Mckoy NA. Robinson KA. Rockville, MD: Agency for Healthcare Research and Quality; 2010. Future research needs for the management of gestational diabetes. [PubMed] [Google Scholar]
- 13.Saldanha IJ. Wilson LM. Mckoy NA. Bennett WL. Nicholson WK. Robinson KA. Identification of research needs from a systematic review: A pilot study. J Clin Epidemio. (in press). [Google Scholar]
- 14.Baptiste-Roberts K. Barone BB. Gary TL, et al. Risk factors for type 2 diabetes among women with gestational diabetes: A systematic review. Am J Med. 2009;122:207–214.e4. doi: 10.1016/j.amjmed.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett WL. Bolen S. Wilson LM. Bass EB. Nicholson WK. Performance characteristics of postpartum screening tests for type 2 diabetes mellitus in women with a history of gestational diabetes mellitus: A systematic review. J Womens Health. 2009;18:979–987. doi: 10.1089/jwh.2008.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golden SH. Bennett WL. Baptist-Roberts K, et al. Antepartum glucose tolerance test results as predictors of type 2 diabetes mellitus in women with a history of gestational diabetes mellitus: A systematic review. Gend Med. 2009;6(Suppl 1):109–122. doi: 10.1016/j.genm.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Witkop CT. Neale D. Wilson LM. Bass EB. Nicholson WK. Active compared with expectant delivery management in women with gestational diabetes: A systematic review. Obstet Gynecol. 2009;113:206–217. doi: 10.1097/AOG.0b013e31818db36f. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson W. Bolen S. Witkop CT. Neale D. Wilson L. Bass E. Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: A systematic review. Obstet Gynecol. 2009;113:193–205. doi: 10.1097/AOG.0b013e318190a459. [DOI] [PubMed] [Google Scholar]
- 19.Delbecq A. Van de Ven A. Gustafson D. Group techniques for program planning: A guide to nominal group and Delphi processes. In: Linstone HA, editor; Turoff M, editor. The Delphi Method: Techniques and applications. Reading, MA: Addison-Wesley Educational Publishers Inc.; 1975. [Google Scholar]
- 20.Chalkidou K. Whicher D. Kary W. Tunis S. Comparative effectiveness research priorities: Identifying critical gaps in evidence for clinical and health policy decision making. Int J Technol Assess Health Care. 2009;25:241–248. doi: 10.1017/S0266462309990225. [DOI] [PubMed] [Google Scholar]
- 21.Brown P. Brunnhuber K. Chalkidou K, et al. How to formulate research recommendations. BMJ. 2006;333:804–806. doi: 10.1136/bmj.38987.492014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson KA. Saldanha IJ. Mckoy NA. Identification of research gaps from evidence-based guidelines: A pilot study in cystic fibrosis. Int J Technol Assess Health Care. 2011;27:247–252. doi: 10.1017/S0266462311000225. [DOI] [PubMed] [Google Scholar]
- 23.Landon MB. Spong CY. Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langer O. Conway DL. Berkus MD. Xenakis EM. Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343:1134–1138. doi: 10.1056/NEJM200010193431601. [DOI] [PubMed] [Google Scholar]
- 25.Clinicaltrials.gov. Diabetes prevention in women with a recent history of gestational diabetes mellitus (GDM) identifier: NCT01158131. [Feb 1;2012 ].
- 26.Congressional research service summary: H.R. 5354: GEDI Act. www.govtrack.us/congress/bill.xpd?bill=h111-5354&tab=summary. [Feb 1;2012 ]. www.govtrack.us/congress/bill.xpd?bill=h111-5354&tab=summary
- 27.National Institutes of Health Consensus Development Program. Bethesda, MD: Oct 29–31, 2012. 2012. [Feb 1;2012 ]. NIH Consensus Development Conference: Diagnosing gestational diabetes mellitus. [Google Scholar]