Abstract
Peripheral artery disease (PAD) is a common progressive atherosclerotic occlusive disease that causes insufficient blood flow to the lower extremities. The symptom that health care professionals most often associate with PAD is claudication. However, patient reporting of claudication is highly variable. A structured literature review was conducted to evaluate how PAD symptoms are identified, defined, and categorized. This review focuses on the development and performance characteristics of PAD symptom questionnaires and the identification of a spectrum of leg symptoms beyond classic claudication. Additionally, potential confounders of PAD symptom report and strategies for a more comprehensive assessment of PAD symptoms are discussed. Overall, there is a lack of consistency in the utilization of PAD claudication questionnaires which impacts PAD symptom reporting and categorization. Based on this review, atypical symptoms are commonly reported, but poorly understood. Additional research is needed to gain a better understanding of the presentation of atypical symptoms, as well as the role of age, gender, race, and comorbid conditions on the symptom experience of patients with PAD.
Peripheral artery disease (PAD) is a progressive atherosclerotic occlusive disease that causes insufficient blood flow to the lower extremities and can result in debilitating, activity-induced, pain even while walking short distances. Estimates vary widely, but currently it is estimated that over 8 million Americans are afflicted with PAD.1–3 The prevalence has been shown to increase with age, particularly in individuals aged 60 years and older.4,5 Therefore, as the population ages, PAD will become increasingly prevalent. Despite the high prevalence of PAD, it remains largely underdiagnosed and undertreated.2,6 Evidence suggests the underutilization of inexpensive and widely available diagnostic screening tools,7 guideline-recommended treatments,8 and lifestyle modifications.8 Early detection of PAD is crucial for timely treatment and prevention of amputation, heart attack, stroke, and death.9–12 Individuals with PAD have 4 to 5 times the risk of dying of a cardiovascular event compared to those without PAD, which translates into a mortality risk that is 2 to 3 times higher.13,14
The presentation and progression of PAD is varied. Some individuals remain asymptomatic despite disease progression, while others consistently experience discomfort upon exertion that subsides when activity ceases. Critical limb ischemia (CLI) is the most severe form of PAD. Individuals with CLI typically experience severe leg pain even while resting that usually occurs in the feet or toes. However, for some individuals with CLI, the first sign of the disease is the presence of tissue loss.15 In patients with CLI, blood flow to the lower extremities is severely reduced, resulting in chronic non-healing wounds and tissue necrosis that if left untreated can lead to amputation.
PAD symptoms have been assessed through a series of questionnaires that have evolved over time.16–18 Of the symptoms reported by individuals with PAD, the symptom that health care professionals most often associate with the disease is claudication, also referred to as classic claudication, Rose intermittent claudication (Rose IC), intermittent claudication (IC), or definite claudication.16 This has been classically defined as a painful, aching, cramping, or tired feeling in the calves that occurs during walking, does not begin at rest, does not subside if walking continues, and is relieved within 10 minutes or less when activity ceases. In this paper, this specific symptom presentation will be referred to as classic claudication. It is the PAD symptom that usually triggers confirmatory diagnostic testing,19 most commonly the ankle-brachial index (ABI), which is the ratio of systolic ankle versus brachial pressure.
Classic claudication, as measured by a variety of questionnaires, is only reported in 7.5%20 to 33%18,21,22 of PAD patients. Thus, heavy reliance on this symptom for screening and detection can result in mis- or under-diagnosis of this serious disease. This under-diagnosis allows the disease to progress undetected, leading to increased morbidity and mortality. In order to increase accurate and timely diagnosis and clinical treatment for the more than 8 million Americans afflicted with PAD, it is necessary to gain a greater understanding of the array of symptoms experienced, including not only classic claudication, but other symptoms that are currently considered an atypical presentation of the disease.
This review critically evaluates how PAD symptoms are identified, defined, and categorized. It focuses on the development and performance characteristics of PAD symptom questionnaires and the identification of a spectrum of leg symptoms beyond classic claudication. Additionally, potential confounders of PAD symptom report and strategies for a more comprehensive assessment of PAD symptoms are discussed.
Methods
Four electronic databases were used for this review: CINAHL, MEDLINE, The Cochrane Library, and Digital Dissertations, utilizing the following keywords: peripheral vascular disease, peripheral artery disease, atherosclerosis, diagnosis, recognition, ankle-brachial index, questionnaires, experience, symptom(s), prevalence, atypical, claudication, intermittent claudication, pain, and asymptomatic. Limits included English language, humans, and adults. No date limits were set and electronic searches were supplemented by cross-referencing. Only empirical studies describing the breakdown of symptom reporting into multiple categories beyond classic claudication prevalence (e.g. Rose claudication and atypical claudication) were included, with the exception of the first claudication questionnaire developed.16 Studies focused solely on PAD prevalence, classic claudication prevalence, quality of life, or asymptomatic disease were excluded. Symptom confounders were of interest, but were not part of the inclusion and exclusion criteria.
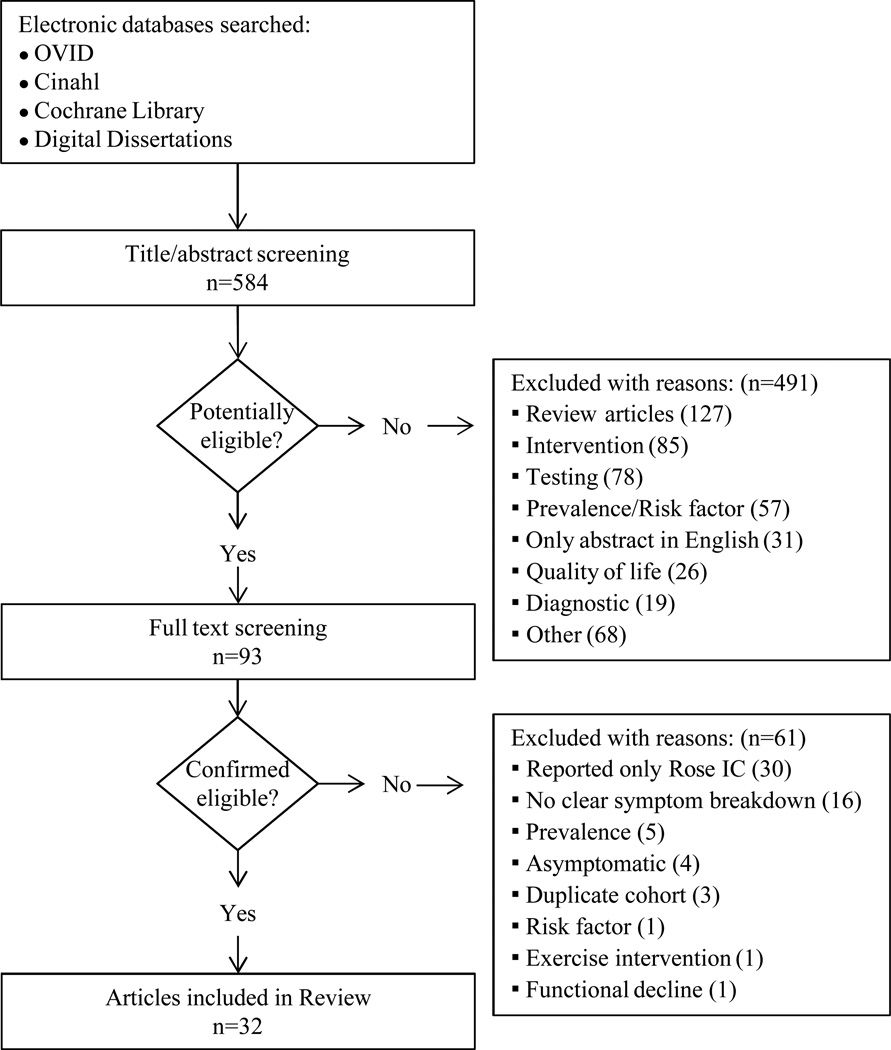
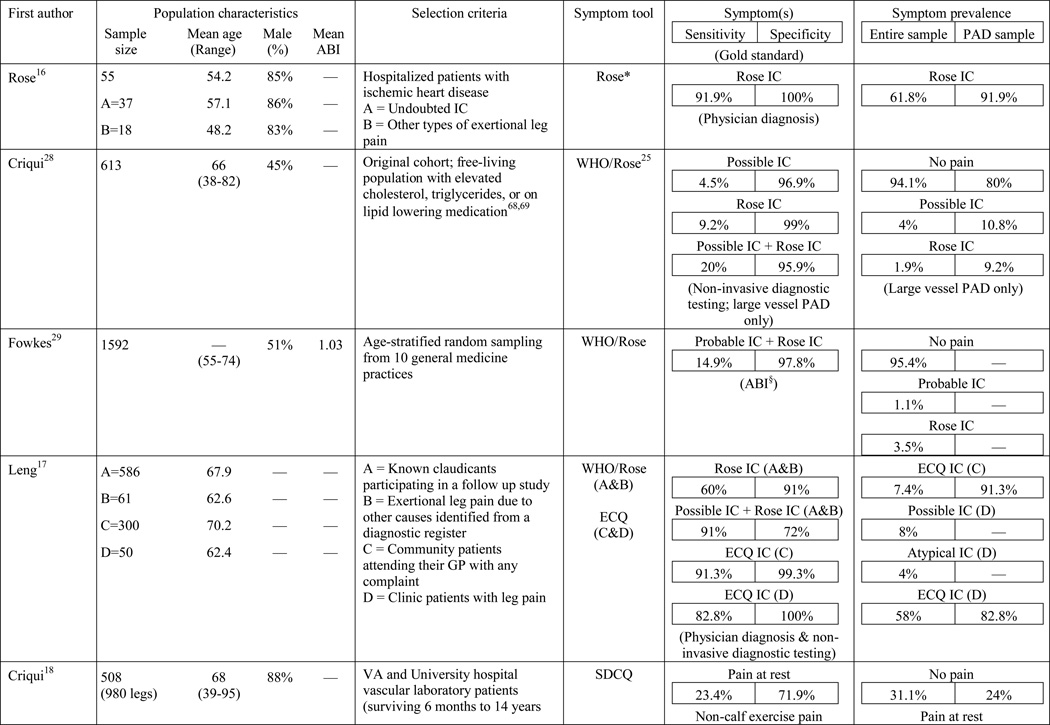
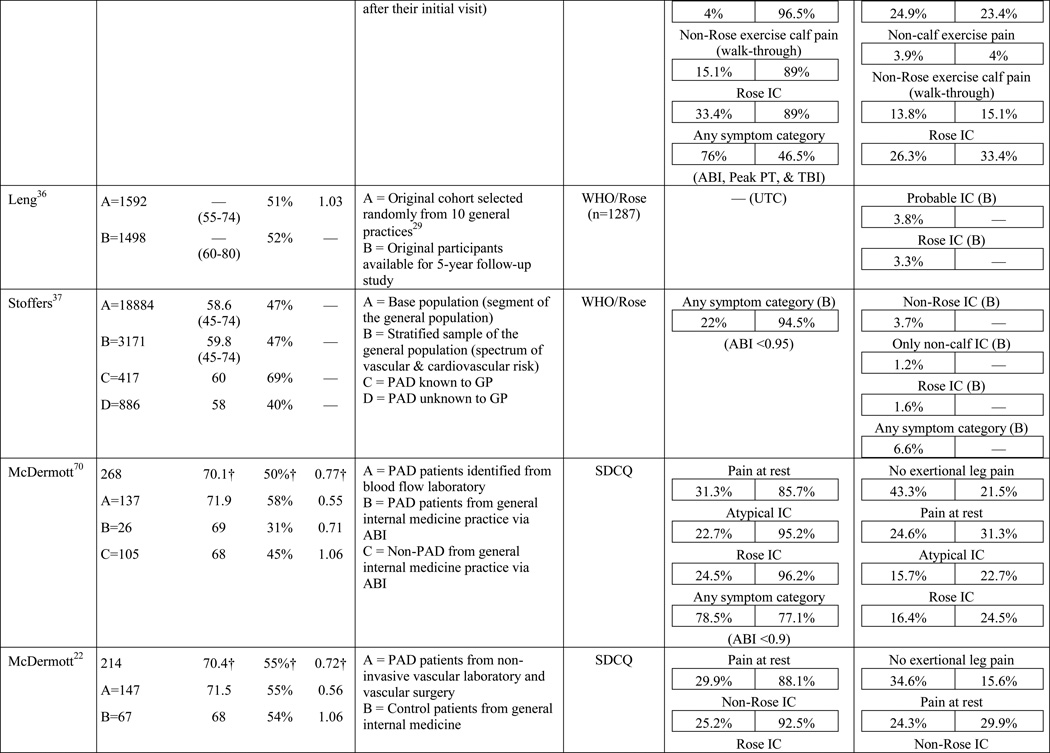
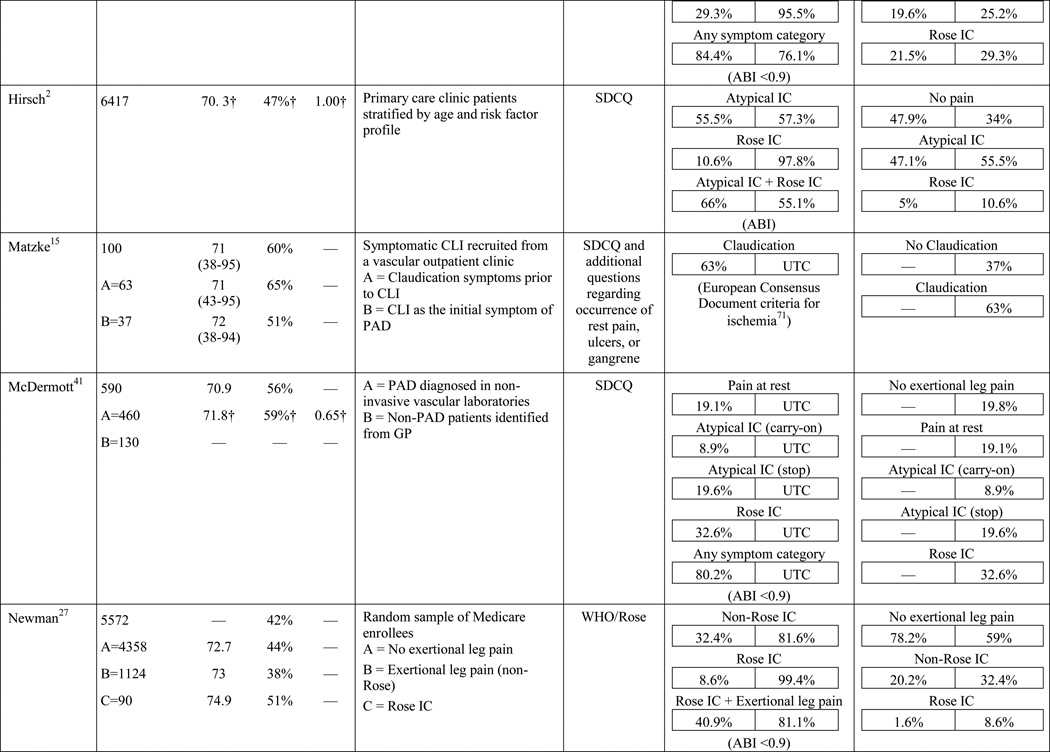
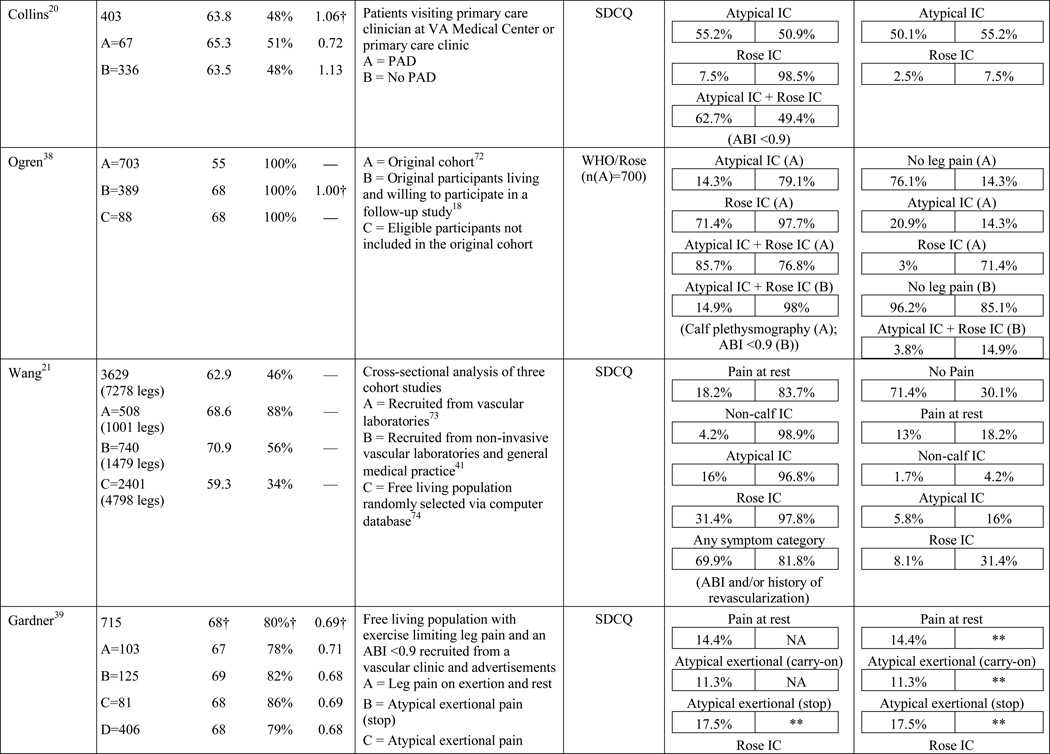
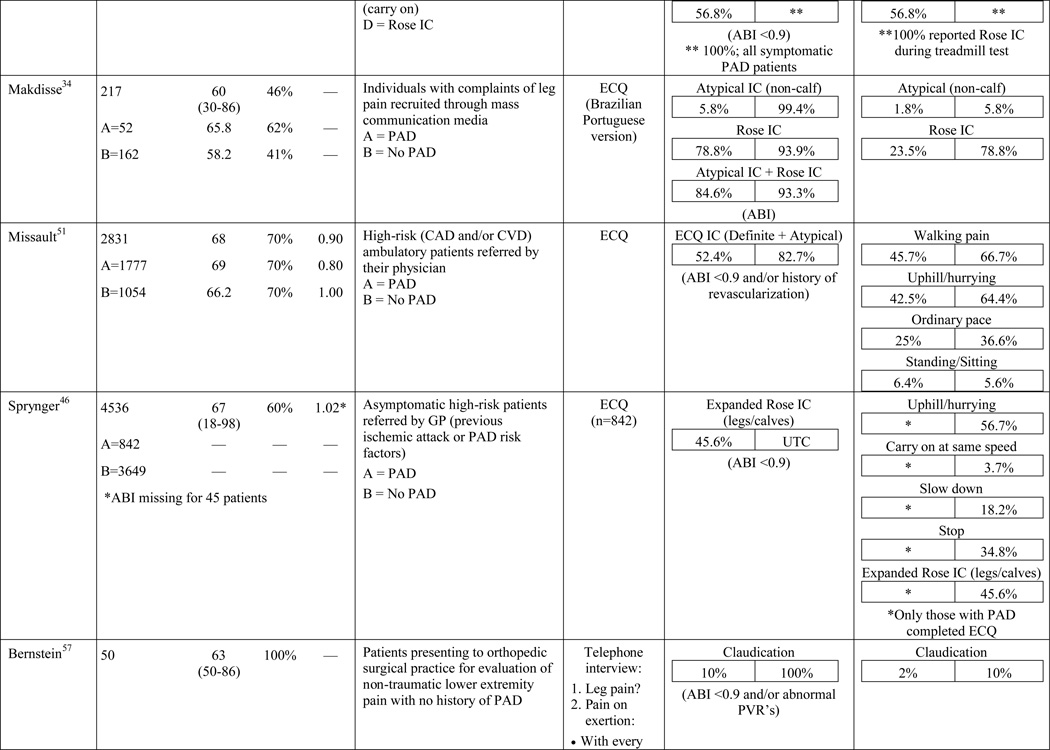
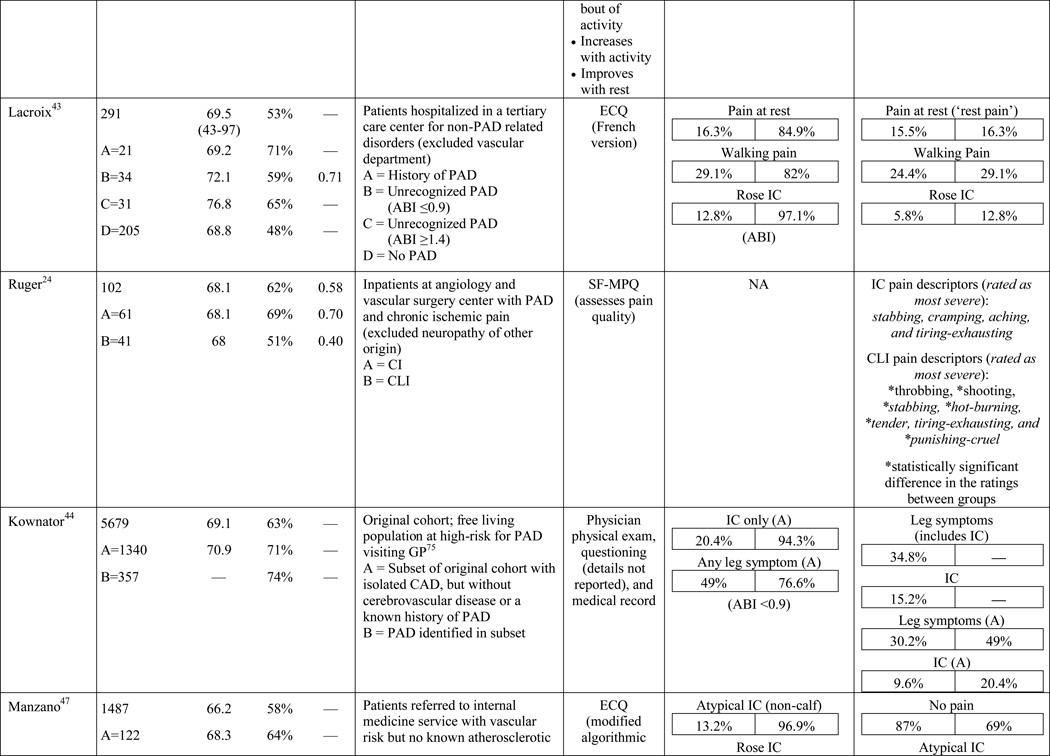
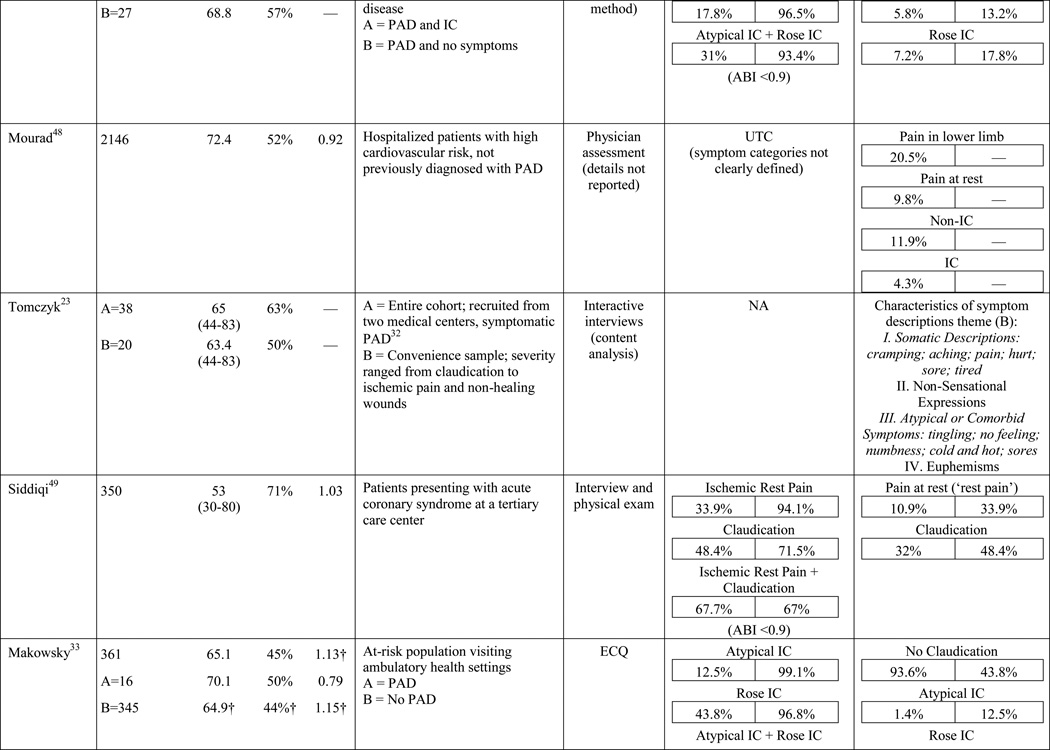
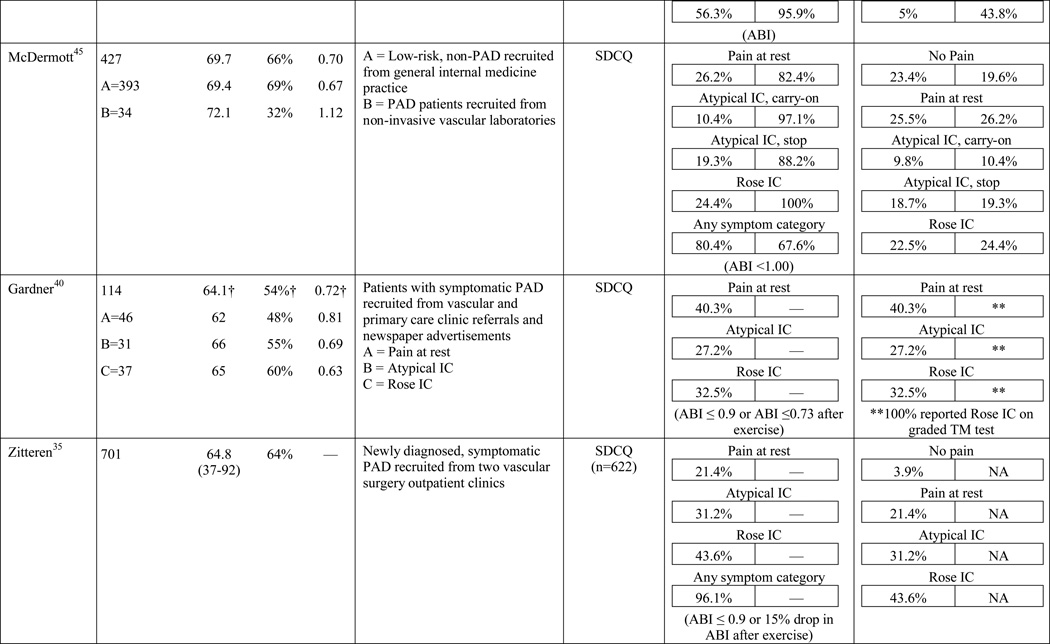
A total of 584 papers were examined. After reviewing the full text of 93 articles, 32 met the inclusion criteria of the review (see Table 1). The literature review search process, including the reasons for exclusion at each stage of screening is presented in Figure 1. The 32 papers included in the review were evaluated in ascending chronological order using a structured abstracting form with eleven topics: first author, year of publication, sample size, mean age/age range, gender, mean ABI, study selection criteria, symptom tool, sensitivity, specificity, and symptom prevalence. Limitations of this review include using English language as a search restriction, thus not including articles published in other languages. Additionally, not including papers and reports unpublished in journals, such as conference abstracts and presentations, may have limited the comprehensiveness of the review.
Table 1.
Results of the structured review.
 |
 |
 |
 |
 |
 |
 |
 |
ABI=ankle-brachial index; PAD=peripheral artery disease; — =not reported; IC=intermittent claudication; *Rose questionnaire adopted by the World Health Organization (WHO) in 1968 for use in epidemiological surveys; §PAD diagnostic criteria was ABI ≤ 0.9 unless indicated otherwise76; GP=General Practitioner; ECQ=Edinburgh Claudication Questionnaire; VA=Veterans Affairs; SDCQ=San Diego Claudication Questionnaire; Peak PT=peak velocity in the posterior tibial artery; TBI=toe-brachial index; UTC=unable to calculate; †=calculated; CLI=critical limb ischemia; CAD=coronary artery disease; CVD=cerebrovascular disease; PVR=pulse volume recordings; CI=chronic ischemia; SF-MPQ=Short-Form McGill Pain Questionnaire; NA=not applicable
Figure 1.
Structured Literature Review Search Process
Results
The results of the structured evaluation are presented in Table 1. The papers included in the review are also denoted with an asterisk in the reference list. Sample sizes ranged from 2023 to 6,4172 participants, with an average of 1,197 participants. Research designs were mostly cross-sectional, but qualitative results were also included.23,24 In instances where population characteristics were only listed for subgroups, the numbers reported for the entire sample were calculated based on the information reported.
PAD Symptom Questionnaires
Symptom assessment often involves a combination approach: an oral report of symptoms to a provider and written completion of a PAD symptom questionnaire by a patient. The Rose questionnaire,16 developed in 1962, was the first PAD symptom questionnaire. It attempted to standardize the one and only symptom thought to be indicative of PAD at the time, claudication. Originally, the Rose questionnaire was developed for use in epidemiologic studies to determine prevalence rates and it was subsequently adopted by the World Health Organization (WHO) in 1968.25 In 1977, minor changes were made to the wording of the questionnaire to make it suitable for self-administration; claudication criteria remained unchanged.26 Results of the initial study revealed 91.9% sensitivity and 100% specificity in 37 patients with undoubted claudication (most verified by arteriograms) and 18 patients with other types of leg pain on walking (mainly sciatica, osteoarthritis, and calf cramps).16 The WHO/Rose questionnaire failed to identify three participants with undoubted claudication, but correctly ruled out all of the participants reporting leg pain unrelated to claudication. Later studies with larger sample sizes, using physician diagnosis as a comparison (usually based on an ABI), resulted in a sensitivity and specificity as low as 8.6%27 and 91%,17 respectively. The low sensitivity in later studies may be explained by failure of the WHO/Rose questionnaire to identify participants reporting symptoms in an atypical location (e.g. buttock) or reporting symptoms in multiple locations, as having claudication. Further, a lower specificity may be explained when participants surveyed present with other types of non-ischemic leg pain and are classified as having claudication.
The low sensitivity and reduced specificity of the WHO/Rose questionnaire led to the development of the Edinburgh claudication questionnaire (ECQ) in 1992.17 The revised questionnaire included a response for non-ambulatory patients and a lower extremity body diagram for patients to indicate leg symptoms in multiple locations. The body diagram allowed for claudication to be classified as definite claudication or atypical claudication depending on involvement (or lack thereof) of the calf. Initial testing of the ECQ revealed 91.3% sensitivity and 99.3% specificity in comparison to the diagnosis of claudication made by a physician.17 The study population consisted of 50 new patients attending a peripheral vascular clinic with leg pain, aged over 55 years and 300 patients aged over 55 years visiting their general practitioner with any complaint.17
A new questionnaire, the San Diego claudication questionnaire (SDCQ),18 was developed in 1996. The SDCQ was a revised and expanded version of the WHO/Rose questionnaire. It included buttock and thigh pain, which was also a component of the ECQ, but unlike the ECQ, the SDCQ inquired specifically whether symptoms were present in the right, left, or both legs. Of all the articles included in the review, the SDCQ was the most frequently used claudication questionnaire. Interestingly, all of the studies that utilized the SDCQ were conducted in the United States, whereas studies conducted abroad used the WHO/Rose and the ECQ.
Claudication questionnaires have undergone several revisions over time, but sensitivity remains low and specificity is variable. All three questionnaires are seemingly insensitive to PAD detection compared to ABI as a gold standard for diagnosis. This indicates the need for further questionnaire refinement to increase the sensitivity and correctly identify patients with disease, but with symptoms differing in location and/or quality compared to those exhibiting classic claudication.
Symptom Definitions
A relatively strict definition of claudication (the ‘typical’ PAD symptom) has persisted over time. As previously described, in its original form,16 classic claudication, is exertional pain restricted to one or both calves that causes a patient to slow down or stop walking, resolves within 10 minutes of standing still, does not resolve while the patient is walking, and does not begin at rest. While the introduction of the ECQ allowed for the presence of symptoms elsewhere in the lower extremities, pain still had to be present in one or both calves to be classified as definite claudication.17,28,29
The creation of the SDCQ allowed for the presence of more specific symptom categories beyond classic claudication, and the assessment of leg-specific symptoms (right versus left).18 The SDCQ consists of five possible symptom categories per leg: Rose claudication, non-Rose exercise calf pain, previously referred to as ‘possible IC’28 and ‘probable IC,’29 non-calf exercise leg pain, pain at rest, and no pain.18 Table 2 summarizes the evolution of claudication questionnaires, including symptom categories and their associated characteristics that most frequently appear in the literature.
Table 2.
Evolution of claudication questionnaires.
| Questionnaire | Year created/ revised |
Symptom category | Symptom characteristics |
|---|---|---|---|
| Rose16 WHO/Rose25 | 1962 |
|
|
| 1985 |
|
|
|
| 1991 |
|
|
|
| ECQ17 | 1992 |
|
|
| SDCQ18 | 1996 |
|
|
Despite the evolution of these questionnaires, patients reporting pain in the hamstrings, feet, shins, joints, or radiating pain in the absence of calf pain would still not classify as ‘symptomatic,’ and subsequently would not be suspected of having PAD. Furthermore, although the number of symptom categories has increased on questionnaires, none allow for the reporting of symptom descriptors such as tingling, numbness, burning, throbbing, or shooting that have been reported by patients with PAD as being part of the symptom experience.23,24
Symptom Report
Typical Symptoms
The symptom most frequently recognized as the hallmark sign of arterial insufficiency is claudication. Claudication comes from the Latin word claudicare, meaning to limp. But, the use of this term is misleading, as patients who experience symptoms other than classic claudication are still shown to be functionally limited30,31 and report a decreased quality of life.32 Aside from confusion about the meaning of claudication, using classic claudication as the gold standard for PAD symptom recognition results in significant under-diagnosis of disease. Over the last ten to fifteen years, the reported prevalence of classic claudication in patients with symptomatic PAD has been highly variable, ranging from 7.5%20 to 33%.18,21,22 Higher prevalence has been reported in smaller populations (43.8%)33 and specific populations including only individuals complaining of leg pain (78.8%),34 or excluding individuals who have non-compressible arteries, CLI, or a history of revascularization (43.6%).35 Overall, study results indicate that there are specific characteristics of individuals who are more likely to report classic claudication. Reporting appears to increase as age increases,21,28,29,36,37 and be more prevalent among men,21,36,37 and in individuals with diabetes,21 hypertension,38 a previous diagnosis of PAD,2,18 or a more severe form of the disease.18,21,37 Disease location may also influence the reporting of classic claudication, with higher prevalence among those with distal lesions35 or large vessel PAD.28
The highest reported prevalence of classic claudication is 100%.39–41 The most recent study conducted by Gardner and colleagues40 included 114 participants with symptomatic PAD recruited from vascular and primary care clinic referrals. Prior to exercise testing, participants fell into the following three symptom categories: leg pain on exertion and rest (40.3%), atypical leg pain (27.2%), and classic claudication (32.5%). However, during a graded treadmill test, all of the participants reported symptoms consistent with classic claudication. In 2007, Gardner and colleagues39 reported similar findings. The study included 715 participants self-reporting exertional leg pain consistent with one of the first four categories on the SDCQ. Initial classic claudication prevalence was 56.8%. As with the 2012 study, during treadmill testing, all of the study participants experienced exertional leg pain that was consistent with classic claudication (i.e. participants stopped walking due to calf pain that resolved with subsequent rest). McDermott and colleagues41 reported similar findings with a group of 57 patients who initially self-reported no symptoms, but over half became symptomatic during a 6-minute walking test. These results raise important questions that have not been previously explored: Are the patients classified in the literature as ‘asymptomatic’ truly not experiencing symptoms, or are they slowing their walking pace or limiting ambulation to prevent the onset and/or progression of leg symptoms which could be revealed under controlled exercise testing? The issue of under-reporting versus true symptom prevalence deserves further attention.
Atypical Symptoms
When Rose16 developed the first claudication questionnaire in 1962, the characteristics of PAD were thought to be well-delineated, which made it suitable for diagnosis in epidemiologic surveys. However, over the last five decades, researchers have discovered a more diverse presentation of PAD symptoms. With classic claudication consistently being reported by less than one-third of patients with PAD, claudication questionnaires have been forced to evolve in order to capture the broad array of symptom experiences.17,18 But, revised claudication questionnaires are still not sufficient, as patients are reporting symptoms and symptom experiences that are not detected by these questionnaires. Until a more comprehensive tool exists, it is essential for clinicians to recognize that patients with underlying PAD are reporting ‘atypical’ symptoms more frequently than classic claudication,2,20,22,27,42–45 and adapt their assessment techniques accordingly.
In the literature reviewed, the prevalence of atypical symptoms was difficult to ascertain compared to classic claudication, despite its increased frequency. The main reasons were the use of a variety of definitions for atypical symptoms and inconsistent use of symptom categories from study to study. In its simplest form, atypical symptoms included any lower extremity symptom that was not consistent with classic claudication2,18 and increased in complexity to include all lower extremity symptoms not located in the calf,17 exercise calf pain not present at rest, but otherwise not fully concordant with the Rose criteria (‘possible IC’),28,46 calf pain, but one Rose criteria not fulfilled (‘probable IC’),29 atypical pain on exertion (non-Rose walk-through pain and non-Rose stop because of pain), and pain on exertion and rest.39,41 Atypical pain was used to refer to ‘walk-through pain’ and/or pain that was not consistently relieved within 10 minutes of rest.38 However, prolonged symptom recovery was also grouped together with pain at rest into a ‘no pain’ category.47 Pain that presented at rest and on exertion was often referred to as ‘leg pain on exertion and rest,’21,39–41,45 but was also referred to as ‘pain at rest,’18,22,42,48 ‘rest pain,’43,49 or ‘symptoms at rest.’35 Some studies subdivided the ‘no symptoms with exertion’ category into active and inactive participants, resulting in a total of six leg categories,41,50 whereas Collins and colleagues20 condensed the five symptom categories of the SDCQ into three: no pain, atypical leg pain, (pain at rest, non-calf exercise pain, and non-Rose exercise calf pain) and Rose claudication. Others followed the original five symptom categories established by the SDCQ21 or used a general category of ‘leg symptoms’44 or ‘symptomatic’ that included lower extremity revascularization, amputation secondary to PAD, or report of claudication regardless of ABI.51 The use of either category, ‘leg symptoms’ or ‘symptomatic,’ limits the understanding of symptom presentation by classifying symptomatic patients as asymptomatic and vice versa.
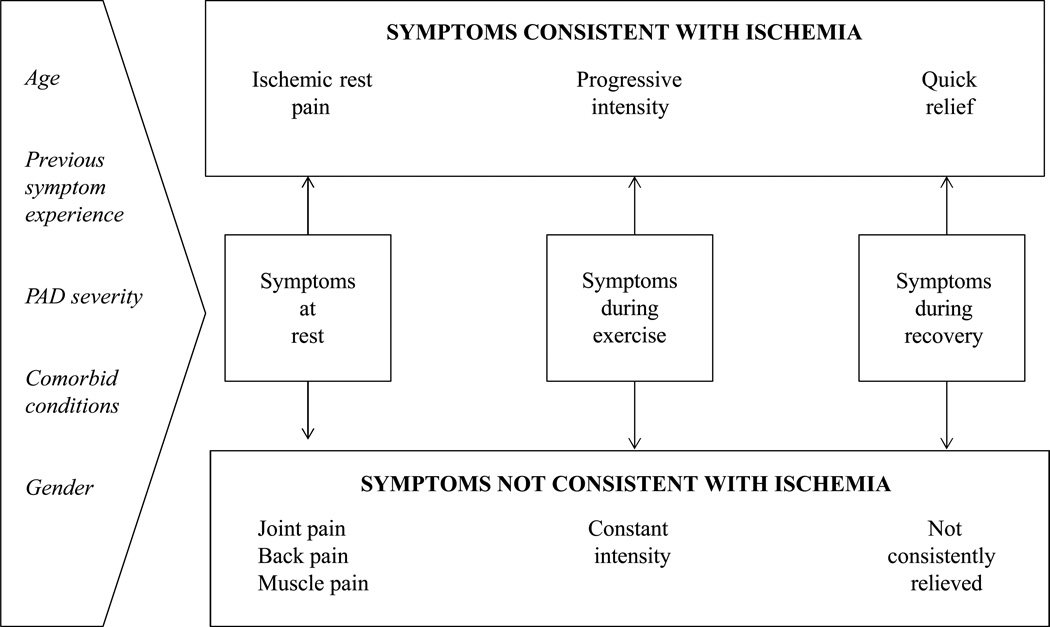
Discovering a wider variety of PAD symptoms has not been entirely without challenge, particularly since responses to some symptom categories can be difficult to interpret. For example, the rest pain category on the SDCQ could represent an individual experiencing ischemic rest pain or pain at rest not associated with PAD, but attributable to a comorbid condition such as arthritis. Additionally, it is imperative that symptoms consistent with ischemia are differentiated from those not consistent with ischemia in order to identify atypical PAD symptoms versus manifestations of comorbid conditions unrelated to PAD (i.e. symptom confounders). Figure 2 illustrates how a symptom can be classified as ischemic or non-ischemic in three phases: at rest, during exercise, and during recovery.
Figure 2.
Conceptual Model of Symptom Differentiation
Potential Symptom Confounders
It has been demonstrated that older adults are more likely to become afflicted with PAD.52,53 Older age also makes it more likely that patients with PAD are afflicted with other age-related conditions that could cause or contribute to lower extremity symptoms. Consideration should also be given to PAD severity and its influence on the symptom experience. While several researchers have recognized the potential influence of comorbid conditions on symptom presentation,24,27,41,42,53–56 the topic has not been thoroughly researched or reported in the literature. Findings from McDermott and colleagues41 revealed an increased prevalence of diabetes, neuropathy, and spinal stenosis in patients who reported pain on exertion and rest. Similarly, Newman and colleagues27 discovered a higher prevalence of arthritis and depression in patients reporting exertional leg pain other than classic claudication. Findings from Bernstein and colleagues57 revealed a low prevalence of classic claudication (2%) among patients with PAD, half of whom were also diagnosed with degenerative joint disease. Insulin resistance without a diagnosis of diabetes has also been identified as a factor influencing claudication prevalence.58 Further support for the effect of comorbid conditions came from a study conducted by Weinberg and colleagues,54 indicating that regional neuropathy is commonly associated with chronic ischemia and CLI.
The neuropathic component of ischemic pain has been examined more closely by researchers and the current understanding is that the character of ischemic pain changes from nociceptive pain in patients with classic claudication to predominately neuropathic pain in patients with CLI.24 Despite the large numbers of patients diagnosed with PAD and reporting neuropathy, the knowledge of the role of ischemia in neuropathic pain remains limited. Overall, these preliminary results suggest that there are differences in the symptoms reported and/or differences in the character of the symptom in the presence of certain comorbid conditions or in those with severe PAD (i.e. CLI). This provides additional evidence that using classic claudication as the defining symptom of PAD is insufficient to capture the breadth of symptoms experienced, particularly in this patient population.
Similarly, differential diagnoses have been described in PAD literature in an attempt to clear the blurring of symptom reporting that occurs in the presence of multiple comorbidities, but it has not been extensively studied.59–65 An understanding of physiology can allow a clinician to locate the site of arterial occlusion based on the location of the symptom(s). For example, pain or discomfort in the calf, ankle, or foot could indicate an obstruction/occlusion in the popliteal or superficial femoral arteries.60 Symptoms located primarily in the calf or thigh could indicate involvement of the femoral arteries or their branches, whereas, symptoms in the buttock, hip, and thigh indicate higher disease in the aorta or iliac artery.
The location of symptoms can serve as a guide, but they do not guarantee the presence or location of a lesion with 100% certainty. Symptoms of a patient with claudication may overlap with symptomatology of other conditions, particularly neurological and musculoskeletal diseases.63 Take for instance a patient reporting calf pain. The pain could indicate claudication secondary to a femoral artery occlusion or it could indicate a venous occlusion, chronic compartment syndrome, nerve root compression, or a Baker’s cyst (a tight bursting pain/dull ache that worsens on standing and resolves with leg elevation).59,61,63,66 The presence of any of these conditions could lead a provider to suspect claudication, which could be ruled out if the symptom is relieved by a change in position. Symptoms in the hip, thigh, or buttock could be related to hip arthritis.59,63 However, arthritis is usually a more persistent pain compared to the intermittent nature of claudication and typically associated with symptoms in other joints.63,67 Spinal cord compression should also be considered, particularly when a patient is reporting a history of back pain, with symptoms that worsen upon standing, but are relieved by positional changes.59 Patients reporting foot symptoms could have an inflammatory condition such as arthritis or Buerger’s disease.63,64 Current clinician recommendations are to conduct a thorough physical exam and symptom assessment that includes the location, duration, and intensity.62 If PAD is suspected based on patient symptom report or a patient’s risk factor profile, a confirmatory ABI should be performed.
Conclusions and Recommendations
Claudication questionnaires have been used extensively to assess the presence of claudication and subsequently to detect the presence of PAD. Although often highly specific, they remain insensitive for the detection and diagnosis of PAD. Additionally, the inconsistent use of one standardized questionnaire, combined with variations in sample characteristics, definition of PAD, diagnostic methods, and definition of claudication and atypical symptoms make comparisons across studies difficult, if not impossible. Although appearing more frequently, the non-specific nature of atypical symptoms further complicates clear symptom categorization and necessitates classification of atypical symptoms as being caused by ischemia or caused by comorbid conditions unrelated to ischemia. Furthermore, age and gender differences may affect the reporting of classic claudication and atypical symptoms on PAD questionnaires. However, the largest confounder of PAD symptom report may be the presence of comorbidities, particularly those that affect mobility, as physical limitations may preclude manifestation of PAD symptoms and delay necessary diagnosis and treatment. As the role of comorbid conditions becomes more clearly defined, follow up questions can be added to existing questionnaires to eliminate false positives and to capture participants who were originally considered false negatives.
Additional research is needed to increase understanding of the role of age, gender, race, and comorbid conditions on the symptom experience of patients with PAD. The next logical step is to validate subjective symptom report with objective physiologic measures that detect ischemia during exercise in an attempt to broaden the current understanding of PAD symptom presentation. Better understanding and differentiation of symptoms that are not consistent with classic claudication or atypical symptoms caused by ischemia, but rather caused by a comorbid condition that is unrelated to ischemia, is essential to enhance understanding of the symptom experience. While previous research has correlated symptom report with PAD disease severity as measured by ABI, to the authors knowledge, they have been conducted in a static state. Simultaneous data collection during dynamic exercise has the potential to provide new symptom descriptors that are necessary to consistently and accurately detect PAD based on patient characteristics and vague symptom reporting, thus expanding the definition of ‘claudication.’ These additional descriptors could be incorporated into existing PAD questionnaires, thus enhancing the sensitivity of these questionnaires, potentially leading to improved detection and treatment of PAD.
References
- 1.Allison MA, Ho E, Denenberg JO, et al. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32(4):328–333. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch AT, Hiatt WR. PARTNERS Steering C.PAD awareness, risk, and treatment: New resources for survival--the USA PARTNERS program. Vasc Med. 2001;6(3 Suppl):9–12. doi: 10.1177/1358836X0100600i103. [DOI] [PubMed] [Google Scholar]
- 4.Ostchega Y, Paulose-Ram R, Dillon CF, et al. Prevalence of peripheral arterial disease and risk factors in persons aged 60 and older: Data from the national health and nutrition examination survey 1999–2004. J Am Geriatr Soc. 2007;55(4):583–589. doi: 10.1111/j.1532-5415.2007.01123.x. [DOI] [PubMed] [Google Scholar]
- 5.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the national health and nutrition examination survey, 1999–2000. Circulation. 2004;110(6):738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 6.Becker GJ, McClenny TE, Kovacs ME, et al. The importance of increasing public and physician awareness of peripheral arterial disease. J Vasc Interv Radiol. 2002;13(1):7–11. doi: 10.1016/s1051-0443(07)60002-5. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira AC, Macedo FY. A review of simple, non-invasive means of assessing peripheral arterial disease and implications for medical management. Ann Med. 2010;42(2):139–150. doi: 10.3109/07853890903521070. [DOI] [PubMed] [Google Scholar]
- 8.Mukherjee D, Cho L. Peripheral arterial disease: Considerations in risks, diagnosis, and treatment. J Natl Med Assoc. 2009;101(10):999–1008. doi: 10.1016/s0027-9684(15)31066-x. [DOI] [PubMed] [Google Scholar]
- 9.Diehm C, Allenberg JR, Pittrow D, et al. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation. 2009;120(21):2053–2061. doi: 10.1161/CIRCULATIONAHA.109.865600. [DOI] [PubMed] [Google Scholar]
- 10.Criqui MH, Ninomiya JK, Wingard DL, et al. Progression of peripheral arterial disease predicts cardiovascular disease morbidity and mortality. J Am Coll Cardiol. 2008;52(21):1736–1742. doi: 10.1016/j.jacc.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caro J, Migliaccio-Walle K, Ishak KJ, et al. The morbidity and mortality following a diagnosis of peripheral arterial disease: Long-term follow-up of a large database. BMC Cardiovasc Disord. 2005;5:14. doi: 10.1186/1471-2261-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326(6):381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 13.Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295(2):180–189. doi: 10.1001/jama.295.2.180. [DOI] [PubMed] [Google Scholar]
- 14.Newman AB, Shemanski L, Manolio TA, et al. Ankle-arm index as a predictor of cardiovascular disease and mortality in the cardiovascular health study. Arterioscler Thromb Vasc Biol. 1999;19(3):538–545. doi: 10.1161/01.atv.19.3.538. [DOI] [PubMed] [Google Scholar]
- 15.Matzke S, Lapantalo M. Claudication does not always precede critical leg ischemia. Vasc Med. 2001;6:77–80. [PubMed] [Google Scholar]
- 16.Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–658. [PMC free article] [PubMed] [Google Scholar]
- 17.Leng GC, Fowkes FG. The Edinburgh claudication questionnaire: An improved version of the WHO/Rose questionnaire for use in epidemiological surveys. J Clin Epidemiol. 1992;45(10):1101–1109. doi: 10.1016/0895-4356(92)90150-l. [DOI] [PubMed] [Google Scholar]
- 18.Criqui MH, Denenberg JO, Bird CE, et al. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1(1):65–71. doi: 10.1177/1358863X9600100112. [DOI] [PubMed] [Google Scholar]
- 19.Olson KW, Treat-Jacobson D. Symptoms of peripheral arterial disease: A critical review. J Vasc Nurs. 2004;22(3):72–77. doi: 10.1016/j.jvn.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Collins TC, Petersen NJ, Suarez-Almazor M, et al. The prevalence of peripheral arterial disease in a racially diverse population. Arch Intern Med. 2003;163(12):1469–1474. doi: 10.1001/archinte.163.12.1469. [DOI] [PubMed] [Google Scholar]
- 21.Wang JC, Criqui MH, Denenberg JO, et al. Exertional leg pain in patients with and without peripheral arterial disease. Circulation. 2005;112(22):3501–3508. doi: 10.1161/CIRCULATIONAHA.105.548099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDermott MM, Mehta S, Liu K, et al. Leg symptoms, the ankle-brachial index, and walking ability in patients with peripheral arterial disease. J Gen Intern Med. 1999;14(3):173–181. doi: 10.1046/j.1525-1497.1999.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomczyk S, Treat-Jacobson D. Claudication symptom experience in men and women: Is there a difference? J Vasc Nurs. 2009;27(4):92–97. doi: 10.1016/j.jvn.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Ruger LJ, Irnich D, Abahji TN, et al. Characteristics of chronic ischemic pain in patients with peripheral arterial disease. Pain. 2008;139(1):201–208. doi: 10.1016/j.pain.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 25.Rose GA, Blackburn H. Cardiovascular survey methods. Monograph series. 1968;56:1–188. [PubMed] [Google Scholar]
- 26.Rose G, McCartney P, Reid DD. Self-administration of a questionnaire on chest pain and intermittent claudication. Br J Prev Soc Med. 1977;31(1):42–48. doi: 10.1136/jech.31.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman AB, Naydeck BL, Sutton-Tyrrell K, et al. The role of comorbidity in the assessment of intermittent claudication in older adults. J Clin Epidemiol. 2001;54(3):294–300. doi: 10.1016/s0895-4356(00)00308-5. [DOI] [PubMed] [Google Scholar]
- 28.Criqui MH, Fronek A, Klauber MR, et al. The sensitivity, specificity, and predictive value of traditional clinical evaluation of peripheral arterial disease: Results from noninvasive testing in a defined population. Circulation. 1985;71(3):516–522. doi: 10.1161/01.cir.71.3.516. [DOI] [PubMed] [Google Scholar]
- 29.Fowkes FG, Housley E, Cawood EH, et al. Edinburgh artery study: Prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991;20(2):384–392. doi: 10.1093/ije/20.2.384. [DOI] [PubMed] [Google Scholar]
- 30.McDermott MM, Ferrucci L, Liu K, et al. Leg symptom categories and rates of mobility decline in peripheral arterial disease. J Am Geriatr Soc. 2010;58(7):1256–1262. doi: 10.1111/j.1532-5415.2010.02941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDermott MM, Liu K, Greenland P, et al. Functional decline in peripheral arterial disease: Associations with the ankle brachial index and leg symptoms. JAMA. 2004;292(4):453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 32.Treat-Jacobson D, Halverson SL, Ratchford A, et al. A patient-derived perspective of health-related quality of life with peripheral arterial disease. J Nurs Scholarsh. 2002;34(1):55–60. doi: 10.1111/j.1547-5069.2002.00055.x. [DOI] [PubMed] [Google Scholar]
- 33.Makowsky M, McMurtry MS, Elton T, et al. Prevalence and treatment patterns of lower extremity peripheral arterial disease among patients at risk in ambulatory health settings. Can J Cardiol. 2011;27(3):389.e11–389.e18. doi: 10.1016/j.cjca.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 34.Makdisse M, Neto RN, Chagas ACP, et al. Cross-cultural adaptation and validation of the Brazilian portuguese version of the Edinburgh claudication questionnaire. Arq Bras Cardiol. 2007;88(5):501–506. doi: 10.1590/s0066-782x2007000500001. [DOI] [PubMed] [Google Scholar]
- 35.Van Zitteren M, Vriens PW, Heyligers JM, et al. Self-reported symptoms on questionnaires and anatomic lesions on duplex ultrasound examinations in patients with peripheral arterial disease. J Vasc Surg. 2012;55(4):1025–1034. doi: 10.1016/j.jvs.2011.10.115. [DOI] [PubMed] [Google Scholar]
- 36.Leng GC, Lee AJ, Fowkes FG, et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1996;25(6):1172–1181. doi: 10.1093/ije/25.6.1172. [DOI] [PubMed] [Google Scholar]
- 37.Stoffers HE, Rinkens PE, Kester AD, et al. The prevalence of asymptomatic and unrecognized peripheral arterial occlusive disease. Int J Epidemiol. 1996;25(2):282–290. doi: 10.1093/ije/25.2.282. [DOI] [PubMed] [Google Scholar]
- 38.Ogren M, Hedblad B, Engstrom G, et al. Leg blood flow and long-term cardiovascular prognosis in men with typical and atypical intermittent claudication. Eur J Vasc Endovasc Surg. 2003;26(3):272–279. doi: 10.1053/ejvs.2002.2008. [DOI] [PubMed] [Google Scholar]
- 39.Gardner AW, Montgomery PS, Afaq A. Exercise performance in patients with peripheral arterial disease who have different types of exertional leg pain. J Vasc Surg. 2007;46(1):79–86. doi: 10.1016/j.jvs.2007.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gardner A, Parker D, Montgomery P, et al. Calf muscle hemoglobin oxygen saturation in patients with peripheral artery disease who have different types of exertional leg pain. J Vasc Surg. 2012;55(6):1654–1661. doi: 10.1016/j.jvs.2011.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: Associated clinical characteristics and functional impairment. JAMA. 2001;286(13):1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 42.McDermott MM, Mehta S, Greenland P. Exertional leg symptoms other than intermittent claudication are common in peripheral arterial disease. Arch Intern Med. 1999;159(4):387–392. doi: 10.1001/archinte.159.4.387. [DOI] [PubMed] [Google Scholar]
- 43.Lacroix P, Aboyans V, Voronin D, et al. High prevalence of undiagnosed patients with peripheral arterial disease in patients hospitalised for non-vascular disorders. Int J Clin Pract. 2008;62(1):59–64. doi: 10.1111/j.1742-1241.2007.01579.x. [DOI] [PubMed] [Google Scholar]
- 44.Kownator S, Cambou JP, Cacoub P, et al. Prevalence of unknown peripheral arterial disease in patients with coronary artery disease: Data in primary care from the IPSILON study. Arch Cardiovasc Dis. 2009;102(8–9):625–631. doi: 10.1016/j.acvd.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 45.McDermott MM, Liu K, Carr J, et al. Superficial femoral artery plaque, the ankle-brachial index, and leg symptoms in peripheral arterial disease: The walking and leg circulation study (WALCS) III. Circ Cardiovasc Imaging. 2011;4(3):246–252. doi: 10.1161/CIRCIMAGING.110.962183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sprynger M, Fassotte C, Verhaeghe R. The ankle-brachial pressure index and a standardized questionnaire are easy and useful tools to detect peripheral arterial disease in non-claudicating patients at high risk. Int Angiol. 2007;26(3):239–244. [PubMed] [Google Scholar]
- 47.Manzano L, Garcia-Diaz JD, Suarez C, et al. Thigh and buttock exertional pain for the diagnosis of peripheral arterial disease. Eur J Intern Med. 2009;20(4):429–434. doi: 10.1016/j.ejim.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 48.Mourad JJ, Cacoub P, Collet JP, et al. Screening of unrecognized peripheral arterial disease (PAD) using ankle-brachial index in high cardiovascular risk patients free from symptomatic PAD. J Vasc Surg. 2009;50(3):572–580. doi: 10.1016/j.jvs.2009.04.055. [DOI] [PubMed] [Google Scholar]
- 49.Siddiqi RO, Paracha MI, Hammad M. Frequency of peripheral arterial disease in patients presenting with acute coronary syndrome at a tertiary care centre in Karachi. JPMA J Pak Med Assoc. 2010;60(3):171–174. [PubMed] [Google Scholar]
- 50.Collins TC, Petersen NJ, Suarez-Almazor M. Peripheral arterial disease symptom subtype and walking impairment. Vasc Med. 2005;10(3):177–183. doi: 10.1191/1358863x05vm615oa. [DOI] [PubMed] [Google Scholar]
- 51.Missault L, Krygier C, Lukito G, et al. Occurrence of peripheral arterial disease in a Belgian cohort of patients with cardiovascular history of atherothrombosis. Acta Chir Belg. 2007;107(5):508–514. doi: 10.1080/00015458.2007.11680112. [DOI] [PubMed] [Google Scholar]
- 52.Murabito JM, D'Agostino RB, Silbershatz H, et al. Intermittent claudication. A risk profile from the Framingham heart study. Circulation. 1997;96(1):44–49. doi: 10.1161/01.cir.96.1.44. [DOI] [PubMed] [Google Scholar]
- 53.He Y, Jiang Y, Wang J, et al. Prevalence of peripheral arterial disease and its association with smoking in a population-based study in Beijing, China. J Vasc Surg. 2006;44(2):333–338. doi: 10.1016/j.jvs.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 54.Weinberg DH, Simovic D, Isner J, et al. Chronic ischemic monomelic neuropathy from critical limb ischemia. Neurology. 2001;57(6):1008–1012. doi: 10.1212/wnl.57.6.1008. [DOI] [PubMed] [Google Scholar]
- 55.Ogren M, Hedblad B, Engstrom G, et al. Prevalence and prognostic significance of asymptomatic peripheral arterial disease in 68-year-old men with diabetes. Results from the population study 'men born in 1914' from Malmo, Sweden. Eur J Vasc Endovasc Surg. 2005;29(2):182–189. doi: 10.1016/j.ejvs.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 56.Dolan NC, Liu K, Criqui MH, et al. Peripheral artery disease, diabetes, and reduced lower extremity functioning. Diabetes Care. 2002;25(1):113–120. doi: 10.2337/diacare.25.1.113. [DOI] [PubMed] [Google Scholar]
- 57.Bernstein J, Esterhai JL, Staska M, et al. The prevalence of occult peripheral arterial disease among patients referred for orthopedic evaluation of leg pain. Vasc Med. 2008;13(3):235–238. doi: 10.1177/1358863X08091970. [DOI] [PubMed] [Google Scholar]
- 58.Pande RL, Park MA, Perlstein TS, et al. Impaired skeletal muscle glucose uptake by [18F]fluorodeoxyglucose-positron emission tomography in patients with peripheral artery disease and intermittent claudication. Arterioscler Thromb Vasc Biol. 2011;31(1):190–196. doi: 10.1161/ATVBAHA.110.217687. [DOI] [PubMed] [Google Scholar]
- 59.Meru AV, Mittra S, Thyagarajan B, et al. Intermittent claudication: An overview. Atherosclerosis. 2006;187(2):221–237. doi: 10.1016/j.atherosclerosis.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 60.Almahameed A. Peripheral arterial disease: Recognition and medical management. Cleve Clin J Med. 2006;73(7):621–626. doi: 10.3949/ccjm.73.7.621. [DOI] [PubMed] [Google Scholar]
- 61.Jang JJ, Halperin JL. Peripheral arterial disease. In: Rosendorf C, editor. Essential cardiology: Principles and practice. 2nd ed. Totowa, NJ: Humana Press; 2005. pp. 807–828. [Google Scholar]
- 62.Lyden SP, Joseph D. The clinical presentation of peripheral arterial disease and guidance for early recognition. Cleve Clin J Med. 2006;73(Suppl 4):S15–S21. doi: 10.3949/ccjm.73.suppl_4.s15. [DOI] [PubMed] [Google Scholar]
- 63.Abul-Khoudoud O. Diagnosis and risk assessment of lower extremity peripheral arterial disease. J Endovasc Ther. 2006;13(Suppl 2):10–18. doi: 10.1177/15266028060130S205. [DOI] [PubMed] [Google Scholar]
- 64.Weitz JI, Byrne J, Clagett GP, et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review. Circulation. 1996;94(11):3026–3049. doi: 10.1161/01.cir.94.11.3026. [DOI] [PubMed] [Google Scholar]
- 65.Jeon CH, Han SH, Chung NS, et al. The validity of ankle-brachial index for the differential diagnosis of peripheral arterial disease and lumbar spinal stenosis in patients with atypical claudication. Eur Spine J. 2012;21(6):1165–1170. doi: 10.1007/s00586-011-2072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dizon-Townson DS, Nelson LM, Jang H, et al. The incidence of the factor V leiden mutation in an obstetric population and its relationship to deep vein thrombosis. Am J Obstet Gynecol. 1997;176(4):883–886. doi: 10.1016/s0002-9378(97)70615-x. [DOI] [PubMed] [Google Scholar]
- 67.Meru AV, Mittra S, Thyagarajan B, et al. Intermittent claudication: An overview. Atherosclerosis. 2006;187(2):221–237. doi: 10.1016/j.atherosclerosis.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 68.Criqui MH, Frankville DD, Barrett-Connor E, et al. Change and correlates of change in high and low density lipoprotein cholesterol after six years: A prospective study. Am J Epidemiol. 1983;118(1):52–59. doi: 10.1093/oxfordjournals.aje.a113616. [DOI] [PubMed] [Google Scholar]
- 69.The lipid research clinics program prevalence study epidemiology committee. Plasma lipid distributions in selected North American populations. 1979;60:427. doi: 10.1161/01.cir.60.2.427. [DOI] [PubMed] [Google Scholar]
- 70.McDermott MM, Mehta S, Greenland P. Exertional leg symptoms other than intermittent claudication are common in peripheral arterial disease. Arch Intern Med. 1999;159(4):387–392. doi: 10.1001/archinte.159.4.387. [DOI] [PubMed] [Google Scholar]
- 71.Second European consensus document on chronic critical leg ischemia. Eur J Vasc Surg. 1992;6(Suppl A):1–32. [PubMed] [Google Scholar]
- 72.Isacsson SO. Venous occlusion plethysmography in 55-year-old men. A population study in Malmo, Sweden. Acta Med Scand. 1972;(Suppl 537):1–62. [PubMed] [Google Scholar]
- 73.Bird CE, Criqui MH, Fronek A, et al. Quantitative and qualitative progression of peripheral arterial disease by non-invasive testing. Vasc Med. 1999;4(1):15–21. doi: 10.1177/1358836X9900400103. [DOI] [PubMed] [Google Scholar]
- 74.Criqui MH, Jamosmos M, Fronek A, et al. Chronic venous disease in an ethnically diverse population: The San Diego population study. Am J Epidemiol. 2003;158(5):448–456. doi: 10.1093/aje/kwg166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cacoub P, Cambou JP, Kownator S, et al. Prevalence of peripheral arterial disease in high-risk patients using ankle-brachial index in general practice: A cross-sectional study. Int J Clin Pract. 2009;63:63–70. doi: 10.1111/j.1742-1241.2008.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: A scientific statement from the American Heart Association. Circulation. 2012;126(24):2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]