Abstract
Metastatic breast cancer cells co-opt the cells of the bone to increase their production of inflammatory cytokines. Here, we sought to identify key cytokines expressed by osteoblasts in vitro and in vivo in the presence of MDA-MB-231 metastatic breast cancer cells, including a bone-seeking variant. We hypothesized that osteoblast-derived cytokines increase in the presence of metastatic breast cancer cell conditioned medium (CM), act as chemoattractants for cancer cells, and enhance osteoclast formation. We detected increases in the concentrations of osteoblast-derived IL-6, MCP-1, VEGF, MIP-2, and KC in vitro in culture supernatants from MC3T3-E1 cells in the presence of metastatic breast cancer cell CM and from cancer-bearing femurs ex vivo. A comparison of cancer cell- and osteoblast-derived cytokines revealed that while breast cancer cells expressed the same or equivalent cytokines as the osteoblasts, the breast cancer cells only produced picogram quantities of MCP-1; osteoblasts expressed nanogram amounts. Bone-derived MCP-1 increased in the proximal metaphysis, an area where breast cancer cells preferentially trafficked following intracardiac inoculation in athymic mice. An MDA-MB-231 bone-seeking variant was not different from parental lines. Osteoblast CM was a potent chemoattractant for metastatic breast cancer cells. Furthermore, culture supernatants of osteoblasts treated with breast cancer cell CM enhanced osteoclast formation. These findings suggest that bone metastatic breast cancer cells utilize osteoblast-derived cytokines to facilitate breast cancer cell colonization and survival upon arrival in the bone microenvironment. J. Cell. Biochem. 111: 1138–1148, 2010.
Keywords: OSTEOBLAST, BREAST CANCER, IL-6, MCP-1, VEGF
Breast cancer, the second leading cause of cancer deaths in American women [Jemal et al., 2008], preferentially metastasizes to the skeleton in nearly 50% of primary and 80% of recurring metastases [Rubens and Mundy, 2000]. Once breast cancer metastasizes to bone, the 5-year survival rate falls from 90% to <10% [Jemal et al., 2008].
In a healthy adult, bone continuously undergoes remodeling. Bone turnover is accomplished by bone-resorbing osteoclasts and bone-depositing osteoblasts, with no net change [Kanis and McCloskey, 1997]. However, when metastatic breast cancer cells enter the bone microenvironment, this balance is disrupted, and bone loss exceeds bone gain [Yoneda, 1996]. Treatment modalities to block osteoclast activity, for example, bisphosphonates, are not curative [Hillner et al., 2000]. Lesion progression is slowed, but existing lesions do not heal [Kukreja et al., 1998]. Bone pain, fractures, and hypercalcemia result [Mundy, 2002].
The available data point to chemokines and cytokines produced by breast cancer cells as key players in metastasis [Bendre et al., 2003b]. While breast cancer factors are undoubtedly important, we have evidence that osteoblasts are directed by breast cancer cells to produce inflammatory cytokines implicated in breast cancer cell migration, survival, and osteoclast activation [Bendre et al., 2003a; Scapini et al., 2004]. We previously reported that MDA-MB-231 human metastatic breast cancer cell-conditioned medium (CM) increased osteoblast production of IL-6, MCP-1, and IL-8 [Kinder et al., 2008]. Here, we sought to identify other factors involved in the osteoblast inflammatory stress response to metastatic breast cancer cells, and determine if this response occurred in vivo. We found that osteoblast-derived cytokines, specifically IL-6, MCP-1, KC/GRO-α, MIP-2/IL-8, and VEGF, were increased in vivo and in vitro in the presence of breast cancer cells or their CM. These molecules may act as chemoattractants, growth, and maintenance factors for cancer cells or osteoclasts. We also hypothesized that the osteoblast-derived cytokine response was greater following culture with a bone-seeking cancer variant. Using an in vitro culture and xenograft model of human metastatic or non-metastatic breast cancer cell variants, we found that osteoblasts increased their production of inflammatory cytokines irrespective of cancer cell variant. These osteoblast-derived cytokines likely aid in bone metastatic breast cancer cell colonization, survival, and osteoclast formation.
MATERIALS AND METHODS
CELLS
Osteoblasts
MC3T3-E1 murine osteoblasts that differentiate and mineralize in culture [Sudo et al., 1983] (Dr. Norman Karin, University of Delaware), were maintained in alpha minimum essential medium (αMEM; Mediatech, Manassas, VA), 10% neonatal FBS (Cansera, Roxdale, ON), and penicillin 100 U/ml/streptomycin 100 μg/ml (Sigma, St. Louis, MO; growth medium). MC3T3-E1 cells were plated at 1 × 105 cells/ml. Twenty-four hours later, the medium was replaced with differentiation medium (growth medium plus 50 μg/ml ascorbic acid, and 10 mM β-glycerophosphate). MC3T3-E1 cells were cultured to three stages of differentiation: growth (4 days), early differentiation (10 days), or late differentiation (20 days) [Lian and Stein, 1992]. Differentiation medium was changed every 3rd day.
Breast cancer cell variants
MDA-MB-231W human metastatic breast cancer cells [Cailleau et al., 1978] were a gift from Dr. Danny Welch, University of Alabama, Birmingham. MDA-MB-231PY cells, comparable to MDA-MB-231W cells [Cailleau et al., 1978], were used to derive MDA-MB-231BO bone-seeking and MDA-MB-231BR brain-seeking variants [Yoneda et al., 2001] (Dr. Toshiyuki Yoneda, University of Texas Health Science Center, San Antonio, Texas).
For intracardiac inoculations, MDA-MB-231W-green fluorescent protein (GFP) and metastasis suppressed MDA-MB-231BRMS1-GFP cells [Phadke et al., 2008] (Dr. Danny Welch) were utilized. MDA-MB-231PY-GFP and MDA-MB-231BO-GFP were obtained from Dr. Patricia Steeg, NIH, Bethesda, Maryland, with permission from Dr. Toshiyuki Yoneda. Cells were maintained antibiotic-free for three passages immediately prior to use, and tested negative for Mycoplasma spp. infection (TaKaRa Bio, Inc., Shiga, Japan). Cells were maintained in DMEM (Mediatech), 5% neonatal FBS, and penicillin 100 U/ml/streptomycin 100 μg/ml, except for MDA-MB-231PY, MDA-MB-231BO, and MDA-MB-231BR which were maintained in 10% neonatal FBS.
Osteoclast precursors
Monocytes were obtained from marrow flushed from femurs and tibia of C57BL/6 mice. Marrow from six femurs and tibiae were combined, centrifuged (300g), and the pellet washed three times by resuspension and centrifugation in αMEM plus 1% penicillin, 100 U/ml/streptomycin 100 μg/ml, before resuspension in a total volume of 2 ml osteoblast growth medium.
Conditioned media preparation
MC3T3-E1 cells, grown for 4, 10, or 20 days, were rinsed, and serum-free αMEM added (20 ml per T-150 flask, ~9.1 × 104 cells/cm2) for 24 h. Osteoblast conditioned medium (OBCM) was collected, centrifuged, and stored at −20°C.
MDA-MB-231 breast cancer cell variants, ~90% confluent, were rinsed, and serum-free αMEM added (20 ml per T-150 flask, ~1.3 × 105 cells/cm2). Twenty-four hours later, breast cancer cell conditioned medium (BCCM) was collected, centrifuged, and stored at −20°C. Vehicle medium (VM) consisting of MC3T3-E1 differentiation medium was used for comparison.
CONDITIONED MEDIA TREATMENTS OF OSTEOBLASTS
MC3T3-E1 cells were treated with CM for 24 h, after which the culture supernatant was collected. CM consisted of one-half volume BCCM and one-half volume 2 × MC3T3-E1 differentiation medium, to ensure that concentrations of serum and differentiation factors were identical for VM and CM.
INTRACARDIAC INOCULATIONS AND FEMUR CULTURES
MDA-MB-231 cell variants, ~90% confluent, were detached, washed, and resuspended in PBS. 3 × 105 cells/200 μl were injected into the left cardiac ventricle of female athymic mice aged 6 weeks (Harlan Sprague–Dawley, Indianapolis, IN) as previously described [Phadke et al., 2006]. Six mice were utilized per experimental group. Three weeks post-injection, mice were euthanized via CO2 inhalation. Mice were maintained under the guidelines of the NIH and The Pennsylvania State University. All protocols were approved and monitored by the Institutional Animal Care and Use Committee.
Femurs were photographed and cut into ends (proximal and distal metaphyses) and shaft. Bone marrow was separately flushed and collected. Isolated bone pieces were crushed and cultured separately in 1 ml α-MEM for 24 h, and culture supernatants collected.
RETRIEVED HUMAN BREAST CANCER CELLS
Breast cancer cells were grown out of the marrow by culture of the bone marrow in 1 ml of the respective breast cancer growth medium. Cancer cells present in the marrow were grown for ≤2 passages post-recovery and BCCM collected.
CYTOKINE ANALYSES
Cytokines were quantified with a Murine Bio-Plex™ Cytokine Assay System (Mouse Group I, Mouse Group II; Bio-Rad, Hercules, CA) or human Bio-Plex™ Cytokine Assay System (Bio-Rad). Cytokine concentrations were normalized to 1 million cells; CM batches had <15% variation in cell number. Cytokine protein levels were verified using species-specific sandwich ELISAs (R&D Systems, Minneapolis, MN) following R&D Systems recommended protocols. Intra-assay variation was typically <15%.
CYTOKINES
Murine IL-6, murine KC, human IL-6 recombinant proteins, and anti-IL-6 murine, anti-KC murine, and anti-TGF-β1,2,3 human neutralizing antibodies were obtained from R&D Systems. Murine and human VEGF recombinant protein and anti-VEGF murine neutralizing antibody were obtained from PeproTech (Rocky Hill, NJ). Anti-IL-6, anti-KC, and anti-VEGF murine neutralizing antibodies were used at 5 ng/ml. Murine IL-6, murine VEGF, and human VEGF recombinant proteins were used at 1 ng/ml. Murine KC recombinant protein was used at 0.1 ng/ml. The antibody concentrations were sufficient to neutralize IL-6, KC, and VEGF concentrations found in OBCM from 10- or 20-day old osteoblasts as determined by Murine Bio-Plex™ Cytokine Assay Systems. Human anti-TGF-β1,2,3 neutralizing antibody, was used at 5 μg/ml (sufficient to neutralize 25 ng/ml TGF-β).
CHEMOATTRACTION
To measure chemoattraction, MDA-MB-231W cells were stained with Vybrant DiI (Molecular Probes, Eugene, OR) and plated at 5 × 104 cells in BD™ Falcon FluoroBlok™ inserts (8 μm pores; Becton Dickinson, Franklin Lakes, NJ) in a transwell system with cytokines in the bottom chamber. Cytokine treatments included: OBCM from 10 or 20 day MC3T3-E1 cells plus 5 ng/ml anti-IL-6, anti-KC, and/or anti-VEGF mouse neutralizing antibody; 0.1 ng/ml murine KC, 1 ng/ml IL-6, and 1 ng/ml VEGF recombinant protein dried to the underside of the membrane inserts; human IL-6 (1 or 25 ng/ml) and VEGF (1 or 100 ng/ml) soluble recombinant protein; or OBCM from 10-day old osteoblasts plus 5 μg/ml anti-TGF-β1,2,3 neutralizing antibody. After 24 h, inserts were cut, mounted, and examined with a fluorescence microscope.
OSTEOCLAST FORMATION
Bone marrow cell suspension (10 μl in 1 ml growth medium) was added to the wells of a 24-well plate. Twenty-four hours later, 100 ng/ml recombinant murine M-CSF (PeproTech) and 50 ng/ml recombinant human RANK-L (PeproTech), or test molecules were added. Experimental treatments included: culture supernatant of 10-day old MC3T3-E1 cells treated with MDA-MB-231W CM for 24 h or 1, 10, or 100 ng/ml murine IL-6 recombinant protein.
One half volume OBCM or BCCM was combined with one half volume 2× osteoclast growth medium, to ensure that concentrations of serum and growth factors were identical for all treatments. Media were changed every 3rd day. Two weeks later, osteoclasts were stained for tartrate-resistant acid phosphatase (TRAP; Sigma) following manufacturer’s instructions. Cells were counter stained with acid hematoxylin for 5 min. TRAP positive, multinucleated (≥3 nuclei) osteoclasts were evaluated using light microscopy.
STATISTICAL ANALYSES
Statistical analyses were carried out using SAS, For Windows (SAS Version 9.2; SAS Institute, Cary, NC). Logarithmic transformed data were used for analyses. For in vitro analyses, an unbalanced analysis of variance was performed for each cytokine, with day, cell type, and their interaction as potential factors. Two-tailed P-values from Dunnett’s test for multiple comparisons against a control are reported. For in vivo analyses, data were analyzed using repeated measures analysis of variance, with Dunnett’s test for comparisons against the controls and Tukey’s correction for comparisons between cell types. Statistical significance was defined as a probability P ≤0.05.
RESULTS
OSTEOBLAST-DERIVED CYTOKINE PRODUCTION WAS INCREASED IN THE PRESENCE OF METASTATIC BREAST CANCER CONDITIONED MEDIUM
We previously reported that IL-6, IL-8, and MCP-1 increased in human osteoblasts exposed to breast cancer cell CM [Kinder et al., 2008]. To assay for additional cytokines related to the osteoblast response to metastatic breast cancer cells, Bio-Rad Bio-Plex™ 32 x-Plex Murine Cytokine Assays were carried out that included cytokines involved in inflammation (Mouse Group I, 23-plex plus Mouse Group II, 9-plex; Supplemental Table I). When MC3T3-E1 cells grown to 4, 10, or 20 days were treated with VM or MDA-MB-231 variant CM for 24 h, osteoblast-derived IL-6, MIP-2 (human IL-8), KC (human GRO-α), MCP-1, and VEGF increased. These results were similar among the three stages of osteoblast differentiation. Differences were most dramatic, however, with 10 days (Fig. 1a) or 20 days (Fig. 1b) osteoblasts.
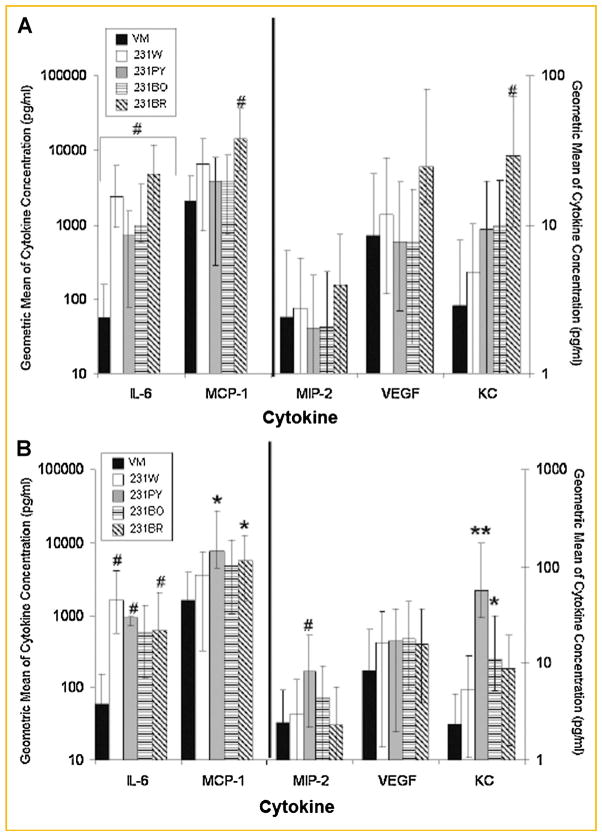
Fig. 1.
Osteoblasts increase inflammatory cytokine production in response to human metastatic breast cancer variants. The culture supernatant of MC3T3-E1 cells grown to 10 or 20 days and treated with breast cancer cell CM was assayed for osteoblast-derived cytokine production using a Bio-Rad Bio-Plex™ Murine Cytokine Assay. The geometric mean ± standard error of MC3T3-E1 cells grown to (A) 10 days; (B) 20 days. Black bar, osteoblast VM treatment; white bar, MDA-MB-231W CM treatment; light gray bar, MDA-MB-231PY CM treatment; bar with horizontal lines, MDA-MB-231BO CM treatment; bar with diagonal lines, MDA-MB-231BR CM treatment. Three biological replicates were cultured per experiment, and the experiment conducted twice for a total of six biological replicates. Cytokines in medium from osteoblasts treated with cancer cells were compared to those treated with VM. *P < 0.05, #P < 0.01, **P < 0.0001.
Osteoblast-derived cytokine expression consistently increased (up to six experiments per condition) when osteoblasts were treated with metastatic breast cancer CM. Specifically, osteoblast-derived IL-6 production increased up to 85-fold and was statistically significant in all cases (P < 0.01) when 10-day old osteoblasts were treated with breast cancer variant CM compared to VM (Fig. 1a, note log scale). In addition, treatment of 10-day old osteoblasts with MDA-MB-231BR breast cancer variant CM yielded nearly seven times the amount of osteoblast-derived MCP-1 than VM (P < 0.01), while osteoblast-derived KC expression increased up to 10 times (P < 0.01, Fig. 1a). MIP-2 and VEGF cytokine expression increased minimally in 10-day old osteoblasts with treatment of metastatic breast cancer CM (Fig. 1a). The largest increase in osteoblast-derived cytokines occurred with treatment of MDA-MB-231BR CM when compared to treatment with MDA-MB-231W, MDA-MB-231PY, or MDA-MB-231BO CM (Fig. 1a).
When osteoblasts were more fully differentiated, the increases in cytokines after treatment with breast cancer CM were greater in some cases than with less differentiated osteoblasts. Specifically, MIP-2 expression increased nearly 3.5-fold when 20-day old osteoblasts were treated with MDA-MB-231PY CM when compared to treatment with VM (P < 0.01), while KC expression increased up to 28.5-fold (P < 0.0001). MCP-1 expression increased up to fivefold (P < 0.05, Fig. 1b) and IL-6 expression increased up to 27-fold in the presence of breast cancer CM compared to VM (P < 0.01, Fig. 1b). VEGF expression increased minimally (Fig. 1b). The greatest increase in osteoblast-derived cytokines typically occurred following treatment of 20-day old osteoblasts with MDA-MB-231PY CM (Fig. 1b). Thus, osteoblasts produced more cytokines in the presence of breast cancer CM regardless of variant type. Furthermore, effects were more pronounced in late stage osteoblasts, suggesting that cancer cells elicit the largest production of osteoblast-derived cytokines in mature osteoblasts.
HUMAN METASTATIC BREAST CANCER CELL VARIANTS EXPRESS NEGLIGIBLE AMOUNTS OF MCP-1
To determine if human breast cancer cells produced the same cytokines as the murine osteoblasts, CM from MDA-MB-231 variants was assessed (Fig. 2). Since human cells do not express murine MIP-2 or KC, IL-8, and GRO-α were assayed [Fitzgerald et al., 2001].
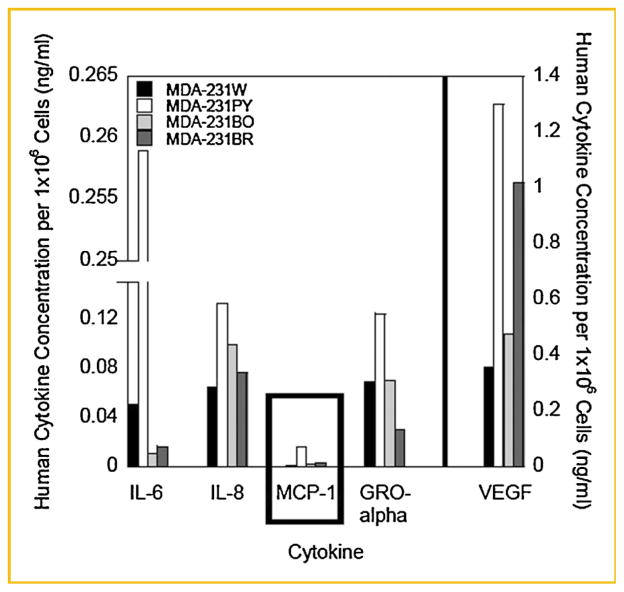
Fig. 2.
Human metastatic breast cancer cells express inflammatory cytokines. MDA-MB-231 BCCM, prepared as described in the Materials and Methods Section, was collected and a Bio-Rad Bio-Plex™ Human Cytokine Assay conducted. Cytokine concentration was normalized to 1 million cells; variation between batches of BCCM was <15%. A representative experiment is shown. Three individual batches of BCCM were assayed per MDA-MB-231 breast cancer variant. MDA-231W BCCM cytokine concentration, black bar; MDA-231PY BCCM, white bar; MDA-231BO BCCM, light gray bar; MDA-231BR BCCM, dark gray bar.
Human metastatic breast cancer VEGF concentration was nearly 400 times more than the concentrations of MCP-1, IL-8, IL-6, or GRO-α (Fig. 2). However, human breast cancer cells produced negligible amounts of MCP-1; 0.003–0.03 ng/ml (Fig. 2). These concentrations were in contrast to comparatively large amounts (~1.6–2.0 ng/ml) of murine-derived MCP-1 produced by MC3T3-E1 cells (Fig. 1a,b). On the other hand, human cancer cell variants produced greater amounts of VEGF; ~14 ng/ml (Fig. 2) compared to smaller amounts produced by MC3T3-E1 cells (0.005–0.014 ng/ml; Fig. 1a,b). Furthermore, human metastatic breast cancer cells expressed up to 50 times more GRO-α (murine KC), 58 times more IL-8 (murine MIP-2), and 4.5 times more IL-6 compared to MC3T3-E1 cells under culture conditions tested (Figs. 1a,b and 2).
BONE-DERIVED CYTOKINE PRODUCTION WAS INCREASED IN CANCER-BEARING MICE
To determine if changes in cytokines found in cell culture could also be seen in vivo, femurs from athymic mice inoculated with MDA-MB-231 human metastatic breast cancer cells were assayed. Three weeks post-inoculation, femurs were harvested, cultured as described in the Materials and Methods Section, and assayed for cytokine expression (Murine Bio-Rad Bio-Plex™ Cytokine Assays). A xenograft model (human cancer cells inoculated into athymic mice) permitted species-specific cytokine detection.
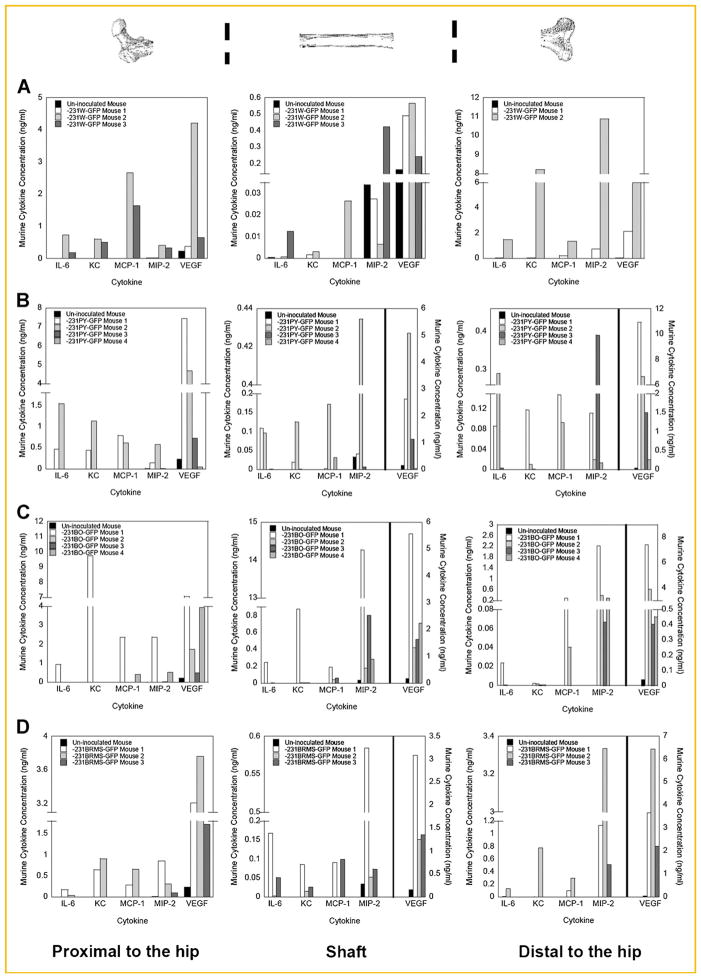
The same five murine cytokines detected in cell culture were increased in the ex vivo cultures of bone from athymic mice inoculated with human metastatic breast cancer cells or with metastasis-suppressed MDA-MB-231BRMS1 cells [Phadke et al., 2008]. Cytokine expression was examined from each end of the bone (distal vs. proximal metaphyses) as well as from the cortical bone (diaphysis). Standard deviation was high when mice were grouped as a whole, and therefore statistical significance could not be obtained (P > 0.05). However, it is clear in many cases that, when compared individually to control, that IL-6, MCP-1, MIP-2, KC, and VEGF cytokine levels increased in cancer-bearing mice (Fig. 3a–d). Bone-derived cytokine expression was greater in the metaphysis compared to the diaphysis of cancer-bearing mice (note scale bar; Fig. 3a–d). Up to a 10-fold increase in bone-derived MCP-1 was found in the proximal metaphysis compared to the bone shaft of MDA-MB-231-GFP variant cancer-bearing mice (Fig. 3a–c). A fivefold increase was also found in bone-derived MCP-1 production in the proximal metaphysis compared to the diaphysis in bones from MDA-MB-231BRMS1-GFP cancer-bearing mice (Fig. 3d). Therefore, MCP-1, produced in large quantities by osteoblasts and in negligible amounts by breast cancer cells, increased in concentration in the proximal metaphysis, an area where breast cancer cells preferentially traffic [Phadke et al., 2006].
Fig. 3.
Bone-derived cytokine production is increased in cancer-bearing mice. Bone-derived cytokine production was quantitated in bone regions proximal to the hip, shaft, and distal to the hip as described in the Materials and Methods Section. Culture supernatants were assayed for cytokine expression using a Bio-Rad Bio-Plex™ Murine Cytokine Assay. Shown is the bone-derived cytokine production for mice inoculated with (A) MDA-MB-231W, (B) MDA-MB-231PY, (C) MDA-MB-231BO, and (D) MDA-MB-231BRMS1 human breast cancer cells for the regions proximal to the hip (left), shaft (middle), and distal to the hip (right). Bone-derived cytokine concentrations of the un-inoculated mouse, black bar; MDA-MB-231-GFP cancer-bearing mouse #1, white bar; MDA-MB-231-GFP cancer-bearing mouse #2, light gray bar; MDA-MB-231-GFP cancer-bearing mouse #3, dark gray bar; MDA-MB-231-GFP cancer-bearing mouse #4, medium gray bar. One femur from each of three mice was used, and 3–4 mice were assayed per MDA-MB-231 breast cancer variant.
THE BONE MICROENVIRONMENT DID NOT ALTER CANCER CELL-DERIVED CYTOKINE PRODUCTION
To determine if the bone microenvironment altered cytokine production of inoculated cancer cells, cells were grown from the bone marrow of femurs of mice. Post-inoculation cancer cells were expanded to at least two passages post-recovery and BCCM subjected to a human multiplex assay.
All breast cancer cell lines were recovered from bone marrow except for MDA-MB-231BRMS1-GFP cells, which did not grow in culture. Normalized to the cytokine expression of 1 million cells, post-inoculation cells recovered from mice did not exhibit significant differences in cancer cell-derived cytokine expression compared with cancer cells pre-inoculation (P > 0.05, Table I). Furthermore, no significant differences were found among BC cell variants post-inoculation (P > 0.05, Table I).
TABLE I.
Cytokine Concentration of Pre-Inoculated and Retrieved Human Metastatic Breast Cancer Cell Variants
| Cytokine concentration (ng/ml) | MDA-231W-GFP Pre | MDA-231W-GFP Post | MDA-231PY-GFP Pre | MDA-231PY-GFP Post | MDA-231BO-GFP Pre | MDA-231BO-GFP Post | MDA-231BRMS-GFP Pre | MDA-231BRMS-GFP Post |
|---|---|---|---|---|---|---|---|---|
| Human metastatic breast cancer cell variant type | ||||||||
| IL-6 | 1.5 (1.5, 1.5) | 0.7 (0.7, 0.7) | 0.2 (0.2, 0.2) | 0.09 (0.03, 0.3) | 0.4 (0.4, 0.4) | 0.09 (0.05, 0.2) | 0.9 (0.9, 0.9) | Did not grow |
| GRO-a | 3.5 (3.5, 3.5) | 0.5 (0.5, 0.5) | 0.3 (0.3, 0.3) | 0.4 (0.4, 0.4) | 1.7 (1.7, 1.7) | 0.4 (0.3, 0.6) | 2.4 (2.4, 2.4) | Did not grow |
| MCP-1 | 0.04 (0.04, 0.04) | 0.02 (0.02, 0.02) | 0.06 (0.06, 0.06) | 0.04 (0, 0.09) | 0.05 (0.05, 0.05) | 0.03 (0.03, 0.03) | 0.02 (0.02, 0.02) | Did not grow |
| IL-8 | 2.3 (2.3, 2.3) | 0.6 (0.6, 0.6) | 0.5 (0.5, 0.5) | 0.5 (0.3, 0.8) | 2.6 (2.6, 2.6) | 0.8 (0.4, 1.9) | 2.3 (2.3, 2.3) | Did not grow |
| VEGF | 13.0 (13.0, 13.0) | 7.9 (7.9, 7.9) | 13.0 (13.0, 13.0) | 9.9 (5.9, 16.0) | 27.0 (27.0, 27.0) | 9.0 (8.3, 9.8) | 9.8 (9.8, 9.8) | Did not grow |
The cytokine concentration of pre-inoculated and retrieved human metastatic breast cancer cell variants was determined using a Bio-Rad Bio-Plex™ human cytokine array. Geometric mean (geometric mean −1 SD, geometric mean +1 SD), values were rounded to the nearest integer. Compilation of cells from at least two mice per region. No significant differences were found (P > 0.05)
OSTEOBLAST CONDITIONED MEDIUM WAS A CHEMOATTRACTANT FOR MDA-MB-231W HUMAN METASTATIC BREAST CANCER CELLS IN VITRO
A transwell assay was utilized to determine if cytokines produced by osteoblasts served to chemoattract cancer cells. OBCM was used as a positive control. OBCM from 10- (Fig. 4a) and 20-day old osteoblasts (Fig. 4b) were potent chemoattractants for MDA-MB-231W cells (used as a representative cell population since no differences were seen among cancer cell variants in other assays). Approximately 12% more MDA-MB-231W cells per field of view were chemoattracted toward OBCM from 20-day osteoblasts (980 cells) than were chemoattracted toward OBCM from 10-day osteoblasts (860 cells; Fig. 4a,b, Supplemental Table II).
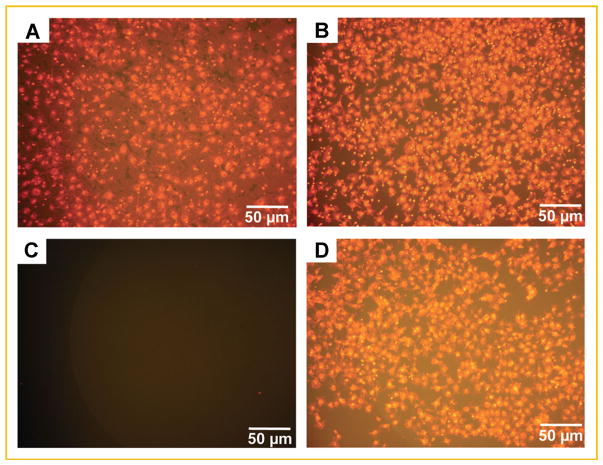
Fig. 4.
Osteoblast conditioned medium was a chemoattractant for MDA-MB-231W human metastatic breast cancer cells. MDA-MB-231W breast cancer cells stained with Vybrant DiI were seeded in the upper chamber of a transwell plate system with treatments as described in the Materials and Methods Section. Twenty-four hours later, membranes were removed and cancer cells on the insert underside were counted. MDA-MB-231W cell migration when subjected to OBCM from MC3T3-E1 cells grown to (A) 10 or (B) 20 days; (C) 25 ng/ml recombinant human IL-6 protein plus 100 ng/ml recombinant human VEGF protein; or (D) OBCM from MC3T3-E1 cells grown to 10 days plus 5 ng/ml each of anti-IL-6, anti-KC, and anti-VEGF mouse neutralizing antibody. Shown are representative experiments. Each treatment was conducted at least twice.
IL-6 and VEGF were assessed for chemoattraction; however, MDA-MB-231W cells were not chemoattracted to these molecules (Fig. 4c, Table II). Furthermore, cancer cell migration was not altered when murine IL-6, KC, and VEGF were neutralized in OBCM alone or in combination (Fig. 4d, Table II). Thus, neither IL-6, KC, or VEGF appeared to chemoattract MDA-MB-231W cells alone or in combination. Therefore, osteoblasts must secrete other molecules, besides IL-6, KC, or VEGF that were chemoattractants for metastatic breast cancer cells [Campo et al., 2006].
TABLE II.
Osteoblast Conditioned Medium Was a Chemoattractant for MDA-MB-231W Human Metastatic Breast Cancer Cells
| Chemoattractant | Mean ± standard deviation of MDA-MB-231W human metastatic breast cancer cells per field of view that migrated toward the chemoattractant |
|---|---|
| Conditioned medium from osteoblasts grown to 10 days | 860 ± 131.2 |
| Conditioned medium from osteoblasts grown to 20 days | 980 ± 138.6 |
| Human IL-6 plus VEGF recombinant protein | 0 |
| Conditioned medium from osteoblasts grown to 10 days incubated with anti-murine IL-6, KC, and VEGF | 970 ± 189.0 |
| Conditioned medium from osteoblasts grown to 10 days incubated with anti-TGFβ1,2,3 | 950 ± 268.6 |
Conditioned medium from osteoblasts grown to 10 or 20 days, human IL-6 plus VEGF recombinant protein, conditioned medium from osteoblasts grown to 10 days incubated with anti-murine IL-6, KC, and VEGF, or conditioned medium from osteoblasts grown to 10 days incubated with anti-TGF-β1,2,3 were assayed for their chemoattractant capabilities with MDA-MB-231W human metastatic breast cancer cells. The number of cells that migrated toward each chemoattractant was visualized on a fluorescent microscope and counted. The mean and standard deviation were calculated following the enumeration of three random fields of view.
TGF-β is a possible mediator of an osteoblast-derived inflammatory stress response to metastatic breast cancer cells [Kinder et al., 2008]. Therefore, a neutralizing antibody to TGF-β1,2,3 was tested in the assay. OBCM from 10-day old osteoblasts was a positive control (Fig. 5a, Table II). Little change (10%) in MDA-MB-231W cell migration towards OBCM was observed with the use of the TGF-β1,2,3 neutralizing antibody (Fig. 5b, Table II). Therefore, neutralization of TGF-β1,2,3 did not alter MDA-MB-231W chemoattraction.
Fig. 5.
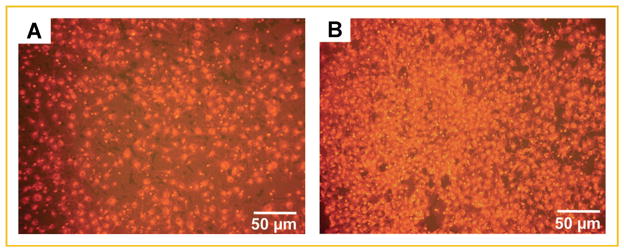
A neutralizing antibody to TGF-β1,2,3 did not alter MDA-MB-231W human metastatic breast cancer cell migration. MDA-MB-231W BC cells stained with Vybrant DiI were seeded in a transwell plate system with treatments as described in the Materials and Methods Section. Twenty-four hours later, membranes were removed and cancer cells on the insert underside were counted. MDA-MB-231W breast cancer cell migration when subjected to (A) OBCM from MC3T3-E1 cells grown to 10 days; and (B) OBCM from MC3T3-E1 cells grown to 10 days plus 5 μg/ml anti-TGF-β1,2,3. Each treatment was conducted at least twice. Shown are representative experiments.
OSTEOBLAST PLUS BREAST CANCER CELL CULTURE SUPERNATANT ELICITED THE FORMATION OF TRAP POSITIVE OSTEOCLASTS
We considered that IL-6, MCP-1, VEGF, KC/GRO-α, and MIP-2/IL-8 are directed towards osteoclastogenesis. Bone marrow monocytes were treated for 14 days with culture supernatant of MC3T3-E1 cells treated with MDA-MB-231W CM. TRAP positive multinucleated osteoclasts (at least three nuclei) formed from bone marrow monocytes following treatment with the culture supernatant from 10-day old osteoblasts treated with breast cancer cell CM (Fig. 6a).
Fig. 6.
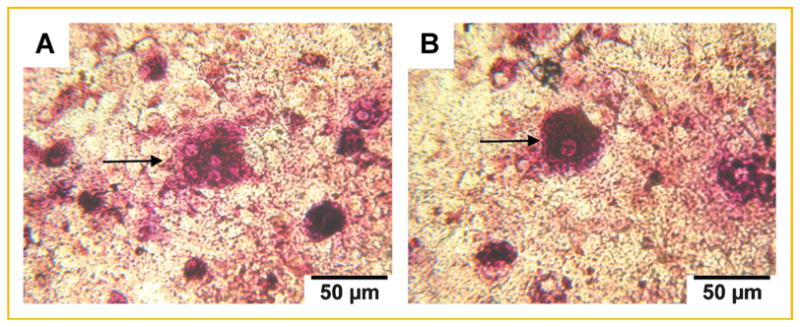
Supernatants from osteoblasts cultured with breast cancer cell conditioned medium elicited the formation of TRAP positive osteoclasts. Bone marrow monocytes were plated and treated as described in the Materials and Methods Section. Fourteen days later, osteoclast formation was determined via TRAP stain. Bone marrow monocytes treated with (A) culture supernatant of 10-day old osteoblasts treated with breast cancer cell CM; arrow indicates multinucleated (at least three nuclei), TRAP positive osteoclasts; (B) 1 ng/ml murine IL-6; arrow indicates mononucleated, TRAP positive cells. Representative images are shown of three biological replicates per condition.
Murine IL-6 was additionally assayed for osteoclast formation. Murine IL-6, added at concentrations of 1, 10, and 100 ng/ml (concentrations representative of IL-6 expression in both MC3T3-E1 cells and MDA-MB-231 cancer cells; Figs. 1a,b and 2), yielded only mononucleated TRAP positive cells (Fig. 6b). The numbers of TRAP positive cells did not increase with 10 or 100 ng/ml murine IL-6. These data suggested that 1 ng/ml murine IL-6 was sufficient to elicit TRAP positive cell formation, but did not lead to multinucleated cells under these assay conditions.
DISCUSSION
The results of this study showed that human metastatic breast cancer cells profoundly affected osteoblast- and bone-derived cytokine production in cell culture in vitro and in bone ex vivo. Osteoblast-derived cytokine production of IL-6, MIP-2 (human IL-8), KC (human GRO-α), MCP-1, and VEGF increased >50-fold in the presence of breast cancer cell CM during both osteoblast proliferation and differentiation. The osteoblast response to cancer cell CM was most substantial when osteoblasts were most mature. This stage of differentiation may best reflect osteoblasts in mature bone microenvironments in vivo.
We hypothesized that the amount of osteoblast-derived cytokines would increase more with CM or contact with a bone-seeking metastatic breast cancer cell variant; however, no differences in osteoblast-derived cytokine concentrations were seen between the variants tested, which included metastasis suppressed MDA-MB-231BRMS1 cells. This finding suggests that the osteoblast response to breast cancer cells may be a generalized inflammatory response to breast cancer cells in the bone, but unrelated to trafficking to bone. Furthermore, the MDA-MB-231BRMS1 do not form large colonies in vivo [Phadke et al., 2008]. Thus, the ratio of cancer cells to osteoblasts is small.
Interestingly, in comparison to the MC3T3-E1 cells, breast cancer cells expressed negligible amounts of MCP-1 (0.003–0.03 ng/ml; Fig. 2). In contrast, MCP-1 was expressed in comparably large amounts by MC3T3-E1 cells (1.6–2.0 ng/ml; Fig. 1a,b). MCP-1, a monomeric polypeptide member of the CC chemokine superfamily [Graves et al., 1999], is involved in inflammation and bone remodeling [Fitzgerald et al., 2001]. MCP-1 is known to recruit osteoclast progenitors from the blood or bone marrow, as well as cells involved in inflammation and angiogenesis, such as monocytes and tumor-associated macrophages [Goede et al., 1999; Fitzgerald et al., 2001]. MCP-1 is also produced by osteoblasts [Horowitz and Lorenzo, 2002], increased in metastatic cell lines [Neumark et al., 2002], and associated with increased breast cancer survival [Neumark et al., 2003]. MCP-1 is particularly important in cancer cell migration and metastasis where increased MCP-1 expression corresponded with increased cancer cell proliferation [Lu et al., 2006], invasion [Koide et al., 2004; Lu et al., 2006; Mestdagt et al., 2006], increased osteoclastogenesis, and bone resorption [Lu et al., 2007a].
When osteoblasts were treated with breast cancer cell CM, MCP-1 osteoblast production increased as much as sevenfold over control (Fig. 1a,b). Furthermore, athymic mice inoculated with human metastatic BC cells showed a nearly 10-fold increase in bone-derived MCP-1 expression in the proximal metaphysis, an area to which metastatic breast cancer cells preferentially traffic [Phadke et al., 2006] (Fig. 3a–d). Recently, it was reported that MCP-1 increases prostate cancer cell proliferation and invasion, as well as promotes prostate and lung cancer-induced osteoclast formation and activity in vivo [Lu et al., 2007b; Cai et al., 2009]. Here, we found that TRAP positive multinucleated osteoclasts were formed after incubation of bone marrow cells with culture supernatant of osteoblasts cultured with BC cell CM (Fig. 6a). These media contained 1.6–2.0 ng/ml MCP-1 (Fig. 1a,b). These data suggested that osteoblast-derived MCP-1 promotes osteoclast-induced formation in bone metastatic breast cancer.
Bone-derived cytokine production was also examined ex vivo. A xenograft model system of human cancer cells inoculated into athymic mice allowed for independent determination of breast cancer-derived (human) and bone-derived (murine) cytokines. Similar to the in vitro model, bone-derived cytokines increased in MDA-MB-231 cancer-bearing mice, irrespective of the breast cancer variant (Figs. 1a,b and 3a–d). Bone-derived cytokine production increased most in the bone metaphyses compared to the diaphysis. In a related study, we found by immunohistochemistry that MCP-1 and VEGF were localized to trabecular bone as opposed to shaft in both un-inoculated mice and cancer-bearing mouse bones [Bussard et al., 2010]. These findings suggested that bone-derived cytokines play a role in cancer cell localization within bone.
While it is clear that breast cancer cells exerted profound effects on bone cells, the bone microenvironment did not appear to alter the cancer cells for the cytokines tested (Table I). However, it may be that study conditions, including time post-inoculation, passage number ex vivo, and sample size were not sufficient for detecting differences in a limited number of cytokines. Furthermore, two of the variants were derived by several sequential passages through the bone or brain and may have already changed.
We found that OBCM was a potent chemoattractant for breast cancer cells (Fig. 4a, Table II). IL-8 (human; MIP-2, murine) is a chemoattractant for MDA-MB-231W cells, but MCP-1 is not [Mierke et al., 2007]. Addition of human and murine IL-6, KC, and VEGF, as well as neutralization of these cytokines separately, and in combination, were tested. None of these cytokines altered breast cancer cell migration to OBCM in a transwell assay.
If not chemoattractants, what is the role of osteoblast-derived cytokines in bone metastatic breast cancer? MDA-MB-231W cells express receptors to the cytokines identified in this study [Muller et al., 2001; Underhill and Heath, 2006; Wu et al., 2006; Raman et al., 2007], and it is also reported that these cytokines, IL-6, IL-8, and VEGF in particular, are maintenance factors for BC cells [Bendre et al., 2002; Starzec et al., 2006; Underhill and Heath, 2006]. Furthermore, these factors stimulate osteoclastogenesis, leading to increased bone resorption and the release from bone of other maintenance factors like TGF-β [Niida et al., 1999; Moonga et al., 2002; Mundy, 2002; Bendre et al., 2003b; Kim et al., 2005]. Thus, these cytokines may be indirectly involved in breast cancer cell maintenance and survival.
To further investigate the possibility that cytokines are involved in osteoclastogenesis and breast cancer cell maintenance, the effect of osteoblast-derived cytokines on osteoclast formation was examined. TRAP positive multinucleated osteoclasts (at least three nuclei) formed upon incubation with culture supernatant treatment of osteoblasts cultured with breast cancer cell CM (Fig. 6a). Osteoclasts were not formed when bone marrow monocytes were treated with IL-6 alone suggesting that cytokines in the culture supernatant other than or in addition to IL-6 (such as MCP-1) are necessary for osteoclast formation. However, these findings imply that osteoclast formation occurs when osteoblasts are affected by metastatic breast cancer cells either via a direct mechanism or by paracrine signaling.
Osteoblast-derived cytokines, particularly IL-6, IL-8/MIP-2, GRO-α/KC, MCP-1, and VEGF, appear to play important roles in bone metastatic BC. Even though some of these cytokines were not identified as chemoattractants, their roles bone metastatic breast cancer may be just as sinister. In addition to the RANK–RANKL initiation of osteoclastogenesis and vicious cycle of bone degradation, osteoclast precursors express receptors for IL-6, IL-8/MIP-2, GRO-α/KC, MCP-1, and VEGF [Niida et al., 1999; Moonga et al., 2002; Bendre et al., 2003b; Kim et al., 2005; Niida et al., 2005]. In the absence of RANK–RANKL pathway, these cytokines can stimulate osteoclastogenesis leading to bone degradation [Niida et al., 1999; Moonga et al., 2002; Bendre et al., 2003b; Kim et al., 2005]. With this knowledge, the following model of cytokine involvement in bone metastatic breast cancer, which extends the vicious cycle of bone degradation, is proposed (Fig. 7): osteoblast-derived IL-6, KC, MCP-1, MIP-2, and VEGF are produced during normal bone remodeling which occurs in the metaphyseal region of long bones. Metastatic breast cancer cells circulate to the bone and enter the marrow easily through endothelial sinusoids [Bussard et al., 2010]. They remain due to chemoattractant(s) other than those investigated here, causing an increase in osteoblast-derived cytokine production, osteoclastogenesis, and subsequently bone resorption. Additional breast cancer maintenance factors (TGF-β [Mundy, 2002]), are released from bone, facilitating breast cancer cell colonization.
Fig. 7.
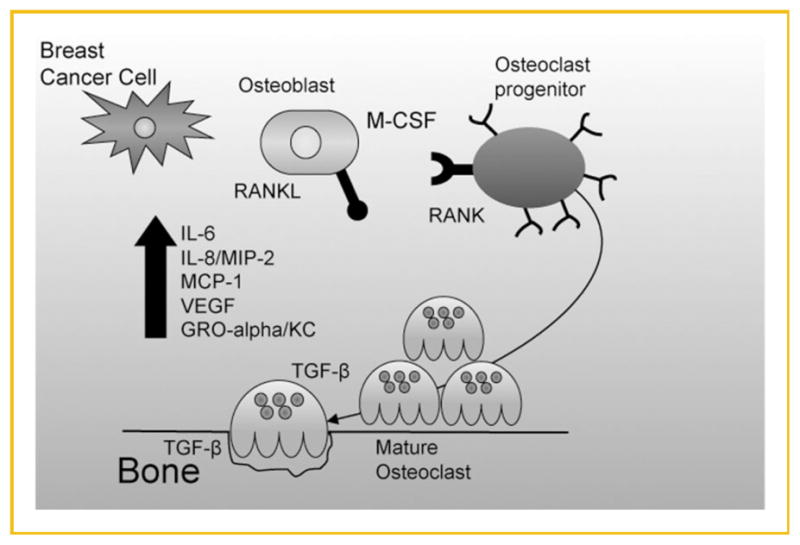
Proposed model of osteoblast-derived cytokine involvement in bone metastatic breast cancer. Osteoblasts naturally secrete basal levels of cyto-kines. At a defined point, the microenvironment is shifted to favor breast cancer cell colonization and survival. Breast cancer cells arrive in the bone microenvironment, causing an increase in osteoblast-derived cytokines. Osteoclastogenesis occurs, yielding increased bone resorption.
These findings support the hypothesis that breast cancer metastases create a unique bone niche by co-opting the normal cells of the bone to favor tumor cell colonization and survival. Herein, we show evidence that supports an addition to the vicious cycle of bone degradation, where the osteoblast-derived cytokines IL-6, VEGF, MCP-1, KC, and MIP-2 stimulate osteoclastogenesis in addition to or in the absence of the RANK–RANKL pathway. While other cells types are undoubtedly involved in metastatic and tumorigenic processes, we demonstrate a direct effect of metastatic BC cells on osteoblasts in addition to their well-known expression of RANKL [Boyce and Xing, 2008].
Supplementary Material
Acknowledgments
The authors would like to thank Yu-chi Chen for her assistance with graphing results. This work was supported by the U.S. Army Medical and Materiel Research Command Breast Cancer Program (DAMD 17-02-1-0358, W81XWH-06-1-0432, and W81XWH-08-1-0448 to A.M.M., and W81XWH-06-1-0363 to K.M.B.); National Foundation for Cancer Research, Center for Metastasis Research; and The Susan G. Komen Breast Cancer Foundation (BCTR0601044 and BCTR104406).
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- Bendre M, Gaddy-Kurten D, Foote-Mon T, Akel NS, Skinner RA, Nicholas RW, Suva LJ. Expression of interleukin 8 and not parathyroid hormone-related protein by human breast cancer cells correlates with bone metastasis in vivo. Cancer Res. 2002;62:5571–5579. [PubMed] [Google Scholar]
- Bendre M, Gaddy D, Nicholas RW, Suva LJ. Breast cancer metastasis to bone. Clin Orthop Rel Res. 2003a;415S:S39–S45. doi: 10.1097/01.blo.0000093844.72468.f4. [DOI] [PubMed] [Google Scholar]
- Bendre M, Montague DC, Peery T, Akel NS, Gaddy D, Suva LJ. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003b;33:28–37. doi: 10.1016/s8756-3282(03)00086-3. [DOI] [PubMed] [Google Scholar]
- Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussard KM, Okita N, Sharkey N, Neuberger T, Webb A, Mastro AM. Localization of osteoblast inflammatory cytokines MCP-1 and VEGF to the matrix of the trabecula of the femur, a target area for metastatic breast cancer cell colonization. Clin Exp Metastasis. 2010;27:331–340. doi: 10.1007/s10585-010-9330-3. [DOI] [PubMed] [Google Scholar]
- Cai Z, Chen Q, Chen J, Lu Y, Xiao G, Wu Z, Zhou Q, Zhang J. Monocyte chemotactic protein 1 promotes lung cancer-induced bone resorptive lesions in vivo. Neoplasia. 2009;11:228–236. doi: 10.1593/neo.81282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailleau R, Olive M, Cruciger QV. Long-term human breast carcinoma cell lines of metastatic origin: Preliminary characterization. In Vitro. 1978;14:911–915. doi: 10.1007/BF02616120. [DOI] [PubMed] [Google Scholar]
- Campo DA, Sosnowski DM, Koblinski JE, Gay CV. Roles of osteonectin in the migration of breast cancer cells into bone. J Cell Biochem. 2006;97:288–302. doi: 10.1002/jcb.20644. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, O’Neill LAJ, Gearing AJH, Callard RE. The cytokine facts book. San Diego: Academic Press; 2001. [Google Scholar]
- Goede V, Brogelli L, Ziche M, Augustin HG. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int J Cancer. 1999;82:765–770. doi: 10.1002/(sici)1097-0215(19990827)82:5<765::aid-ijc23>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Graves DT, Jiang Y, Valente AJ. The expression of monocyte chemoattractant protein-1 and other chemokines by osteoblasts. Front Biosci. 1999;4:D571–D580. doi: 10.2741/graves. [DOI] [PubMed] [Google Scholar]
- Hillner BE, Ingle JN, Berenson JR, Janjan NA, Albain KS, Lipton A, Yee G, Biermann JS, Chlebowski RT, Pfister DG. American society of clinical oncology guideline on the role of bisphosphonates in breast cancer. J Clin Oncol. 2000;18:1378–1391. doi: 10.1200/JCO.2000.18.6.1378. [DOI] [PubMed] [Google Scholar]
- Horowitz MC, Lorenzo JA. Local regulators of bone: IL-1, TNF, Lymphotoxin, Interferon-γ, IL-8, IL-10, IL-4, the LIF/IL-6 family, and additional cytokines. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of bone biology. San Diego: Academic Press; 2002. pp. 961–978. [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Kanis JA, McCloskey EV. Bone turnover and biochemical markers in malignancy. Cancer. 1997;80:1538–1545. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1538::aid-cncr3>3.3.co;2-v. [DOI] [PubMed] [Google Scholar]
- Kim MS, Day CJ, Morrison NA. MCP-1 is induced by RANKL, promotes human osteoclast fusion and rescues GM-CSF suppression of osteoclast formation. J Biol Chem. 2005;280:16163–16169. doi: 10.1074/jbc.M412713200. [DOI] [PubMed] [Google Scholar]
- Kinder M, Chislock EM, Bussard KM, Shuman LA, Mastro AM. Meta-static breast cancer induces an osteoblast inflammatory response. Exp Cell Res. 2008;314:173–183. doi: 10.1016/j.yexcr.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Koide N, Nishio A, Sato T, Sugiyama A, Miyagawa S. Significance of macrophage chemoattractant protein-1 expression and macrophage infiltration in squamous cell carcinoma of the esophagus. Am J Gastroenterol. 2004;99:1667–1674. doi: 10.1111/j.1572-0241.2004.30733.x. [DOI] [PubMed] [Google Scholar]
- Kukreja SC, Rosol TJ, Shevrin DH, York PA. Quantitative bone histomorphometry in nude mice bearing a human squamous cell lung cancer. J Bone Min Res. 1998;3:341–346. doi: 10.1002/jbmr.5650030314. [DOI] [PubMed] [Google Scholar]
- Lian JB, Stein GS. Concepts of osteoblast growth and differentiation: Basis for modulation of bone cell development and tissue formation. Crit Rev Oral Biol Med. 1992;3:269–305. doi: 10.1177/10454411920030030501. [DOI] [PubMed] [Google Scholar]
- Lu Y, Cai Z, Galson DL, Xiao G, Liu Y, George DE, Melhem MF, Yao Z, Zhang J. Monocyte chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate. 2006;66:1311–1318. doi: 10.1002/pros.20464. [DOI] [PubMed] [Google Scholar]
- Lu Y, Cai Z, Xiao G, Keller ET, Mizokami A, Yao Z, Roodman GD, Zhang J. Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Res. 2007a;67:3646–3653. doi: 10.1158/0008-5472.CAN-06-1210. [DOI] [PubMed] [Google Scholar]
- Lu Y, Xiao G, Galson DL, Nishio Y, Mizokami A, Keller ET, Yao Z, Zhang J. PTHrP-induced MCP-1 production by human bone marrow endothelial cells and osteoblasts promotes osteoclast differentiation and prostate cancer cell proliferation and invasion in vitro. Int J Cancer. 2007b;121:724–733. doi: 10.1002/ijc.22704. [DOI] [PubMed] [Google Scholar]
- Mestdagt M, Polette M, Buttice G, Noel A, Ueda A, Foidart J-M, Gilles C. Transactivation of MCP-1/CCL2 by β-catenin/TCF-4 in human breast cancer cells. Int J Cancer. 2006;118:35–42. doi: 10.1002/ijc.21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierke CT, Zitterbart DP, Kollmannsberger P, Raupach C, Schlotzer-Schrehardt U, Goecke TW, Behrens J, Fabry B. Breakdown of the endothelial barrier function in tumor cell transmigration. Biophys J. 2007;94:2832–2846. doi: 10.1529/biophysj.107.113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonga BS, Adebanjo OA, Wang H-J, Li X, Wu XB, Troen B, Inzerillo A, Abe E, Minkin C, Huang CL-H, Zaidi M. Differential effects of interleukin-6 receptor activation on intracellular signaling and bone resorption by isolated rat osteoclasts. J Endocrinol. 2002;173:395–405. doi: 10.1677/joe.0.1730395. [DOI] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Mundy GR. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- Neumark E, Cohn MA, Lukanidin E, Witz IP, Ben-Baruch A. Possible co-regulation of genes associated with enhanced progression of mammary adenocarcinomas. Immunol Lett. 2002;82:111–121. doi: 10.1016/s0165-2478(02)00026-3. [DOI] [PubMed] [Google Scholar]
- Neumark E, Sagi-Assif O, Shalmon B, Ben-Baruch A, Witz IP. Progression of mouse mammary tumors: MCP-1-TNF-alpha cross regulatory pathway and clonal expression of promalignancy and antimalignancy factors. Int J Cancer. 2003;106:879–886. doi: 10.1002/ijc.11337. [DOI] [PubMed] [Google Scholar]
- Niida S, Kaku M, Amano H, Yoshida H, Kataoka H, Nishikawa S, Tanne K, Maeda N, Nishikawa S-I, Kodama H. Vascular endothelial growth factor can substitute for macrophage colony-stimulating factor in the support of osteoclastic bone resorption. J Exp Med. 1999;190:293–298. doi: 10.1084/jem.190.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niida S, Kondo T, Hiratsuka S, Hayashi S-I, Amizuka N, Noda T, Ikeda K, Shibuya M. VEGF receptor 1 signaling is essential for osteoclast development and bone marrow formation in colony-stimulating factor-1 deficient mice. Proc Nat Aca Sci USA. 2005;102:14016–14021. doi: 10.1073/pnas.0503544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadke PA, Mercer RR, Harms JF, Yujiang J, Frost AR, Jewell JL, Bussard KM, Nelson S, Moore C, Kappes JC, Gay CV, Mastro AM, Welch DR. Kinetics of metastatic breast cancer cell trafficking in bone. Clin Cancer Res. 2006;12:1431–1440. doi: 10.1158/1078-0432.CCR-05-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadke PA, Vaidya KS, Nash KT, Hurst DR, Welch DR. BRMS1 suppresses breast cancer experimental metastasis to multiple organs by inhibiting several steps of the metastatic process. Am J Pathol. 2008;172:809–817. doi: 10.2353/ajpath.2008.070772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–165. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens RD, Mundy GR. Cancer and the skeleton. London: Martin Dunitz; 2000. [Google Scholar]
- Scapini P, Morini M, Tecchio C, Minghelli S, Di Carlo E, Tanghetti E, Albini A, Lowell C, Berton G, Noonan DM, Cassatella MA. CXCL1/macrophage inflammatory protein-2-induced angiogenesis in vivo is mediated by neutrophil-derived vascular endothelial growth factor-A. J Immunol. 2004;172:5034–5040. doi: 10.4049/jimmunol.172.8.5034. [DOI] [PubMed] [Google Scholar]
- Starzec A, Vassy R, Martin A, Lecouvey M, Benedetto MD, Crepin M, Perret GY. Antiangiogenic and antitumor activities of peptide inhibiting the vascular endothelial growth factor binding to neuropilin-1. Life Sci. 2006;79:2370–2381. doi: 10.1016/j.lfs.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Sudo H, Kodama HA, Amagai Y, Yamamoto S, Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983;96:191–198. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill N, Heath JK. Oncostatin M (OSM) cytostasis of breast tumor cells: Characterization of an OSM receptor beta-specific kernel. Cancer Res. 2006;66:10891–10901. doi: 10.1158/0008-5472.CAN-06-1766. [DOI] [PubMed] [Google Scholar]
- Wu Y, Hooper AT, Zhong Z, Witte L, Bohlen P, Rafii S, Hicklin DJ. The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survivial of human breast carcinoma. Int J Cancer. 2006;119:1519–1529. doi: 10.1002/ijc.21865. [DOI] [PubMed] [Google Scholar]
- Yoneda T. Mechanisms of preferential metastasis of breast cancer to bone. Int J Oncol. 1996;9:103–109. doi: 10.3892/ijo.9.1.103. [DOI] [PubMed] [Google Scholar]
- Yoneda T, Williams PJ, Hiraga T, Niewolna M, Nishimura R. A bone-seeking clone exhibits different biological properties from the MDA-MD-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res. 2001;16:1486–1495. doi: 10.1359/jbmr.2001.16.8.1486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.