Abstract
Recently, the simian type 1 transforming growth factor beta (TGF-beta 1) cDNA was expressed at high levels in Chinese hamster ovary (CHO) cells by dihydrofolate reductase-induced gene amplification (L.E. Gentry, N.R. Webb, G.J. Lim, A.M. Brunner, J.E. Ranchalis, D.R. Twardzik, M.N. Lioubin, H. Marquardt, and A.F. Purchio, Mol. Cell. Biol. 7:3418-3427, 1987). We have now purified and characterized the recombinant proteins released by these cells. Analyses of the precursor proteins by amino acid sequencing identified potentially important proteolytic processing sites. Signal peptide cleavage occurs at the Gly-29-Leu-30 peptide bond of pre-pro-TGF-beta 1, yielding pro-TGF-beta 1 (30 to 390). In addition, proteolytic processing of the precursor to yield mature TGF-beta 1 occurs at the dibasic cleavage site immediately preceding Ala-279, indicating that CHO cells possess the appropriate processing enzyme. Greater than 95% of the biological activity detected in the conditioned medium of the CHO transfectant was due to mature, properly processed growth factor. Highly purified recombinant TGF-beta 1 had the same specific biological activity as natural TGF-beta 1. The concentration of TGF-beta 1 required for half-maximal inhibition of Mv1Lu mink lung epithelial cell growth was approximately 1 to 2 pM. Purified precursor inhibited mink lung cell proliferation at 50 to 60 pM concentrations. The purified precursor preparation was shown to consist of pro-TGF-beta 1 (30 to 390), the pro region of the precursor (30 to 278), and mature TGF-beta 1 (279 to 390) interlinked by at least one disulfide bond with the pro portion of the precursor. These recombinant forms of TGF-beta1 should prove useful for further structural and functional studies.
Full text
PDF


Images in this article
Selected References
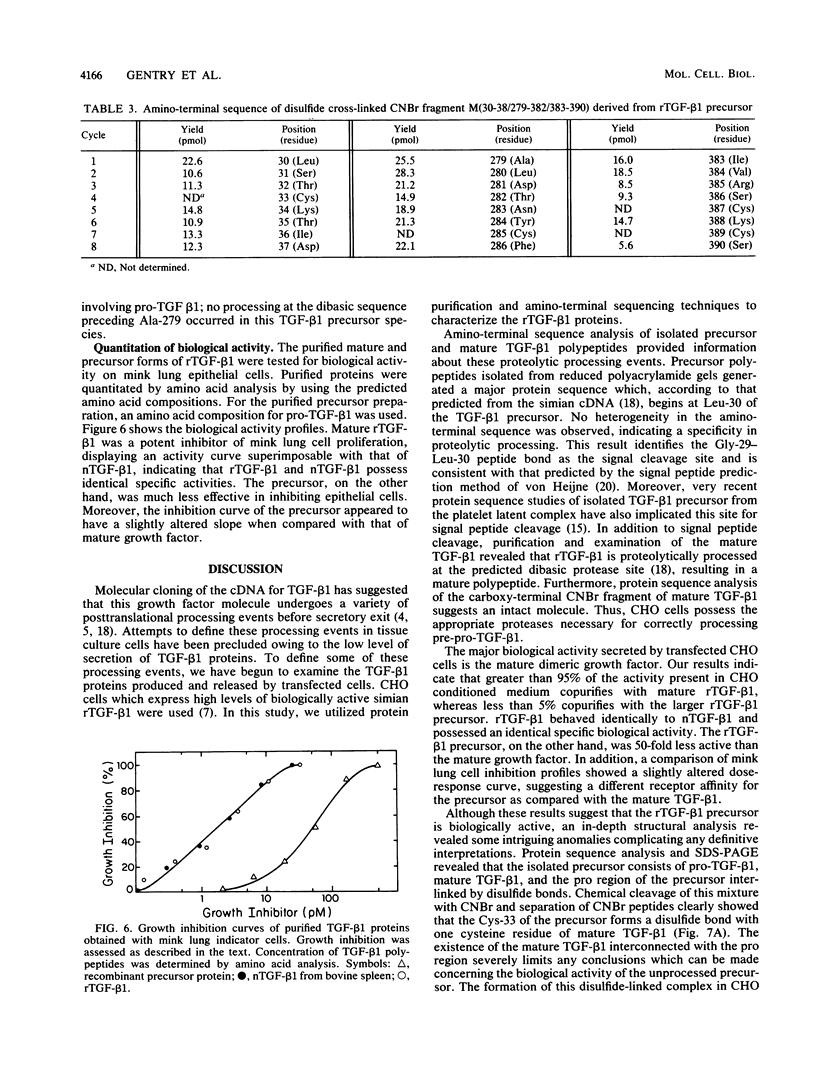
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner A. M., Gentry L. E., Cooper J. A., Purchio A. F. Recombinant type 1 transforming growth factor beta precursor produced in Chinese hamster ovary cells is glycosylated and phosphorylated. Mol Cell Biol. 1988 May;8(5):2229–2232. doi: 10.1128/mcb.8.5.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Goeddel D. V. The murine transforming growth factor-beta precursor. J Biol Chem. 1986 Apr 5;261(10):4377–4379. [PubMed] [Google Scholar]
- Frolik C. A., Dart L. L., Meyers C. A., Smith D. M., Sporn M. B. Purification and initial characterization of a type beta transforming growth factor from human placenta. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3676–3680. doi: 10.1073/pnas.80.12.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS E., WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [PubMed] [Google Scholar]
- Gentry L. E., Webb N. R., Lim G. J., Brunner A. M., Ranchalis J. E., Twardzik D. R., Lioubin M. N., Marquardt H., Purchio A. F. Type 1 transforming growth factor beta: amplified expression and secretion of mature and precursor polypeptides in Chinese hamster ovary cells. Mol Cell Biol. 1987 Oct;7(10):3418–3427. doi: 10.1128/mcb.7.10.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes J., Weissmann C. Constitutive, long-term production of human interferons by hamster cells containing multiple copies of a cloned interferon gene. Nucleic Acids Res. 1983 Feb 11;11(3):687–706. doi: 10.1093/nar/11.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T., Lioubin M. N., Marquardt H. Human transforming growth factor type beta 2: production by a prostatic adenocarcinoma cell line, purification, and initial characterization. Biochemistry. 1987 May 5;26(9):2406–2410. doi: 10.1021/bi00383a002. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Wasley L. C., Spiliotes A. J., Gossels S. D., Latt S. A., Larsen G. R., Kay R. M. Coamplification and coexpression of human tissue-type plasminogen activator and murine dihydrofolate reductase sequences in Chinese hamster ovary cells. Mol Cell Biol. 1985 Jul;5(7):1750–1759. doi: 10.1128/mcb.5.7.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keski-Oja J., Leof E. B., Lyons R. M., Coffey R. J., Jr, Moses H. L. Transforming growth factors and control of neoplastic cell growth. J Cell Biochem. 1987 Feb;33(2):95–107. doi: 10.1002/jcb.240330204. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Lioubin M. N., Ikeda T. Complete amino acid sequence of human transforming growth factor type beta 2. J Biol Chem. 1987 Sep 5;262(25):12127–12131. [PubMed] [Google Scholar]
- Miyazono K., Hellman U., Wernstedt C., Heldin C. H. Latent high molecular weight complex of transforming growth factor beta 1. Purification from human platelets and structural characterization. J Biol Chem. 1988 May 5;263(13):6407–6415. [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Meyers C. A., Wideman J., Blacher R., Pan Y. C., Stein S., Lehrman S. R., Smith J. M., Lamb L. C. Purification and properties of a type beta transforming growth factor from bovine kidney. Biochemistry. 1983 Dec 6;22(25):5692–5698. doi: 10.1021/bi00294a002. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Katz F. N., Lodish H. F. Glycosylation of a membrane protein is restricted to the growing polypeptide chain but is not necessary for insertion as a transmembrane protein. Cell. 1978 Dec;15(4):1447–1454. doi: 10.1016/0092-8674(78)90068-5. [DOI] [PubMed] [Google Scholar]
- Sharples K., Plowman G. D., Rose T. M., Twardzik D. R., Purchio A. F. Cloning and sequence analysis of simian transforming growth factor-beta cDNA. DNA. 1987 Jun;6(3):239–244. doi: 10.1089/dna.1987.6.239. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., Assoian R. K. Transforming growth factor-beta: biological function and chemical structure. Science. 1986 Aug 1;233(4763):532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- Wakefield L. M., Smith D. M., Flanders K. C., Sporn M. B. Latent transforming growth factor-beta from human platelets. A high molecular weight complex containing precursor sequences. J Biol Chem. 1988 Jun 5;263(16):7646–7654. [PubMed] [Google Scholar]
- Walter P., Gilmore R., Blobel G. Protein translocation across the endoplasmic reticulum. Cell. 1984 Aug;38(1):5–8. doi: 10.1016/0092-8674(84)90520-8. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]