Abstract
To define high risk acute graft-versus-host disease (GVHD) at onset, we examined the initial GVHD stage and grade of 864 patients at the University of Minnesota who received uniform therapy with prednisone 60 mg/m2/d. We compared the prognostic utility of the Minnesota (MN) (modified from Consensus) versus Center for International Blood and Marrow Transplant Research (CIBMTR) GVHD organ stage-derived grading systems. As neither GVHD grading system optimally predicted outcomes, a novel acute GVHD risk score was devised by combining the MN and CIBMTR systems. Using multiple regression analysis, we could dichotomize patients into high risk (HR, n=86) acute GVHD with initial grade IIIC, IIID or IVD who were less likely to respond to steroid therapy by day 28 (RR, 0.3, p<.001) and had a higher risk for transplant related mortality (RR. 2.0, p<.001) than patients with standard risk (SR, initial grade IA–IIIB, n=778) GVHD. Using this novel acute GVHD Risk Score, HR GVHD is either skin stage 4, lower gastrointestinal (GI) stage 3+, liver stage 3+, or skin stage 3 and lower GI or liver stage 2+ GVHD. Patients with HR acute GVHD have a poor prognosis, require alternative initial therapy and should be the focus of novel therapeutic trials.
Keywords: acute GVHD, TRANSPLANTATION, STEROIDS, initial therapy, prognosis, risk
Introduction
Despite many advances in haematopoietic cell transplantation (HCT), acute graft-versus-host disease (GVHD) remains a major cause of morbidity and mortality. Corticosteroids are the conventional first-line systemic therapy, but only 1/2 of patients will respond and only 1/3 will have a durable response (MacMillan, et al 2002b, Weisdorf, et al 1990a). It is well established that patients with severe (clinical grade III–IV) acute GVHD are less responsive to steroids leading to poor survival and high transplant related mortality (TRM). However, clinical observations suggest that there is also a subset of moderately severe (grade II) patients who fair poorly and warrant alternative upfront therapy. Early identification of these patients with high risk (HR) acute GVHD may allow for more appropriate and potentially effective upfront therapy. We have previously shown improved survival in patients with steroid resistant GVHD who receive early second line therapy (MacMillan, et al 2002a) and we hypothesize that early recognition of those destined to fail upfront therapy may also improve outcomes.
In order to define HR acute GVHD at diagnosis, we examined the outcomes of 864 patients at the University of Minnesota by their initial GVHD organ stage and grade. All patients received prednisone 60 mg/m2/d for 14 days, followed by an 8-week taper. We compared the prognostic utility of the Minnesota (Przepiorka, et al 1995, Weisdorf, et al 1990b) versus the Center for International Blood and Marrow Transplant Research (CIBMTR) (Rowlings, et al 1997) grading systems to predict both response and treatment-related (non-relapse) mortality (TRM). We previously reported that day 28 complete or partial response (CR + PR) is the best early endpoint for predicting 2-year TRM (MacMillan, et al 2010) and therefore used day 28 response, TRM and overall survival as the endpoints for our analysis.
Patients and Methods
Study design
Clinical and laboratory data were systematically and prospectively collected on all our patients undergoing HCT and entered into the University of Minnesota Blood and Marrow Transplant Database. All HCT protocols were reviewed and approved by the Masonic Cancer Center Protocol Review Committee and the Institutional Review Board (IRB) at the University of Minnesota. All patients and/or guardians signed IRB approved informed consent for HCT and data collection in accordance with the Declaration of Helsinki.
Between January 1990 and December 2007, 2406 consecutive patients underwent allogeneic HCT at the University of Minnesota. Of these, 1149 developed grade I–IV acute GVHD and 864 treated with prednisone 60 mg/m2 PO (or methylprednisolone 48 mg/m2 IV) as initial therapy were subjects for this analysis. Others received only topical corticosteroids or combination therapies dictated by active therapeutic protocols and were not included in this analysis. At the time of analysis, surviving patients had 0.8 – 17.1 years of follow-up (median 6.9 years).
Patient and Transplant Characteristics
Patient demographics including year of transplant, recipient age, gender, CMV serostatus, and underlying diagnosis are shown in Table 1 and are described previously (MacMillan, et al 2010). Median patient age was 32 years (range, 0.2 – 69) with 35% <18 years of age. Standard disease risk was defined as acute leukaemia in first or second complete remission, chronic myelogenous leukaemia in first chronic phase, or myelodysplastic syndrome without excess blasts. All other patients were considered high risk.
Table 1.
Patient and Transplant Characteristics
| Factors | N (%) |
|---|---|
| Total | 864 |
| Year of Transplant | |
| 1990–1995 | 289 (33%) |
| 1996–2000 | 278 (32%) |
| 2001–2005 | 238 (28%) |
| 2006–2007 | 59 (7%) |
| Age | |
| <10 years | 193 (22%) |
| 10–17 years | 108 (13%) |
| 18–35 years | 191 (22%) |
| ≥36 years | 372 (43%) |
| Median (range) | 32 (0.2–69) |
| Gender | |
| Male | 530 (61%) |
| Female | 334 (39%) |
| CMV Serostatus | |
| Recipient negative, donor negative | 340 (39%) |
| Recipient negative, donor positive | 107 (12%) |
| Recipient positive | 417 (48%) |
| Disease | |
| ALL | 141 (16%) |
| AML | 193 (22%) |
| CML | 179 (21%) |
| MDS/MPD | 72 (8%) |
| NHL | 63 (7%) |
| Other Malignancies | 52 (7%) |
| SAA/FA | 55 (6%) |
| Immune Deficiency | 25 (3%) |
| Storage Disorder | 82 (9%) |
| Disease Risk* | |
| Standard | 431 (50%) |
| High | 433 (50%) |
| Donor Type | |
| HLA-Matched Sibling | 315 (36%) |
| Mismatched Relative | 24 (3%) |
| Well Matched URD | 79 (9%) |
| Partially Matched URD | 108 (13%) |
| Mismatched URD | 126 (15%) |
| Single UCB | 89 (10%) |
| Double UCB | 123 (14%) |
| Conditioning | |
| Myeloablative | 736 (85%) |
| Non-Myeloablative | 128 (15%) |
| GVHD Prophylaxis | |
| CSA or tacrolimus containing | 719 (83%) |
| T-cell depletion | 112 (13%) |
| MTX ±ATG/Prednisone | 33 (4%) |
ALL = acute lymphoblastic leukaemia; AML = acute myelogenous leukaemia, CML = chronic myelogenous leukaemia; MDS = myelodysplastic syndrome; MPD = myeloproliferative disease; NHL = Non-Hodgkins lymphoma; SAA = severe aplastic anemia; FA = Fanconi anaemia. Standard risk = acute leukemia in CR1 or CR2, CML in first chronic phase, MDS without excess blasts or non-malignant diseases. High risk = all others. CSA = cyclosporine A; MTX = methotrexate; MMF = mycophenolate mofetil; ATG = anti-thymocyte globulin.
S tandard risk indicates acute leukemia in CR1 or CR2, CML in first chronic phase, or MDS without excess blasts. High risk indicates all others.
Transplant characteristics including donor type, preparative therapy, and GVHD prophylaxis are shown in Table 1. Related sibling donors and recipients were typed at antigen level for HLA-A, -B, and -DRB1 unless adequate family typing was not available to determine haplotypes, which were then confirmed by allele level DNA typing. For unrelated donors (URD), donors and recipients were initially typed for HLA-A and -B at antigen level and allele level typing at HLA-DRB1 until 2004 when allele typing for HLA-C and other loci was fully implemented. HLA-matching followed the definitions used by the CIBMTR (Weisdorf, et al 2008). For umbilical cord blood (UCB) transplants, patients and donors were typed for HLA-A and -B at antigen level and for DRB1 at allele level (Barker, et al 2001, Barker, et al 2005). HLA-DQ and -DP were not considered in URD or UCB donor selection.
Graft sources included HLA identical sibling bone marrow (BM) or peripheral blood stem cells (PBSC; n = 315), HLA mismatched sibling or related donor BM or PBSC, (n = 24), well matched (Weisdorf, et al 2008) URD BM or PBSC (n = 79), partially matched URD BM or PBSC (n = 108), mismatched URD BM or PBSC (n=126), single unit (n = 89) or double UCB (n=123).
Details of the preparative therapy, GVHD prophylaxis and supportive therapy techniques have been previously reported (MacMillan, et al 2010). The majority (89.5%) of patients received a total body irradiation (TBI) based regimen and 10.5% patients received chemotherapy alone. GVHD prophylaxis was assigned differently between HCT from different stem cell sources as shown in Table 1 and consisted of cyclosporine A (CSA) or tacrolimus based therapy in 83% of patients, ex vivo T-cell depletion in 13% of patients, and methotrexate (MTX) alone in 4% of patients.
Diagnosis, Staging and Grading of GVHD
Signs and symptoms of acute GVHD were graded by the Minnesota (MN) grading system2 and the CIBMTR severity index (Table 2) (Rowlings, et al 1997). The MN grading system uses standard clinical criteria derived from organ staging (Glucksberg, et al 1974), modified to include upper gastrointestinal (GI) acute GVHD per the GVHD consensus conference (MacMillan, et al 2002b, Przepiorka, et al 1995, Weisdorf, et al 1990b). Grade of GVHD refers to clinical (not histologic) grade throughout this report. Initial grade was calculated using the maximum stage in each organ within a 10 day window (−5 to +5 days) of initiation of steroid therapy. Prospective real-time organ staging and grading of GVHD was determined weekly by the attending physician, supported by laboratory and clinical information and histologic confirmation when possible. The grading scheme was consistent throughout the study period. While all patients’ GVHD diagnoses and maximum GVHD grades were retrospectively reviewed by the Acute GVHD Grading Committee (MLM and DJW), the initial and overall grade by either schema (MN or CIBMTR) used for this analysis was determined by a computer algorithm, incorporating all available clinical and pathologic GVHD organ staging data as originally and prospectively recorded, not modified by our retrospective review. Responses at weekly or biweekly endpoints were determined by review of the prospectively recorded staging and grading data.
Table 2.
Acute GVHD Grading Systems
| Grade* | Skin† | Liver | LGI | UGI |
|---|---|---|---|---|
| Minnesota (MacMillan, et al 2002b, Przepiorka, et al 1995, Weisdorf et al 1990b) | ||||
| I | 1–2 | 0 | 0 | 0 |
| II | 3 | 1 | 1 | 1 |
| III | - | 2–4** | 2–3** | |
| IV | 4 | - | 4 | |
| CIBMTR(Rowlings, et al 1997)§ | ||||
| A | 1 | 0 | 0 | 0 |
| B | 2 | 1–2 | 1–2 | 1 |
| C | 3 | 3 | 3 | |
| D | 4 | 4 | 4 |
Each grade is based on maximum stage for each involved organ
Each column identifies minimum organ stage for overall grade.
Modified as shown to include UGI GVHD.
Consensus Grading differs from Minnesota Grading only by assigning stage 2–4 LGI as grade III and stage 4 liver as grade IV.(Przepiorka, et al 1995)
GVHD Therapy
All patients received daily, thrice divided doses of prednisone 60 mg/m2/day PO (or methylprednisolone IV equivalent, 48 mg/m2) for 7 consecutive days, followed by daily, single dose, prednisone for 7 days as initial therapy for acute GVHD. Patients were maintained on therapeutic levels of CSA in 795 (93%) patients or tacrolimus in 15 (2%) patients. Additionally, patients with acute skin GVHD were treated with topical 0.1% triamcinolone cream or 1% hydrocortisone cream (for facial rash) three times daily. If a response to prednisone was observed, patients continued therapy with oral prednisone 60 mg/m2/day thru day 14 and then commenced a taper of steroids over 8 weeks (Hings, et al 1993).
Measurement of GVHD Response to Prednisone
Response to therapy was evaluated by the attending physician and prospectively recorded weekly in the University of Minnesota BMT Database by determining the GVHD clinical stage score for each time point (±3 days) (Hings, et al 1994). Response was determined from the maximum acute GVHD stage and grade in each organ at day 28 (±7 days) after prednisone treatment was initiated. Complete response (CR) was defined as the complete resolution of acute GVHD symptomatology in all organs, without secondary GVHD therapy. Partial response (PR) was defined as improvement in GVHD stage in all initial GVHD target organs without complete resolution, without worsening in any other GVHD target organs and without secondary GVHD therapy. No response (NR) was defined as the same severity of GVHD in any organ or death, or the addition of secondary GVHD therapy. Progression was defined as worsening GVHD in ≥1 organ with or without improvement in any organ. Steroid resistant acute GVHD was defined as progression of acute GVHD after 4 days of treatment with prednisone or no improvement after 7 days of treatment. Patients with steroid resistant GVHD were treated with secondary therapy and were considered to have no response. If patients experienced a flare of acute GVHD before day 28 and required therapy with a boost of steroids or the additional GVHD therapy, they were also considered to have no response.
Supportive Care
Patients received antibiotic prophylaxis suitable for the functionally hyposplenic state accompanying GVHD. Broad-spectrum intravenous antibacterial and as indicated antifungal antimicrobials were used when patients developed fever. Patients received acyclovir prophylaxis if they were seropositive for herpes simplex virus and/or cytomegalovirus (CMV). Oral trimethoprim-sulfamethoxazole was given for pneumocystis carinii pneumonia prophylaxis. CMV-seronegative recipients received CMV-safe (seronegative or filtered) blood products.
Statistical Analysis
Univariate assessment of day 28 response by the Minnesota and CIBMTR and a combined MN-CIBMTR index score were performed by the Cochran-Armitage test for trend by index grade (I–IV, or A–D, or IA–IVD) (Armitage 1955). Logistic regression was used to examine the independent effect of each index (at initial treatment for acute GVHD) in predicting the endpoint of response. Positive and negative predictive values of response using simple proportions were also used to compare index scores by categorizing standard risk versus high risk for each score (I–II versus III–IV for Minnesota, A–B versus C–D for CIBMTR and IA–IIIB versus IIIC–IVD for the combined MN-CIBMTR score). Different sample sizes in the high risk and standard risk groups among indices precluded the use of sensitivity and specificity. The positive and negative predictive values were used instead since they are not affected by different group sizes across the indices. Our goal was to identify high risk patients who require alternate upfront therapy not directly to identify standard risk patients who will do well with steroids.
For the endpoints of survival and TRM, the indices were tested at the time of treatment as well as by the maximum GVHD grade. Overall survival and the positive and negative predictive values for survival by the grading index after treatment were estimated by Kaplan-Meier curves (Kaplan and Meier 1958). TRM and the positive and negative predictive values were estimated using cumulative incidence treating relapse as a competing risk (Lin 1997). Comparisons of the endpoints were completed with the log-rank test for trend.
Cox regression was used to assess the independent effect of the indices on two-year overall survival (Cox 1972) and Fine and Gray proportional hazards regression was used to assess the independent effect of the indices on TRM (Fine and Gray 1999). Factors tested in the regression models were: Minnesota grade (I versus II versus III versus IV) or CIBMTR grading index (A versus B versus C versus D) or the combined MN-CIBMTR index (IA versus IB versus IIB versus IIC versus IIIB versus IIIC versus IIID versus IVD), donor type (sibling versus URD well matched versus URD partially matched versus URD mismatched versus UCB), days from HCT to steroid treatment (<28 versus >=28 days), GVHD prophylaxis (MTX versus CSA or tacrolimus, versus T-cell depletion), CMV serostatus (patient/donor negative versus patient negative/donor positive versus patient positive), conditioning (TBI containing myeloablative versus no TBI myeloablative versus non-myeloablative), age (<10 versus 10–17 versus 18–35 versus >35 years) and disease risk (standard versus high).
Results
Initial GVHD stage in each organ is shown in Table 3. Initial GVHD organ involvement was skin only (n = 498; 57%), upper and/or lower GI only (n = 146; 17%), liver only (n = 7; 1%) or multiorgan (n = 213; 25%). Median time to onset of GVHD was 32 days after HCT (range, 8 – 99). Median time to treatment with prednisone was 33 days (range, 8 – 99).
Table 3.
GVHD Organ Stage and Clinical Grade in 864 Patients at Onset of Prednisone Therapy
| Organ Stage | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Skin | 165 (19%) | 111 (13%) | 224 (26%) | 357 (41%) | 7 (1%) |
| Liver | 797 (92%) | 22 (3%) | 27 (3%) | 13 (2%) | 5 (1%) |
| Lower GI | 668 (77%) | 113 (13%) | 40 (5%) | 39 (5%) | 4 (<1%) |
| Upper GI | 644 (75%) | 220 (25%) | |||
| Initial CIBMTR Grade | |||||
| Initial MN Grade | A | B | C | D | |
| I | 68 | 163 | 0 | 0 | |
| II | 0 | 183 | 321 | 0 | |
| III | 0 | 43 | 71 | 5 | |
| IV | 0 | 0 | 0 | 10 | |
Prior to initiation of steroid therapy, initial GVHD MN grades were grade I in 231 (27%) patients, grade II in 504 (58%) patients, grade III in 119 (14%) patients, and grade IV in 10 (1%) patients. Initial CIBMTR grades were grade A in 68 (8%) patients, grade B in 389 (45%) patients, grade C in 392 (45%) patients, and grade D in 15 (2%) patients.
Overall response (CR+PR) was observed in 563 of 864 patients (65%, 95% CI 62–68%) patients at day 28 after steroid initiation. For the entire cohort of 864 patients, survival at 2 years after initiation of steroid therapy was 48% (95% CI 44–51%) and TRM at 2 years after initiation of steroid therapy was 39% (95% CI 36–42%).
Minnesota and CIBMTR Grading Systems
Response to steroid therapy, survival and TRM using either grading system are shown in Table 4. Grading using either the MN or the CIBMTR systems showed significantly less frequent response to steroids in patients with higher GVHD grades, and increased TRM with higher CIBMTR grades. However, as the cohorts are of differing size and heterogeneity, there is only limited predictive value for response to steroid therapy and mortality for each increase in grade.
Table 4.
Day 28 CR/PR, 2 year Survival and TRM by Grading System
| Initial GVHD Grade | N | CR/PR at day 28 (%) | P | 2 Year Survival (95% CI) | P | 2 Year TRM (95% CI) | P |
|---|---|---|---|---|---|---|---|
| Minnesota | <0.001 | 0.34 | 0.26 | ||||
| I | 231 | 164 (71%) | 49% (43–56%) | 39% (33–45%) | |||
| II | 504 | 332 (66%) | 48% (44–52%) | 38% (34–42%) | |||
| III | 119 | 65 (55%) | 33% (35–52%) | 43% (34–52%) | |||
| IV | 10 | 2 (20%) | 50% (18–75%) | 50% (19–81%) | |||
| Minnesota | <0.001 | 0.22 | 0.08 | ||||
| I&II | 735 | 496 (67%) | 49% (45–52%) | 38% (35–41%) | |||
| III&IV | 129 | 67 (52%) | 44% (35–53%) | 43% (34–52%) | |||
| CIBMTR | 0.003 | 0.06 | 0.04 | ||||
| A | 68 | 47 (69%) | 54% (42–65%) | 35% (24–36%) | |||
| B | 389 | 269 (69%) | 50% (45–55%) | 36% (31–41%) | |||
| C | 392 | 244 (62%) | 45% (40–50%) | 42% (37–41%) | |||
| D | 15 | 3 (20%) | 47% (21–69%) | 53% (27–79%) | |||
| CIBMTR | 0.01 | 0.07 | 0.05 | ||||
| A&B | 457 | 316 (69%) | 51% (46–55%) | 36% (32–40%) | |||
| C&D | 407 | 247 (61%) | 45% (40–50%) | 42% (37047%) | |||
| MN-CIBMTR | |||||||
| Standard Risk | |||||||
| IA | 68 | 47 (69%) | <0.001 | 54% (42–65%) | 0.03 | 35% (23–47%) | 0.06 |
| IB | 163 | 117 (72%) | 47% (39–55%) | 40% (32–48%) | |||
| IIB | 183 | 122 (67%) | 51% (44–58%) | 35% (28–42%) | |||
| IIC | 321 | 210 (65%) | 46% (41–52%) | 40% (35–45%) | |||
| IIIB | 43 | 30 (70%) | 53% (38–67%) | 28% (15–41%) | |||
| High Risk | |||||||
| IIIC | 71 | 34 (48%) | 38% (47–49%) | 51% (39–63%) | |||
| IIID | 5 | 1 (20%) | 40% (5–75%) | 60% (19–81%) | |||
| IVD | 10 | 2 (20%) | 50% (18–75%) | 50% (20–80%) | |||
| Standard Risk | 778 | 526 (68%) | <0.001 | 49% (45–52%) | 0.03 | 38% (35–41%) | <0.001 |
| High Risk | 86 | 37 (43%) | 40% (29–50%) | 51% (40–62%) |
A comparison of the initial GVHD MN and CIBMTR organ stage involvement and grade is shown in Table 3. The CIBMTR index yields a higher, but heterogeneous GVHD grade for each given combination of GVHD stages, most notably for CIBMTR grades B and C. Of the 389 patients scored as having CIBMTR grade B acute GVHD, 163 (42%) were scored as grade I, 183 (47%) as grade II and 43 (11%) as grade III using the MN grading system. Of the 392 patients scored as having CIBMTR grade C acute GVHD, 321 (82%) were scored as grade II and 71 (18%) as grade III using the MN grading system. There was a stronger correlation between the two scoring systems with the mildest and the most severe GVHD, but inconsistency in the intermediate (grade B or C) cohorts. All patients scored as CIBMTR grade A were scored as MN grade I. Of the 15 patients with CIBMTR grade D, 5 scored as MN grade III and 10 as MN grade IV.
Proposed New Acute GVHD Risk Score
A combined MN-CIBMTR grading system was then devised by combining the initial GVHD grade as determined by the MN and CIBMTR grade (as shown at the bottom of Table 3). For example, 231 patients had an initial MN grade I GVHD. Of these patients, 68 were scored as CIBMTR grade A and scored as IA in our new system; and 163 initial MN grade I patients were scored as CIBMTR grade B and now scored as IB in our new system. As shown in the bottom of Table 3, this new scoring system highlights the heterogeneity of the individual MN and CIBMTR grading systems; particularly MN grades II and III and CIBMTR grades B and C.
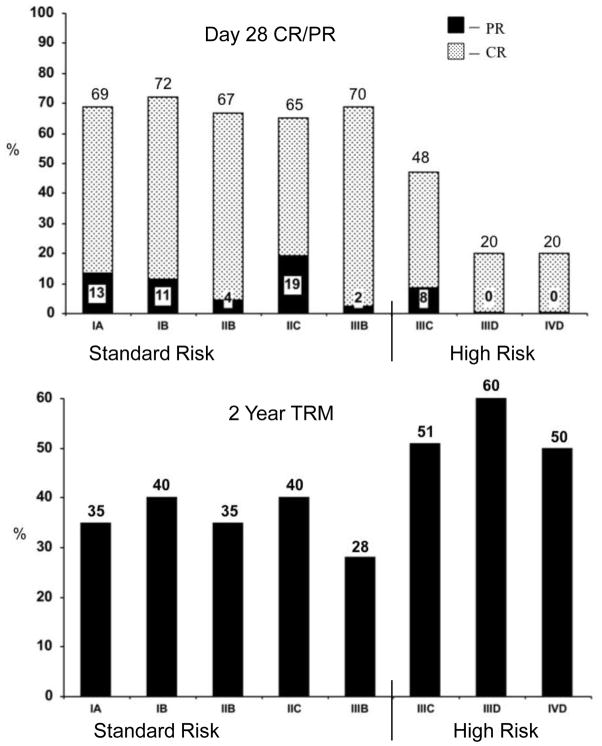
This new MN-CIBMTR acute GVHD risk score was used to determine the probability of day 28 response (CR + PR) and 2 year TRM (Table 4). Patients with initial grade IIIC, IIID or IVD were combined as high risk (HR) acute GVHD and were significantly less likely to respond to steroids than those with less severe, standard risk (SR) GVHD (43 [95% CI 33–53%] vs. 68% [95% CI 65–71%], CR + PR at day 28, p <.001) as shown in Figure 1. SR patients had an earlier time of onset of acute GVHD at a median of 29 days (range 8–99) after HCT compared to HR patients who had a median onset at 35 days (range 9–100) after HCT (p<0.01).
Figure 1.
Probability of CR/PR at day 28 (upper panel) and 2 year TRM (lower panel) after initiation of steroid therapy for acute GVHD by risk.
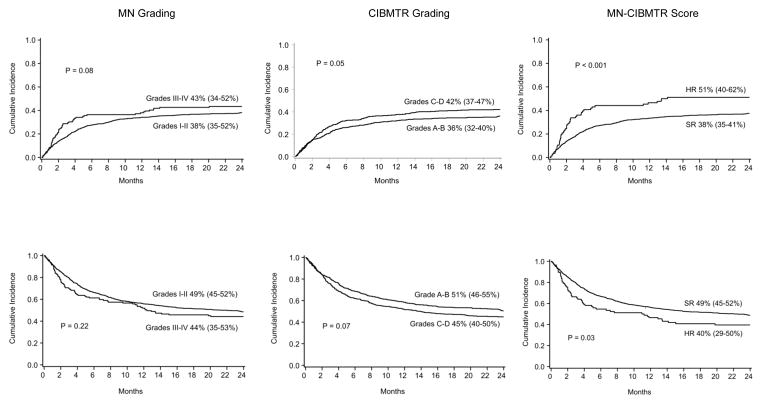
As shown in Figure 2, the MN-CIBMTR risk score significantly demarcates TRM and overall survival in high versus standard risk patients. Neither the conventional MN or CIBMTR indexes alone demonstrate differences for both endpoints. The probability of TRM at 2 years after steroid initiation was significantly higher in the 86 patients with initially HR acute GVHD than the 778 patients with SR acute GVHD (51% [95% CI 40–62%] vs. 38% [95% CI 35–41%], p <.001), Figure 2. The probability of survival at 2 years was also significantly lower in the HR group than those with SR acute GVHD (40% [95% CI 29–50%] vs. 49% [95% CI 45–52%], p <.03).
Figure 2.
Cumulative incidence (+ 95% CI) of transplant related mortality (upper panel) and overall survival (lower panel) at 2 years after initiation of steroid therapy for acute GVHD by risk group and grading system.
We then examined the reclassification of risk as defined by our new MN-CIBMTR risk score by conventional scoring system. Of the 129 patients with HR (grade III–IV) acute GVHD in the MN system, 43 (33%) were reclassified as SR using the new combined MN-CIBMTR risk score. In contrast, 321 of 407 (79%) of the high risk (grade C–D) CIBMTR patients were reclassified as SR. No SR patients moved into the HR category from either the MN or CIBMTR grading systems.
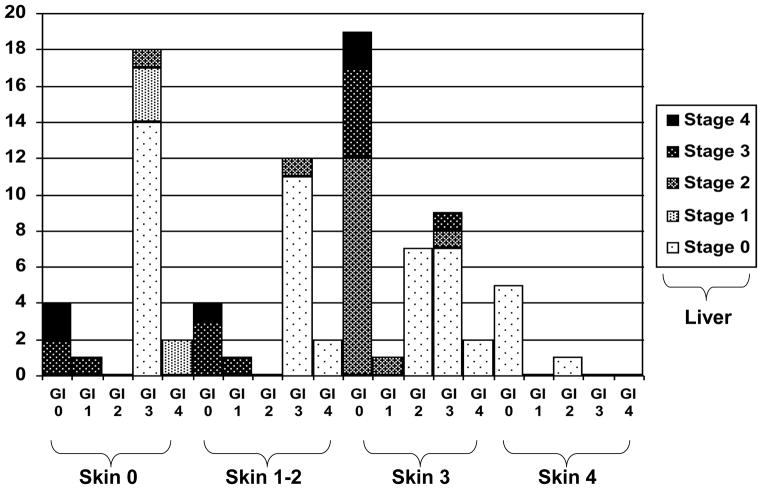
To further define those with HR GVHD as defined by our new MN-CIBMTR risk score, the initial organ stages included in HR GVHD (grades IIIC, IIID or IVD) are shown in Figure 3. The 86 patients with HR GVHD had either: lower GI stage 3–4 GVHD, and/or liver stage 3 + skin stage 0–3 (n=60); skin stage 3–4 and liver stage 2 + lower GI stage 0–1 (n=13); skin stage 3 and lower GI stage 2 (n=7); or skin stage 4 GVHD + lower GI stage 0–1 (n=6). Therefore HR GVHD includes either skin stage 4, lower GI stage 3+, liver stage 3+, or skin stage 3 and lower GI or liver stage 2+ GVHD as shown in Figure 3.
Figure 3.
Organ stage involvement for 86 patients with high risk acute GVHD at diagnosis. Shown are the % of patients with each combination of organ stages.
In multiple regression analysis, the odds of achieving CR + PR by the patients with HR acute GVHD were 3 times less than the odds of responding by the patients with SR acute GVHD (Odds Ratio (OR), 0.3, 95% CI, 0.2–0.5, p<.001, Table 5). In addition, recipients of HLA-mismatched URD BM or PBSC were less likely to respond (OR, 0.4, 95% CI, 0.3–0.6, p<.001). Factors associated with a greater likelihood of response included later initiation of steroid therapy (≥28 days from HCT, OR, 1.5, 95% CI, 1.1–2.1, p<.007) and use of CSA prophylaxis (OR, 3.6, 95% CI, 1.5–8.7, p<.005) or HCT with T cell depletion (OR, 3.6, 95% CI, 1.5–8.7, p<.005).
Table 5.
Factors Associated with Day 28 CR/PR, 2 Year Mortality and TRM: Multivariate Analysis
| Factors | Odds Ratio of CR/PR (95% CI) | P | Relative Risk of Mortality (95% CI) | P | Relative Risk of TRM (95% CI) | P |
|---|---|---|---|---|---|---|
| Age | ||||||
| <10* | 1.0 | 1.0 | 1.0 | |||
| 10–17 | 1.0 (0.6–1.8) | 0.87 | 1.1 (0.8–1.7) | 0.68 | 1.3 (0.9–1.9) | 0.19 |
| 18–35 | 1.0 (0.7–1.6) | 0.84 | 1.5 (1.1–2.4) | 0.006 | 1.3 (1.0–1.9) | 0.09 |
| >35 | 1.0 (0.9–1.1) | 0.82 | 1.7 (1.3–2.3) | <0.001 | 1.4 (1.1–2.0) | 0.01 |
| Disease Risk** | ||||||
| Standard* | 1.0 | 1.0 | 1.0 | |||
| High | 1.0 (0.7–1.3) | 0.83 | 1.4 (1.2–1.7) | <0.001 | 1.4 (1.2–1.8) | 0.001 |
| Donor Type | ||||||
| Sibling* | 1.0 | 1.0 | 1.0 | |||
| URD well match | 1.2 (0.7–2.2) | 0.49 | 1.0 (0.7–1.5) | 0.86 | 1.2 (0.8–1.8) | 0.42 |
| URD partial match | 0.7 (0.4–1.2) | 0.19 | 1.3 (1.0–1.8) | 0.09 | 1.5 (1.1–2.2) | 0.02 |
| URD mismatch | 0.4 (0.3–0.6) | <0.001 | 1.7 (1.3–2.2) | <0.001 | 2.2 (1.6–3.0) | <0.001 |
| UCB | 1.0 (0.6–1.4) | 0.85 | 0.9 (0.7–1.2) | 0.38 | 0.8 (0.6–1.1) | 0.17 |
| GVHD Prophylaxis | ||||||
| MTX alone* | 1.0 | 1.0 | 1.0 | |||
| CSA | 4.9 (2.2–10.3) | <0.001 | 0.8 (0.5–1.4) | 0.88 | 0.7 (0.4–1.3) | 0.31 |
| CSA with TCD | 3.6 (1.5–8.7) | 0.005 | 1.0 (0.5–1.7) | 0.48 | 0.9 (0.5–1.7) | 0.69 |
| Days from HCT to Initial Steroid Rx | ||||||
| <28 days* | 1.0 | 1.0 | 1.0 | |||
| ≥28 days | 1.5 (1.1–2.1) | 0.007 | 1.0 (0.8–1.2) | 0.95 | 0.8 (0.7–1.0) | 0.08 |
| MN-CIBMTR GVHD Risk Score | ||||||
| Standard Risk* | 1.0 | <0.001 | 1.0 | 0.004 | 1.0 | <0.001 |
| High Risk*** | 0.3 (0.2–0.5) | 1.5 (1.1–2.1) | 2.0 (1.4–2.8) |
reference group
Standard risk indicates acute leukemia in CR1 or CR2, CML in first chronic phase, MDS without excess blasts, or non-malignant disease. High risk indicates all others.
As defined in Table 4 with High risk = Initial GVHD Grade IIIC, IIID or IVD.
In multivariate analysis, similar factors were associated with 2 year survival and TRM (Table 5). Patients with HR acute GVHD had a 2 fold increase in risk of TRM (RR, 2.0, 95% CI, 1.4–2.8, p<.001). TRM was also higher in recipients of HLA-partially matched URD donor BM or PBSC (RR, 1.5, 95% CI, 1.1–2.2, p=.02) or HLA-mismatched URD grafts (RR, 2.2, 95% CI, 1.6–3.0, p<.001), in patients older than 35 years (RR, 1.5, 95% CI, 1.1–2.0, p<.01) and those with high risk underlying disease (RR, 1.4, 95% CI, 1.2–1.8, p=.001).
Our new MN-CIBMTR score at presentation had improved positive predictive value. High risk acute GVHD had a positive predictive value for failure of response of 57%, 51% for 2 year TRM, and 60% for 2 year all cause mortality. Standard risk acute GVHD had negative predictive values for response failure of 68%; 62% for absence of 2 year TRM 49% for 2 year survival. Severe GVHD as determined by the Minnesota (grades III–IV) and CIBMTR (grades C and D) grading systems had less positive predictive values but equivalent negative predictive values (Table 6).
Table 6.
Predictive Values of High Risk GVHD by Scoring Models
| Positive Predictive Value | Negative Predictive Value | |
|---|---|---|
| Day 28 CR/PR | ||
| MN* | 48% | 67% |
| CIBMTR** | 39% | 69% |
| MN-CIBMTR*** | 57% | 68% |
| 2 Year Mortality | ||
| MN | 56% | 49% |
| CIBMTR | 55% | 51% |
| MN-CIBMTR | 60% | 49% |
| 2 Year TRM | ||
| MN | 43% | 62% |
| CIBMTR | 42% | 64% |
| MN-CIBMTR | 51% | 62% |
Minnesota grade III–IV
CIBMTR grade C–D
MN-CIBMTR HR grade IIIC, IIID or IVD
Discussion
Using our novel acute GVHD Risk Score, we have identified initial HR acute GVHD as either skin stage 4, lower GI stage 3+, liver stage 3+, or skin stage 3 and lower GI or liver stage 2+ GVHD. Patients with this HR GVHD are less likely to respond to steroid therapy and have a 2 fold increase risk in TRM compared to patients with SR GVHD. Therefore patients with HR acute GVHD at onset will likely fail initial therapy, require alternative treatment and should be the subjects for novel therapeutic trials.
Our new risk score better identifies high risk patients who warrant more intensive initial therapy than prednisone alone. Thirty-three percent of the HR group in the MN scoring system and 79% of the HR in the CIBMTR systems were reclassified as being SR. This is due in large part to the heterogeneity of MN grade II and CIBMTR index C groups. A more accurate definition of HR disk will allow for more appropriate tailored up front therapy.
The predictive value of GVHD scoring systems have been evaluated in 4 previous published reports (Cahn, et al 2005, MacMillan, et al 2002b, Martino, et al 1999, Weisdorf, et al 2003). In our earlier analysis of 443 steroid treated patients, we showed that initial MN grade was not associated with significant differences in overall response whereas patients with initial CIBMTR grade B or C had a higher likelihood of response (MacMillan, et al 2002b). In the current analysis we observed that both the MN and CIBMTR grading systems were predictive of response, but were both imperfect. Neither grading system adequately predicted TRM as well as our new acute GVHD Risk Score. The heterogeneity of CIBMTR grades B–C (which include MN grades I–III) likely contributes to the weaker predictive utility of either system used independently.
Three other studies have reported the prognostic significance of maximum, but not initial GVHD grade on survival. Martino et al compared the predictive value of the Glucksberg and CIBMTR grading systems by assessing the maximum stage of acute GVHD in 100 patients (Martino, et al 1999). The CIBMTR severity index had better predictive value for survival, but only when comparing patients with A or B to those with C or D. Similar results were observed in a multicenter analysis of 607 HCT recipients who were scored prospectively and weekly for acute GVHD using both the Glucksberg (which discounts upper GI) and CIBMTR grading systems. Only the maximum observed GVHD grade (using either system) had prognostic significance for survival (Cahn, et al 2005). Weisdorf et al analyzed of 404 patients in a randomized trial of T-cell depletion versus conventional GVHD prophylaxis in which prospective grading was performed using the Consensus GVHD staging and grading system (Przepiorka, et al 1995). The Consensus grading is nearly identical to the MN grading system which scores stage 4 gut as grade IV acute GVHD while the Consensus system scores stage 4 liver (but not GI stage 4) as obligatory grade IV (MacMillan, et al 2002b). As previously reported, the maximum acute GVHD grade had a major impact on survival (Weisdorf, et al 2003). Our proposed definition of initially HR GVHD is a more practical predictor of outcomes which allows for immediate identification of HR GVHD and facilitates tailoring and intensification of upfront GVHD therapy.
In these studies examining the prognostic significance of GVHD grading schema, patients received steroids as initial therapy; by far the most commonly used treatment. Subsequent studies should retest these models to determine whether the predictive nature of our acute GVHD Risk Score varies with different upfront therapies and thus we suggest our new GVHD Risk Score should be validated using data from prospective multicenter trials. In addition, future studies may also determine predictive factors for HR acute GVHD as defined by our new Risk Score.
We have shown that replacing the MN, Consensus or CIBMTR schema which yield substantial heterogeneity in grade II–III or B–C acute GVHD with an integrated new acute GVHD Risk Score can better define HR GVHD and identify those patients who might best benefit from alternative upfront therapy. We suggest that these HR GVHD patients be the focus for novel initial GVHD therapies along with the conventionally identified HR patients who do not respond to initial steroid treatment. Additionally, SR GVHD patients may benefit from less intensive therapies and this warrants further investigation.
Acknowledgments
The authors thank the nurses, nurse coordinators, and physicians who cared for these patients and their families. In addition, we gratefully acknowledge the research nurses whose dedicated efforts facilitated the prospective collection of the GVHD data.
Footnotes
Author Contributions
All authors contributed equally to the conception, design, and interpretation of data, and the final manuscript. T.E.D. performed the statistical analysis; and M.L.M. had primary responsibility for drafting the manuscript.
The authors have no conflicts of interest to declare.
LITERATURE CITED
- Armitage P. Tests for linear trends in proportions and frequencies. Biometrics. 1955;11:375–386. [Google Scholar]
- Barker JN, Davies SM, DeFor T, Ramsay NK, Weisdorf DJ, Wagner JE. Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: results of a matched-pair analysis. Blood. 2001;97:2957–2961. doi: 10.1182/blood.v97.10.2957. [DOI] [PubMed] [Google Scholar]
- Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, Verfaillie CM, Wagner JE. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- Cahn JY, Klein JP, Lee SJ, Milpied N, Blaise D, Antin JH, Leblond V, Ifrah N, Jouet JP, Loberiza F, Ringden O, Barrett AJ, Horowitz MM, Socie G. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: a joint Societe Francaise de Greffe de Moelle et Therapie Cellulaire (SFGM-TC), Dana Farber Cancer Institute (DFCI), and International Bone Marrow Transplant Registry (IBMTR) prospective study. Blood. 2005;106:1495–1500. doi: 10.1182/blood-2004-11-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables. Journal of the Royal Stastistical Society. 1972:187–220. [Google Scholar]
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- Hings IM, Filipovich AH, Miller WJ, Blazar BL, McGlave PB, Ramsay NK, Kersey JH, Weisdorf DJ. Prednisone therapy for acute graft-versus-host disease: short- versus long-term treatment. A prospective randomized trial. Transplantation. 1993;56:577–580. doi: 10.1097/00007890-199309000-00016. [DOI] [PubMed] [Google Scholar]
- Hings IM, Severson R, Filipovich AH, Blazar BR, Kersey JH, Ramsay NK, McGlave PB, Weisdorf DJ. Treatment of moderate and severe acute GVHD after allogeneic bone marrow transplantation. Transplantation. 1994;58:437–442. doi: 10.1097/00007890-199408270-00008. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- MacMillan ML, DeFor TE, Weisdorf DJ. The best endpoint for acute GVHD treatment trials. Blood. 2010;115:5412–5417. doi: 10.1182/blood-2009-12-258442. [DOI] [PubMed] [Google Scholar]
- MacMillan ML, Weisdorf DJ, Davies SM, DeFor TE, Burns LJ, Ramsay NK, Wagner JE, Blazar BR. Early antithymocyte globulin therapy improves survival in patients with steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant. 2002a;8:40–46. doi: 10.1053/bbmt.2002.v8.pm11858189. [DOI] [PubMed] [Google Scholar]
- MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NK, Davies SM, Blazar BR. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002b;8:387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- Martino R, Romero P, Subira M, Bellido M, Altes A, Sureda A, Brunet S, Badell I, Cubells J, Sierra J. Comparison of the classic Glucksberg criteria and the IBMTR Severity Index for grading acute graft-versus-host disease following HLA-identical sibling stem cell transplantation. International Bone Marrow Transplant Registry. Bone Marrow Transplant. 1999;24:283–287. doi: 10.1038/sj.bmt.1701899. [DOI] [PubMed] [Google Scholar]
- Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Henslee-Downey PJ, Cahn JY, Calderwood S, Gratwohl A, Socie G, Abecasis MM, Sobocinski KA, Zhang MJ, Horowitz MM. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- Weisdorf D, Haake R, Blazar B, Miller W, McGlave P, Ramsay N, Kersey J, Filipovich A. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood. 1990a;75:1024–1030. [PubMed] [Google Scholar]
- Weisdorf D, Spellman S, Haagenson M, Horowitz M, Lee S, Anasetti C, Setterholm M, Drexler R, Maiers M, King R, Confer D, Klein J. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisdorf DJ, Hurd D, Carter S, Howe C, Jensen LA, Wagner J, Stablein D, Thompson J, Kernan NA. Prospective grading of graft-versus-host disease after unrelated donor marrow transplantation: a grading algorithm versus blinded expert panel review. Biol Blood Marrow Transplant. 2003;9:512–518. doi: 10.1016/s1083-8791(03)00162-9. [DOI] [PubMed] [Google Scholar]
- Weisdorf DJ, Snover DC, Haake R, Miller WJ, McGlave PB, Blazar B, Ramsay NK, Kersey JH, Filipovich A. Acute upper gastrointestinal graft-versus-host disease: clinical significance and response to immunosuppressive therapy. Blood. 1990b;76:624–629. [PubMed] [Google Scholar]