Abstract
We present a consolidated view of the complexity and challenges of designing studies for measurement of energy metabolism in mouse models, including a practical guide to the assessment of energy expenditure, energy intake and body composition and statistical analysis thereof. We hope this guide will facilitate comparisons across studies and minimize spurious interpretations of data. We recommend that division of energy expenditure data by either body weight or lean body weight and that presentation of group effects as histograms should be replaced by plotting individual data and analyzing both group and body-composition effects using analysis of covariance (ANCOVA).
The epidemic of obesity has generated a large interest in understanding the physiological mechanisms regulating energy metabolism1. This understanding has been facilitated by the knockout (KO), knockdown or overexpression of specific genes, many of which result in altered body weight (BW) and/or body composition (BC) owing to a perturbation of energy balance. These advances, however, have led to some controversy over how best to assess these phenotypes and how to interpret the data2–4 (Supplementary Table 1). In this Perspective we provide information on the best available and standardized measures for both energy intake and energy expenditure. Such an approach will allow progress in understanding complex biological questions about the consequences of genetic or pharmacological manipulations for energy balance and BW. This standardization is particularly important considering ambitious large-scale operations ongoing in the United States and Europe with the goal of assessing the consequences of all mouse and rat gene deletions.
Study design and general considerations
Genetically modified, pharmacologically treated and nutritionally challenged laboratory mice, as well as spontaneous mutants, often exhibit alterations of BW and/or BC. It is often unclear whether food intake or energy expenditure (or both) is the major determinant of the phenotype because a small imbalance between intake and expenditure, if maintained over the course of weeks or months, can result in dramatic changes in body weight and fat mass. Furthermore, once obesity or leanness has developed, behavioral and metabolic alterations triggered by the BW change may obscure or confound the processes that caused the changes in BW and/or BC in the first place. Thus, assessing BW and BC phenotypes requires a careful analysis of the various factors that might affect these phenotypes.
Type of study
Recommending a single approach to metabolic phenotyping for all different types of studies, models and scientific questions is almost impossible. Nevertheless, several general considerations can help minimize artifacts and achieve comparability of data across studies and laboratories and therefore help understand the overall biology underlying the perturbation in BW and BC.
Studies defining the causal factors leading to altered BW and BC should ideally be initiated early in the life of the mouse, when BW and BC are still identical or minimally different. If changes in caloric intake or energy expenditure are observed before the change in BW or fat mass, it is more likely that one can attribute the difference to these changes. To dissect these mechanisms, it is sometimes helpful to conduct pair-feeding studies, in which mice from a ‘pair-fed’ group are given an amount of food that matches that eaten by the comparison group, which is given ad libitum access to the same food. If the pair-fed group consumes all the food and maintains a normal physiological status (for example, does not enter torpor) and if the body-fat differences persist under conditions of equalized caloric intake, one has prima facie evidence that the alteration in BW is due to altered energy expenditure.
A second approach, which does not require normalization, is careful quantification and subtraction of the cumulative calories burned over a given period of time from the cumulative number of ingested and assimilated calories in the experimental and control groups. Also, mathematical modeling of cumulative intake and BW over several weeks can provide considerable insight into differences in energy balance5. If a mutant mouse differs in weight from wild-type littermates at birth or weaning, the above methodological approach is more difficult. In either case, measurements of energy intake and energy expenditure should be performed over several days and nights, and, if possible, at more than one time point during growth. Additional data on BC, body temperature, physical activity, body length, organ and tissue weights and ex vivo tissue-specific oxygen consumption data are all of value. In such cases, one should then analyze these parameters together with energy intake and energy expenditure data considering relevant covariates in an ANCOVA or generalized linear model as described below.
Genetic background
As genetic backgrounds can lead to dramatic differences in response to experimental procedures or genetic manipulations, studying mice with a homogeneous genetic background simplifies the interpretation of the energy balance data. In contrast, differences between mouse strains can be of great interest. The C57Bl/6 (B6) mouse is the most commonly used strain in obesity and metabolism research because it is prone to diet-induced obesity with high-fat diets and develops severe insulin resistance. By contrast, A/J, FVB, C3HeB/FJ and 129v strains are relatively resistant to diet-induced obesity and exhibit lower weight gain on a high-fat diet6,7. As 129/Sv and FVB mice are often used to create knockout and transgenic lines, many investigators perform extensive backcrossing onto the B6 background to eliminate these background gene effects. In such cases, one needs to backcross for at least 8–10 generations to establish a sufficiently homogeneous background. For some studies, it is important to determine the effect of these background genes, and in these cases, comparative studies on different genetic backgrounds, mixed genetic background or outbred backgrounds can provide additional information.
Housing, calorimeter and environmental conditions
Institutional and governmental guidelines define housing conditions for laboratory mice, and these conditions can affect energy balance. The general recommendations of the EUMORPHIA Consortium for animal housing are useful as a starting point7. Mice are most frequently housed with a constant light and dark phase of 12 hours at 20–23 °C. Because the lower critical temperature of the mouse is around 30 °C this means in most circumstances mice are housed under mild thermoregulatory stress, which may not be the ideal ambient temperature for energy-metabolism studies aiming to mimic the situation in humans, who normally exist much closer to thermoneutral conditions (details and additional considerations for indirect calorimetry measurements of energy expenditure are available in Supplementary Note 1). The temperature in a cage may be substantially higher than the ambient temperature outside of a cage, and this temperature gradient increases from singly housed to multiply housed mice.
It is important to realize that mouse facilities, including commercial breeders, may use different ‘normal’ chow diets. One example source for diet inconsistencies would be the soy-isoflavone content, which is often only partially removed when proteins are extracted. Seasonal differences in diet soy-isoflavone content can affect study outcomes based on their effects on estrogen receptor activation and AMPK. Such influences can make a considerable difference in baseline measurements and affect the interpretation of the effect of changing to high-fat or other experimental diets. It is therefore critical for the investigator to know what constitutes normal chow, especially if mice are housed in more than one facility or are being compared to those in the previous literature. Unfortunately, there also is no clear ‘gold standard’ for high-fat diets. Protein and carbohydrate content should ideally be kept constant within and between studies. High-energy diets should be modeled to principally match diets relevant for human metabolic disease, but it can be advantageous to also match diets used in published studies to allow for relevant comparisons of mouse phenotypes.
Most experimental procedures can induce stress responses in animals, and proper acclimation and recovery intervals should be given before food intake or energy expenditure are recorded. Mice with impaired health often decrease their food consumption, and mice with fever have elevated energy expenditure. Although mice in energy-metabolism studies are normally housed singly, measurements can also be derived from group-housed mice if housing space is a limiting factor, but this is far from ideal. There is little evidence that single housing induces stress because fecal corticosterone levels of singly housed mice do not differ from those of group-housed individuals8–10. However, singly housed mice may have altered activity patterns relative to group-housed individuals11. In contrast, if mice are group-housed, factors such as spilling of food, aggression behavior or social dominance can introduce considerable bias and variability in a range of traits, including those linked to energy balance12–17. Moreover, group-housed individuals can huddle, reducing their thermoregulatory requirements, and this may have a major effect on energy expenditure and intake. Cage position (top of rack versus lower) and group size have been shown to affect development of diabetes in mice18. Ideally, when group-housed, mice should be littermates and together from early age14, and social groups should not be disrupted. Removing individual mice from a group to measure energy intake and/or energy expenditure can cause problems when that mouse is reintroduced to the group, especially in groups of males. Moving boxes of mice around different rooms or rack positions seems not to be related to significant effects on stress or BW19 (P > 0.05).
Methodological recommendations and pitfalls
Practical issues: body composition
Gold-standard methods for determining BC of animals were established over 50 years ago and involve killing the mouse20 (Supplementary Note 2). For longitudinal measures, there are various options such as isotope dilution, dual-energy X-ray absoptiometry, total body electrical conductivity, magnetic resonance spectroscopy, magnetic resonance imaging and computed tomography (Supplementary Note 2). Generally it is good practice to use both destructive and non-destructive methods as they provide complementary information, including individual organ and fat depot weights as well as measures of fat cell size and number. When high accuracy is required, destructive methods of BC analysis are the methods of choice. Magnetic resonance spectroscopy and dual-energy X-ray absoptiometry systems provide the next best alternatives, in that order. Total body electrical conductivity and isotope-dilution methods are relatively poor. All indirect methods should be calibrated against destructive methods to yield the best results.
Practical issues: energy (food) intake
Exact measurement of food intake is a key component in analysis of energy metabolism. Techniques are based on weighing of food and vary greatly in accuracy, reproducibility, practicality and cost. Not all ingested calories are introduced into metabolism, making assimilation efficiency studies (for example, using bomb calorimetry) a recommended addition. Investigators need to be aware of other factors that can complicate estimates of energy intake, including the exact form in which diets are delivered to mice (Supplementary Note 3).
Practical issues: energy expenditure
With the development of several different commercial systems, measures of energy expenditure in mice are now much more common than 10–15 years ago, but results are sometimes misinterpreted2–4. Using direct calorimetry, energy expenditure is assessed by the direct measurement of the body’s heat production in a calorimeter21–23. Despite high reproducibility and measurement errors of only 1–3%, these calorimeters are expensive, have slow response time22 and do not provide information about the nature of the oxidized substrates. In indirect calorimetry, energy expenditure is calculated based on the amount of oxygen consumed and carbon dioxide produced (Supplementary Note 1). The most common indirect calorimeter types are ventilated, open-circuit systems, in which the animals are placed in gas-tight metabolic cages through which a flow of fresh air is passed. The system collects and mixes the expired air, measures the flow rate and analyzes the gas concentration of the incoming and outgoing air for both O2 and CO2 (ref. 22). Another indirect method of calorimetry is the doubly labeled water method, an isotope-elimination technique developed in the 1950s (refs. 24–26). This method has been traditionally used to measure the metabolic rate of small free-living animals, which are released in the field between two time points: it is often referred to as field metabolic rate27. In the laboratory, the main advantage of the method is that it allows the measurement of energy demands of an animal embedded in a social environment28. However, the time intervals between blood sampling are often too long to permit measurements of short-term or diurnal changes of the metabolic rate.
Statistical aspects
In most circumstances, the analysis of energy balance should be made on the raw data expressed per mouse. In many circumstances, however, researchers are interested in whether a particular difference between mice occurs simply because the mice differ in BW, or because treatment or genetic differences affect energy intake or energy expenditure. Often corrections for BW differences are made only on energy expenditure measurements, and this can lead to confusion, especially when combined with analyses of energy intake that have been made without normalization. There is no reason to limit normalization to energy expenditure because energy intake also varies with BW. However, it is important that methods of normalization not be mixed on the two sides of the energy-balance equation2.
One of the most debated issues is how to normalize energy intake and energy expenditure for differences in not only BW but also BC. This is far from a new issue as it has been debated for over a century29–31. The most extensively used method for normalization of metabolic rate data is dividing oxygen consumption (or energy expenditure) by BW or body surface area (Supplementary Table 1). These approaches, however, are problematic because metabolic rate is not linearly proportional to BW, and the regression line between metabolic rate and body size does not go through the zero intercept. In 1883, Rubner found empirically that metabolic rate between different strains of dog was proportional to BW raised to the power 0.66, as anticipated if heat was lost via the skin surface31. However, data collected during the first half of the twentieth century led to the conclusion, again empirical, that mammalian basal metabolic rate was proportional to BW raised to the power of 0.75 (ref. 29). Subsequently, many studies have addressed the issue of the best fit to the interspecies data, generating values between 0.66 and 0.75 (refs. 32,33). In fact, the relationship between metabolic rate and BW seems different between small and large mammals34. Many studies in mice have used the three-quarter power derived from interspecies comparisons to normalize energy metabolism data within species (Supplementary Table 1) and BW raised to the 0.75 power is often called the ‘metabolic BW’. Applying an interspecific coefficient to normalize intraspecific data is questionable because the same scaling exponent seldom applies within species.
As different tissues have different metabolic rates, BC also has a role when the metabolic rate is compared between lean and obese animals. In obese animals, higher BW is mostly due to an expansion of white adipose tissue, a tissue known to have a much lower metabolic activity than tissues composing the fat-free mass35,36. Dividing metabolic rate by BW (or by ‘metabolic BW’) does not accurately take into account differences in BC. To account for such differences in BC, the ratio of the metabolic rate to the fat-free mass or lean body mass has often been calculated in both human37–39 and mouse studies (Supplementary Table 1).
There are several problems with this approach. Although it is true that adipose tissue is metabolically much less active than the brain or liver when measured in vitro35,36, adipose tissue is a much larger proportion of body mass in obese animals, and thus can still make a contribution to the total metabolic rate. This is complicated by the fact that some of the fat mass is brown fat, and when fully activated, brown fat can be the most metabolically active tissue in the body. Third, white adipose tissue is an endocrine tissue, and secreted adipokines, such as leptin and adiponectin, may drive metabolism of the lean tissues4. This can lead to an overestimate of the metabolic activity of fat40 but nevertheless needs to be accounted in the analysis. This cannot be achieved by a simple division by BW or lean mass. This has led to yet another empirical approach using a divisor that combines different tissue effects.
In humans, skeletal muscle, liver and brown fat represent about 50%, 2% and <0.3% of the total fat-free mass, respectively41,42. The simple division of metabolism by BW or lean body mass (or an ‘adjusted’ combination of fat and lean) generally generates as many problems as it solves because this approach only results in normalization of the mass effect when the intercept of the relation between mass (total or lean) and expenditure is zero. In any other situation, which includes virtually all measurements of energy expenditure in small rodents, this simple division actually generates a spurious difference between groups. In fact, this problem was already well known in studies of humans, leading to the suggestion in the 1990s that dividing by lean mass should be abandoned in favor of using analysis of covariance37–39.
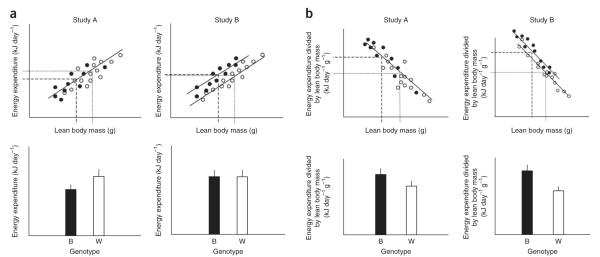
The problem of dividing by lean body mass can be illustrated using theoretical data of two studies comparing two different genotypes with their wild-type equivalents (Fig. 1). In both studies there is a lean body mass difference between the two genotypes. In study A, however, the data for energy expenditure lie on a common line in relation to lean body mass. There is no difference in the energy expenditure of the two genotypes apart from an effect owing to lean body mass. In study B, the data for expenditure lie on two separate lines relative to lean body mass (Fig. 1a). In this situation, there is an effect of the genotype on expenditure independent of any mass effect. The challenge is to find an analysis that separates these two situations. If we use the raw data and average across the individuals for each genotype, the results shown below the plots in histograms reveal that there is a significant difference in study A, with energy expenditure of the wild type being lower than that of the experimental genotype, whereas in study B, there is no significant difference in energy expenditure between the wild type and experimental genotypes. This result is the complete opposite of what is actually apparent in the plots of the individual data, that in study A there is no genotype effect while in study B there is a genotype effect. To see whether there is a genotype effect on expenditure independent of any effect of lean body mass, we may divide the energy expenditure by lean body mass (kJ g lean−1 day−1) (Fig. 1b). The result in study B now reveals that expenditure in the wild-type genotype is higher than that of the experimental genotype. However, this division also reveals a significant effect in study A, where none actually exists. The problem is that the division by lean mass overcompensates for the mass effect. A solution to this issue is to analyze the data using ANCOVA (Supplementary Note 4).
Figure 1.
Problems of analysis and interpretation of energy expenditure demonstrated on hypothetical data. (a) Experimental genotype (W, white) is compared to the wild-type genotype (B, black). Study A and study B are different experimental manipulations of genotype. Energy expenditure per whole animal (kJ day−1) is plotted against lean body mass (top). In all panels, lines show fitted regressions. Average of raw data across the individuals for each genotype plotted in a histogram (bottom). (b) Energy expenditure divided by lean body mass versus lean body mass is plotted for each data point (top) and shown as histogram (bottom). Values represent means ± s.e.m.
There are two take-home messages from the data in Figure 1 and Supplementary Note 4. First, plotting the data as histograms obscures rather than clarifies the effect of the genotypes on energy expenditure, and it is better to plot the actual data in relation to lean body mass. Second, ANCOVA (Supplementary Note 4) or generalized linear modeling37–39,43 is the most appropriate statistical approach to accommodate discrete (genotype) and continuous (body mass) traits, rather than using a simple division by BW or lean BW.
An empirical example of the consequences of using different approaches to the statistical analysis of energy-expenditure data concerns 20 control mice fed ad libitum and 58 mice fed a calorie restricted diet (60% of ad libitum) for three months44 (Fig. 2). These data show that different approaches lead to different interpretations of the treatment effect on energy metabolism. Analyzing these data by ANCOVA reveals that there is, in fact, no treatment-group effect (P = 0.65) and in addition shows that fat-free mass has a significant positive effect on the resting metabolic rate (P < 0.001) and that there is a more minor effect of fat mass (P = 0.04). Moreover this analysis reveals that the gradient of the fat-free mass effect (0.62) is about 3× greater than the gradient of the fat mass effect (0.20). Hence, although dividing by fat-free mass also produces the correct answer (that there is no treatment effect), the use of ANCOVA with body mass as a covariate reveals there is a strong effect of body mass (P < 0.001), but that treatment group (restricted or fed ad lib) has no significant effect (P = 0.16). Both the uncorrected analysis and ‘corrected analysis’ (by dividing by BW) led to spurious interpretations that there was a significant group effect, which the ANCOVA analysis revealed was only because of the covariation of resting metabolic rate with BW. It has been suggested that ANCOVA cannot be used in studies of energy metabolism because sample sizes are often small3. However, the necessary sample size to evaluate any statistical difference is evaluated by power analysis (Supplementary Note 5) and generally if the sample is insufficiently powered for analysis by ANCOVA, it will also be too small for analysis by any other statistical approach, so this is not a valid reason to avoid using ANCOVA for the analysis. Notably, consistent with our Perspective, Schwartz and colleagues recently compared different methods of energy expenditure normalization in a large mouse cohort and recommended a regression-based approach45.
Figure 2.
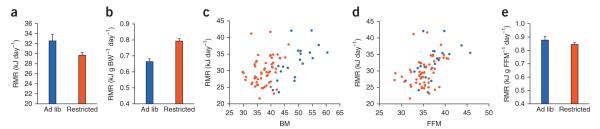
A practical example of the use of different approaches to the analysis of energy metabolism in the mouse. (a) Twenty control mice were fed ad libitum (ad lib) and 58 mice were fed a calorie-restricted diet (60% of ad libitum) for three months44. Raw data with the average metabolic rate were calculated without any correction for body mass. Note that the mice fed a calorie-restricted diet had a significantly (one-way ANOVA: P = 0.01) lower metabolic rate than the ones fed ad lib. (b) Metabolic rate expressed per gram of BW. Note that the opposite result was found: the mice on calorie-restricted diet had significantly (one-way ANOVA: P < 0.001) higher metabolic rates. (c) Resting metabolic rate (RMR) data as a function of body mass. Note that there is some overlap between the groups and a general positive trend of greater RMR at higher body masses (BM). (d) RMR versus fat-free mass (FFM) (measured by dual energy X-ray absorptiometry) shows a much greater overlap between the groups. (e) Dividing RMR by FFM revealed no significant effect of the treatment group on RMR (one-way ANOVA: P = 0.275). Values represent means ± s.e.m.
Separating the body into lean and fat compartments for statistical analysis acknowledges the fact that these compartments have different metabolic rates. This concept could be extended to encompass all the different tissues that have separate metabolic rates44,46–54. If the mice are killed after the energy measurement is made, one can use all the individual organ weights as separate predictor variables (covariates) in the analysis. In its generalized form, ANCOVA can accommodate this type of analysis by including the masses of each individual organ as a separate covariate. In effect this creates a predictive model from the control group for what the metabolic rate should be for an animal with a certain size of liver, brain, skeletal muscle etc. This model can then be used to predict what the metabolism of a target group should be according to their organ sizes and conclude whether the prediction is higher or lower compared to the actual metabolism. This approach has been used, for example, to investigate whether the metabolic rates of animals under caloric restriction are different from the expectation based on their altered BC55 or whether the scaling exponent across animal species can be explained by changes in BC54,56. There is, however, a limitation with this approach because it assumes that the predictors are not correlated with each other. This is not typically true of organ sizes, which are generally correlated with each other across animals of different body sizes. Moreover the more organs that are included the larger the sample needs to be to include them as predictors.
Flowchart for typical mouse energy metabolism studies
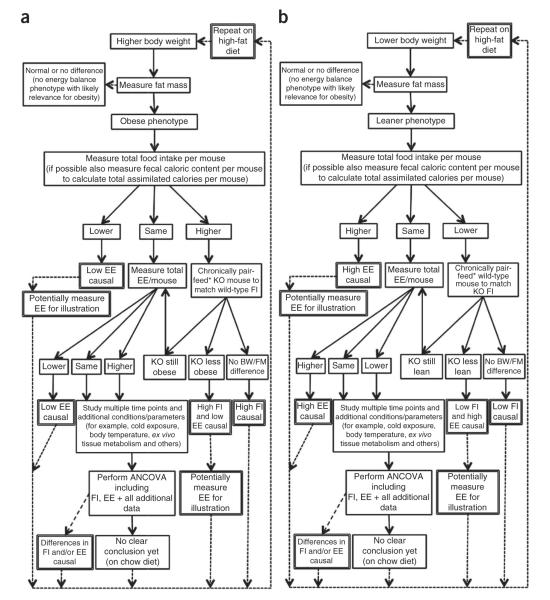
We propose algorithms for guidance on how to optimally organize and sequentially align experiments and analyses with the aim to reach a clear conclusion regarding the physiological processes responsible for BW difference in mice (Fig. 3). Additional refinement such as replacement of food intake measurements by quantifying assimilated calories (subtracting fecal caloric content measured by bomb calorimetry from ingested number of calories) should be integrated if possible. First, a study following this algorithm using sufficiently backcrossed, age- and gender-matched mice should be performed on a standard chow diet. If such a study does not lead to a clear metabolic phenotype that explains the abnormal BW, then a second study following the same principal steps, this time with a high-fat diet, is recommended. Although this flowchart provides an overall context for energy-metabolism studies, each mouse model will require a specifically adjusted experimental design including in-depth analysis in which unique phenotypic differences are detected.
Figure 3.
Flowchart for mouse energy metabolism phenotype analysis for mouse models. (a) Analysis with higher BW in comparison with wild-type littermate controls. (b) Analysis for mouse models with lower BW in comparison with wild-type littermate controls. EI, energy intake; EE, energy expenditure; FI, food intake; KO, knockout. *For discussion of advantages and pitfalls of pair-feeding, see Supplementary note 3.
Supplementary Material
Footnotes
Note: Supplementary information is available on the Nature Methods website.
AUTHOR CONTRIBUTIONS M.H.T., J.R.S., C.R.K. and E.R. conceptualized and wrote the manuscript. J.R.S.A. provided original data and biomathematics advice. J.A. edited the manuscript and co-wrote sections on environment-genetics interactions. J.C.B. and T.L.H. edited aspects of the manuscript relevant for neuronal control of energy metabolism. L.C., R.H.E., G.I.S. and R.V.F. Jr. wrote and edited sections on nutrient partitioning and energy metabolism measurements. J.E.G. generated the table. M.A.H., B.B.K. and E.M.,-F. contributed to the sections on quantification of BC, locomotor activity and food intake. C.H. provided the data for Figure 2. T.D.M. wrote sections on housing and husbandry, and integrated all references. H.M. contributed advice and sections on study design and diets. P.T.P. generated the flowcharts together with M.H.T. and co-wrote sections on practical aspects of calorimetry and study design. L.P. co-edited the manuscript and added practical examples and calculations. M.L.R. and K.R. contributed advice on sections regarding BC and thermogenesis. J.A., S.C.K. and G.T. added input regarding relevant pitfalls arising based on the use of mouse genetics. All authors edited and agreed on the final version of the manuscript.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Woods SC, Schwartz MW, Baskin DG, Seeley RJ. Food intake and the regulation of body weight. Annu. Rev. Psychol. 2000;51:255–277. doi: 10.1146/annurev.psych.51.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Arch JR, Hislop D, Wang SJ, Speakman JR. Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int. J. Obes. 2006;30:1322–1331. doi: 10.1038/sj.ijo.0803280. [DOI] [PubMed] [Google Scholar]
- 3.Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes. 2010;59:323–329. doi: 10.2337/db09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaiyala KJ, et al. Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes. 2010;59:1657–1666. doi: 10.2337/db09-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo J, Hall KD. Estimating the continuous-time dynamics of energy and fat metabolism in mice. PLoS Comput. Biol. 2009;5:e10005111. doi: 10.1371/journal.pcbi.1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almind K, Kahn CR. Genetic determinants of energy expenditure and insulin resistance in diet-induced obesity in mice. Diabetes. 2004;53:3274–3285. doi: 10.2337/diabetes.53.12.3274. [DOI] [PubMed] [Google Scholar]
- 7.Champy MF, et al. Genetic background determines metabolic phenotypes in the mouse. Mamm. Genome. 2008;19:318–331. doi: 10.1007/s00335-008-9107-z. [DOI] [PubMed] [Google Scholar]
- 8.Arndt SS, et al. Individual housing of mice–impact on behaviour and stress responses. Physiol. Behav. 2009;97:385–393. doi: 10.1016/j.physbeh.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Champy MF, et al. Mouse functional genomics requires standardization of mouse handling and housing conditions. Mamm. Genome. 2004;15:768–783. doi: 10.1007/s00335-004-2393-1. [DOI] [PubMed] [Google Scholar]
- 10.Hunt C, Hambly C. Faecal corticosterone concentrations indicate that separately housed male mice are not more stressed than group housed males. Physiol. Behav. 2006;87:519–526. doi: 10.1016/j.physbeh.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Martin AL, Brown RE. The lonely mouse: verification of a separation-induced model of depression in female mice. Behav. Brain Res. 2010;207:196–207. doi: 10.1016/j.bbr.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Bartolomucci A, et al. Metabolic consequences and vulnerability to diet-induced obesity in male mice under chronic social stress. PLoS ONE. 2009;4:e4331. doi: 10.1371/journal.pone.0004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartolomucci A. Social stress, immune functions and disease in rodents. Front. Neuroendocrinol. 2007;28:28–49. doi: 10.1016/j.yfrne.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Bartolomucci A, et al. Age at group formation alters behavior and physiology in male but not female CD-1 mice. Physiol. Behav. 2004;82:425–434. doi: 10.1016/j.physbeh.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Cohn DWH, Sa-Rocha LC. Sickness and aggressive behavior in dominant and subordinate mice. Ethology. 2009;115:112–121. [Google Scholar]
- 16.Moles A, et al. Psychosocial stress affects energy balance in mice: modulation by social status. Psychoneuroendocrinology. 2006;31:623–633. doi: 10.1016/j.psyneuen.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt MV, et al. Persistent neuroendocrine and behavioral effects of a novel, etiologically relevant mouse paradigm for chronic social stress during adolescence. Psychoneuroendocrinology. 2007;32:417–429. doi: 10.1016/j.psyneuen.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Ader DN, Johnson SB, Huang SW, Riley WJ. Group size, cage shelf level, and emotionality in non-obese diabetic mice: impact on onset and incidence of IDDM. Psychosom. Med. 1991;53:313–321. doi: 10.1097/00006842-199105000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Dahlin J, et al. Body weight and faecal corticosterone metabolite excretion in male Sprague-Dawley rats following short transportation and transfer from group-housing to single-housing. Scand. J. Lab Anim. Sci. 2009;36:205–213. [Google Scholar]
- 20.Reynolds DS, Kunz TH. Standard methods for destructive body composition analysis. In: Speakman JR, editor. Body Composition Analysis of Animals: A Handbook of Non-Destructive Methods. Cambridge University Press; 2001. pp. 39–55. [Google Scholar]
- 21.Faber P, Lammert O, Johansen O, Garby L. A fast responding combined direct and indirect calorimeter for human subjects. Med. Eng. Phys. 1998;20:291–301. doi: 10.1016/s1350-4533(98)00037-x. [DOI] [PubMed] [Google Scholar]
- 22.Levine JA. Measurement of energy expenditure. Public Health Nutr. 2005;8:1123–1132. doi: 10.1079/phn2005800. [DOI] [PubMed] [Google Scholar]
- 23.Spinnler G, Jequier E, Favre R, Dolivo M, Vannotti A. Human calorimeter with a new type of gradient layer. J. Appl. Physiol. 1973;35:158–165. doi: 10.1152/jappl.1973.35.1.158. [DOI] [PubMed] [Google Scholar]
- 24.Lifson N, Gordon GB, McClintock R. Measurement of total carbon dioxide production by D2O18. J. Appl. Physiol. 1955;7:704–710. doi: 10.1152/jappl.1955.7.6.704. [DOI] [PubMed] [Google Scholar]
- 25.Lifson N, McClintock R. Theory of use of turnover rates of body water for measuring energy and material balance. J. Theor. Biol. 1966;12:46–74. doi: 10.1016/0022-5193(66)90185-8. [DOI] [PubMed] [Google Scholar]
- 26.Speakman JR. Doubly-Labelled Water: Theory and Practice. Kluwer Academic Publishers; 1997. [Google Scholar]
- 27.Speakman JR, Krol E. Maximal heat dissipation capacity and hyperthermia risk: neglected key factors in the ecology of endotherms. J. Anim. Ecol. 2010;79:726–746. doi: 10.1111/j.1365-2656.2010.01689.x. [DOI] [PubMed] [Google Scholar]
- 28.Krol E, Murphy M, Speakman JR. Limits to sustained energy intake. X. Effects of fur removal on reproductive performance in laboratory mice. J. Exp. Biol. 2007;210:4233–4243. doi: 10.1242/jeb.009779. [DOI] [PubMed] [Google Scholar]
- 29.Kleiber M. Body size and metabolism. Hilgardia. 1932;6:315–353. [Google Scholar]
- 30.Kleiber M. The Fire of Life: An Introduction to Animal Energetics. Wiley and Co.; 1961. [Google Scholar]
- 31.Rubner M. Über den einfluss der körpergrösse auf stoff- und kraftwechsel. Z. Biol. 1883;19:536–562. [Google Scholar]
- 32.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. [Google Scholar]
- 33.White CR, Blackburn TM, Seymour RS. Phylogenetically informed analysis of the allometry of mammalian basal metabolic rate supports neither geometric nor quarter-power scaling. Evolution. 2009;63:2658–2667. doi: 10.1111/j.1558-5646.2009.00747.x. [DOI] [PubMed] [Google Scholar]
- 34.Kolokotrones T, Savage V, Deeds EJ, Fontana W. Curvature in metabolic scaling. Nature. 2010;464:753–756. doi: 10.1038/nature08920. [DOI] [PubMed] [Google Scholar]
- 35.Elia M. Organ and tissue contribution to metabolic rate. In: Elia M, Kinney JM, Tucker HN, editors. Energy Metabolism: Tissue Determinants and Cellular Corollaries. Raven; 1992. pp. 61–80. [Google Scholar]
- 36.Krebs HA. Body size and tissue respiration. Biochim. Biophys. Acta. 1950;4:249–269. doi: 10.1016/0006-3002(50)90032-1. [DOI] [PubMed] [Google Scholar]
- 37.Allison DB, Paultre F, Goran MI, Poehlman ET, Heymsfield SB. Statistical considerations regarding the use of ratios to adjust data. Int. J. Obes. 1995;19:644–652. [PubMed] [Google Scholar]
- 38.Poehlman ET, Toth MJ. Mathematical ratios lead to spurious conclusions regarding age-related and sex-related differences in resting metabolic-rate. Am. J. Clin. Nutr. 1995;61:482–485. doi: 10.1093/ajcn/61.3.482. [DOI] [PubMed] [Google Scholar]
- 39.Ravussin E, Bogardus C. Relationship of genetics, age, and physicalfitness to daily energy-expenditure and fuel utilization. Am. J. Clin. Nutr. 1989;49:968–975. doi: 10.1093/ajcn/49.5.968. [DOI] [PubMed] [Google Scholar]
- 40.Johnstone AM, Murison SD, Duncan JS, Rance KA, Speakman JR. Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am. J. Clin. Nutr. 2005;82:941–948. doi: 10.1093/ajcn/82.5.941. [DOI] [PubMed] [Google Scholar]
- 41.Rolfe DFS, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 42.Virtanen KA, et al. Brief report: functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt MV, et al. A novel chronic social stress paradigm in female mice. Horm. Behav. 2010;57:415–420. doi: 10.1016/j.yhbeh.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Speakman JR, Hambly C. Starving for life: what animal studies can and cannot tell us about the use of caloric restriction to prolong human lifespan. J. Nutr. 2007;137:1078–1086. doi: 10.1093/jn/137.4.1078. [DOI] [PubMed] [Google Scholar]
- 45.Kaiyala KJ, Schwartz MW. Toward a more complete (and less controversial) understanding of energy expenditure and its role in obesity pathogenesis. Diabetes. 2011;60:17–23. doi: 10.2337/db10-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daan S, Masman D, Groenewold A. Avian basal metabolic rates—their association with body-composition and energy-expenditure in nature. Am. J. Physiol. 1990;259:R333–R340. doi: 10.1152/ajpregu.1990.259.2.R333. [DOI] [PubMed] [Google Scholar]
- 47.Gallagher D, et al. Small organs with a high metabolic rate explain lower resting energy expenditure in African American than in white adults. Am. J. Clin. Nutr. 2006;83:1062–1067. doi: 10.1093/ajcn/83.5.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gallagher D, et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol-Endoc M. 1998;38:E249–E258. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 49.Heymsfield SB, et al. Body-size dependence of resting energy expenditure can be attributed to nonenergetic homogeneity of fat-free mass. Am J Physiol-Endoc M. 2002;282:E132–E138. doi: 10.1152/ajpendo.2002.282.1.E132. [DOI] [PubMed] [Google Scholar]
- 50.Konarzewski M, Diamond J. Evolution of basal metabolic rate and organ masses in laboratory mice. Evolution. 1995;49:1239–1248. doi: 10.1111/j.1558-5646.1995.tb04450.x. [DOI] [PubMed] [Google Scholar]
- 51.Meyer CW, et al. Expanding the body mass range: associations between BMR and tissue morphology in wild type and mutant dwarf mice (David mice) J. Comp. Physiol. B. 2007;177:183–192. doi: 10.1007/s00360-006-0120-9. [DOI] [PubMed] [Google Scholar]
- 52.Wang ZM, et al. Resting energy expenditure-fat-free mass relationship: new insights provided by body composition modeling. Am J Physiol-Endoc M. 2000;279:E539–E545. doi: 10.1152/ajpendo.2000.279.3.E539. [DOI] [PubMed] [Google Scholar]
- 53.Wang ZM, Heshka S, Heymsfield SB, Shen W, Gallagher D. A cellular-level approach to predicting resting energy expenditure across the adult years. Am. J. Clin. Nutr. 2005;81:799–806. doi: 10.1093/ajcn/81.4.799. [DOI] [PubMed] [Google Scholar]
- 54.Wang ZM, O’Connor TP, Heshka S, Heymsfield SB. The reconstruction of Kleiber’s law at the organ-tissue level. J. Nutr. 2001;131:2967–2970. doi: 10.1093/jn/131.11.2967. [DOI] [PubMed] [Google Scholar]
- 55.Speakman JR, et al. FTO effect on energy demand versus food intake. Nature. 2010;464:E1–E5. doi: 10.1038/nature08807. [DOI] [PubMed] [Google Scholar]
- 56.Raichlen DA, Gordon AD, Muchlinski MN, Snodgrass JJ. Causes and significance of variation in mammalian basal metabolism. J. Comp. Physiol. B. 2010;180:301–311. doi: 10.1007/s00360-009-0399-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.