Abstract
A new study examining the neural circuitry regulating sleep in Drosophila has identified a pair of dopamine neurons that signal to the fan-shaped body to suppress sleep. These neurons are separate from the dopamine neurons that regulate motivation, memory, and feeding, suggesting that independent populations of dopamine neurons regulate distinct behaviors.
The neurotransmitter dopamine plays a central role in motivation, feeding, memory and sleep–wake regulation across phyla. Fruit flies mutant for the dopamine transporter, the primary target for cocaine and methamphetamine, have reduced sleep, revealing dopamine to be a potent suppressor of sleep [1]. Flies with reduced dopamine signaling have deficits in associative memory and feeding behaviors. How does a single transmitter regulate such diverse behavioral and cognitive processes? One possibility is through a diversity of receptors. Alternatively, the distinct effects of dopamine may be mediated through neural connectivity. The fly brain contains approximately 200 dopamine neurons with widespread projections throughout the central brain [2,3]. Identifying the specific dopamine neurons that modulate individual behavioral processes is difficult and limited by the availability of genetic tools capable of labeling individual classes of neurons.
In this issue of Current Biology, Liu et al. [4] identify two dopamine neurons that suppress sleep. The authors transgenically activate dopamine neurons in a temperature-dependent fashion by genetically expressing the heat-inducible cation channel transient-receptor-potential A1 (TRPA1) in small populations of dopamine neurons. Expressing TRPA1 under control of the Tyrosine Hydroxylase promoter allows for inducible activation of all dopamine neurons and results in a dramatic reduction in sleep [5]. Liu et al. generated driver lines using fragments of the Tyrosine Hydroxylase genomic locus to transgenically label and manipulate specific populations of dopamine neurons. One line labeled a single neuron in each of the protocerebral posterolateral (PPL1) clusters that was not labeled in other lines, and activation of these neurons resulted in reduced sleep. These findings suggest that a pair of neurons in the PPL1 cluster is capable of conferring sleep loss.
Careful morphological analysis revealed that the sleep-suppressing PPL1 neurons ramify throughout the Drosophila fan-shaped body (Figure 1), a region within the central complex that was previously shown to regulate sleep and locomotion [6,7]. The fruit fly genome encodes four dopamine receptors, and mutants for the type 1 dopamine receptor, DopR, have increased sleep [8]. DopR appears to be the primary dopamine receptor required for arousal, because loss of DopR fully suppresses the wake-promoting effects of dopamine [4]. DopR is expressed predominantly in the mushroom bodies and central complex, two brain regions previously implicated in sleep–wake regulation [9]. Liu et al. [4] found that selectively rescuing DopR in the fan-shaped body alone rescues the long-sleeping phenotype of these mutants. These results suggest that a pair of PPL1 dopamine neurons stimulate DopR, thereby elevating cAMP levels in the fan-shaped body. Flies with genetic manipulations that mimic the effects of enhanced cAMP signaling in the fan-shaped body also suppress sleep, fortifying the notion that dopamine signaling to the fan-shaped body promotes wakefulness [4].
Figure 1.
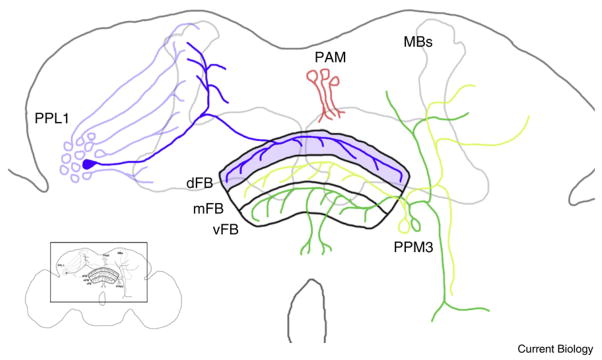
Schematic of sleep-suppressing dopamine neurons in the fly brain.
The fan-shaped body (FB) consists of three layers: dorsal (dFB), medial (mFB), and ventral (vFB). One dopamine neuron from each PPL1 cluster (PPL-dFB) suppresses sleep. Other PPL1 neurons innervate the mushroom bodies (MBs), which have previously been implicated in sleep and memory. Liu et al. [4] find that neurons from PPM3 neuronal cluster innervating vFB and mFB do not regulate sleep. Neurons from PAM cluster innervate medial lobes of MBs and signal positive and aversive reinforcement during learning. Inset depicts the magnified region within the fly brain.
There appears to be extensive redundancy in the neuroanatomy controlling sleep–wake regulation in Drosophila. A previous report found that rescue of DopR in the circadian pacemaker neurons labeled by Pigment Dispersing Factor (PDF) neuropeptide rescues sleep [8], suggesting that dopamine may regulate arousal through multiple loci. Identifying how distinct populations of DopR neurons regulate sleep will be critical for understanding dopamine function.
How does the activity of dopamine neurons contribute to sleep–wake regulation? One possibility is that wake-promoting dopamine neurons are more active during the day. To test this hypothesis the authors employed physiological imaging of PPL1 neurons during the subjective day and night. A neuropeptide fusion protein consisting of rat Atrial Naturetic Factor (ANF) fused to a green fluorescent protein reporter was used as a marker for imaging chronic neuronal activity in dopamine neurons [10]. These experiments suggest the PPL1 neurons innervating the fan-shaped body were more active during day than night [4]. Previous imaging studies of dopamine neurons using a cAMP-sensitive reporter have identified a dopamine-mediated increase in cAMP levels in circadian neurons [5]. While the effects of dopamine application on cAMP levels in the fan-shaped body was not measured by Liu et al. [4], this technique could be applied to examine dopamine-related signaling in the fan-shaped body.
Taken together, Liu et al. reveal neural circuitry that is sufficient for suppression of sleep in the fruit fly. These results are particularly important because they suggest a single pair of neurons is capable of regulating sleep–wake behavior. These findings present a number of questions that may provide critical insight into the neural circuitry regulating sleep. Which neurons activate the PPL1 fan-shaped body neurons and how are these neurons modulated in accordance with circadian cues and sleep need? Furthermore, the target neurons of the fan-shaped body remain unclear. The fan-shaped body is composed of three distinct layers of neurons that are differentially involved in visual memory and locomotion, suggesting these neurons may directly control locomotor and search behaviors. Future experiments addressing these questions may help to complete the dopamine-mediated, sleep-suppressing circuit in the fruit fly brain.
It is particularly interesting that single dopamine neurons appear to regulate distinct behaviors. A number of previous studies have elegantly demonstrated unique populations of dopamine neurons required for distinct memory tasks. Appetitive conditioning involving the pairing of sugar with a novel odor requires starvation prior to training [11]. PPL1 neurons expressing the receptor for the orexigenic transmitter, neuropeptide F, regulate appetitive conditioning by inhibiting appetitive memories in satiated flies [11]. These neurons are distinct from the sleep-regulating neurons because they exclusively innervate the mushroom bodies and do not affect sleep [4,11]. The pairing of electric-shock punishment with a novel odor is dependent on another population of PPL1 neurons that innervate the vertical lobes of the mushroom bodies [3]. The PAM population of dopamine neurons is critical for conveying aversive reinforcement in olfactory conditioning [12] but a small subset of the same neurons also signal sugar reward to the mushroom bodies [13]. Taken together, these studies suggest at least three different populations of dopamine neurons innervate the mushroom bodies to confer distinct signals during classical conditioning and each of these dopamine neuron populations is distinct from the wake-promoting PPL1 neurons that innervate the fan-shaped body.
A recent study has also reported a single dopamine neuron that is necessary and sufficient for the induction of feeding behavior. Flies extend their proboscis (mouth) in response to sucrose presentation and this response is reduced in flies with genetically inactivated dopamine neurons. Activation of a single dopamine neuron in the subesophageal ganglion is sufficient to trigger proboscis extension in the absence of food presentation [14]. Therefore, small numbers, or even single dopamine neurons are capable of mediating distinguishable effects on behavior.
The identification of a sleep-suppressing role for PPL1 neuron signaling to the fan-shaped body reveals important neural circuitry underlying sleep–wake regulation. Future work examining how the activity of individual dopamine neurons that control distinct behavioral processes are regulated may reveal fundamental information about how the brain regulates and chooses among behaviors.
References
- 1.Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J Neurosci. 2005;25:7377–7384. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Budnik V, White K. Catecholamine-containing neurons in Drosophila melanogaster: distribution and development. J Comp Neurol. 1988;268:400–413. doi: 10.1002/cne.902680309. [DOI] [PubMed] [Google Scholar]
- 3.Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, Hirsh J, Miesenbock G. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Q, Liu S, Kodama L, Driscoll MR, Wu M. Two dopaminergic neurons signal to the dorsal fan-shaped body to promote wakefulness in Drosophila. Curr Biol. 2012;22:2114–2123. doi: 10.1016/j.cub.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shang Y, Haynes P, Pirez N, Harrington KI, Guo F, Pollack J, Hong P, Griffith LC, Rosbash M. Imaging analysis of clock neurons reveals light buffers the wake-promoting effect of dopamine. Nat Neurosci. 2011;14:889–895. doi: 10.1038/nn.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donlea JM, Thimgan MS, Suzuki Y, Gottschalk L, Shaw PJ. Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science. 2011;332:1571–1576. doi: 10.1126/science.1202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin JR, Raabe T, Heisenberg M. Central complex substructures are required for the maintenance of locomotor activity in Drosophila melanogaster. J Comp Physiol A. 1999;185:277–288. doi: 10.1007/s003590050387. [DOI] [PubMed] [Google Scholar]
- 8.Lebestky T, Chang JS, Dankert H, Zelnik L, Kim YC, Han KA, Wolf FW, Perona P, Anderson DJ. Two different forms of arousal in Drosophila are oppositely regulated by the dopamine D1 receptor ortholog DopR via distinct neural circuits. Neuron. 2009;64:522–536. doi: 10.1016/j.neuron.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YC, Lee HG, Seong CS, Han KA. Expression of a D1 dopamine receptor dDA1/DmDOP1 in the central nervous system of Drosophila melanogaster. Gene Expr Patterns. 2003;3:237–245. doi: 10.1016/s1567-133x(02)00098-4. [DOI] [PubMed] [Google Scholar]
- 10.Shakiryanova D, Tully A, Hewes RS, Deitcher DL, Levitan ES. Activity-dependent liberation of synaptic neuropeptide vesicles. Nat Neurosci. 2005;8:173–178. doi: 10.1038/nn1377. [DOI] [PubMed] [Google Scholar]
- 11.Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aso Y, Siwanowicz I, Bracker L, Ito K, Kitamoto T, Tanimoto H. Specific dopaminergic neurons for the formation of labile aversive memory. Curr Biol. 2010;20:1445–1451. doi: 10.1016/j.cub.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C, Placais PY, Yamagata N, Pfeiffer BD, Aso Y, Friedrich AB, Siwanowicz I, Rubin GM, Preat T, Tanimoto H. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012;488:512–516. doi: 10.1038/nature11304. [DOI] [PubMed] [Google Scholar]
- 14.Marella S, Mann K, Scott K. Dopaminergic modulation of sucrose acceptance behavior in Drosophila. Neuron. 2012;73:941–950. doi: 10.1016/j.neuron.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]


