Abstract
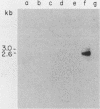
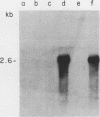
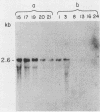
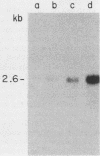
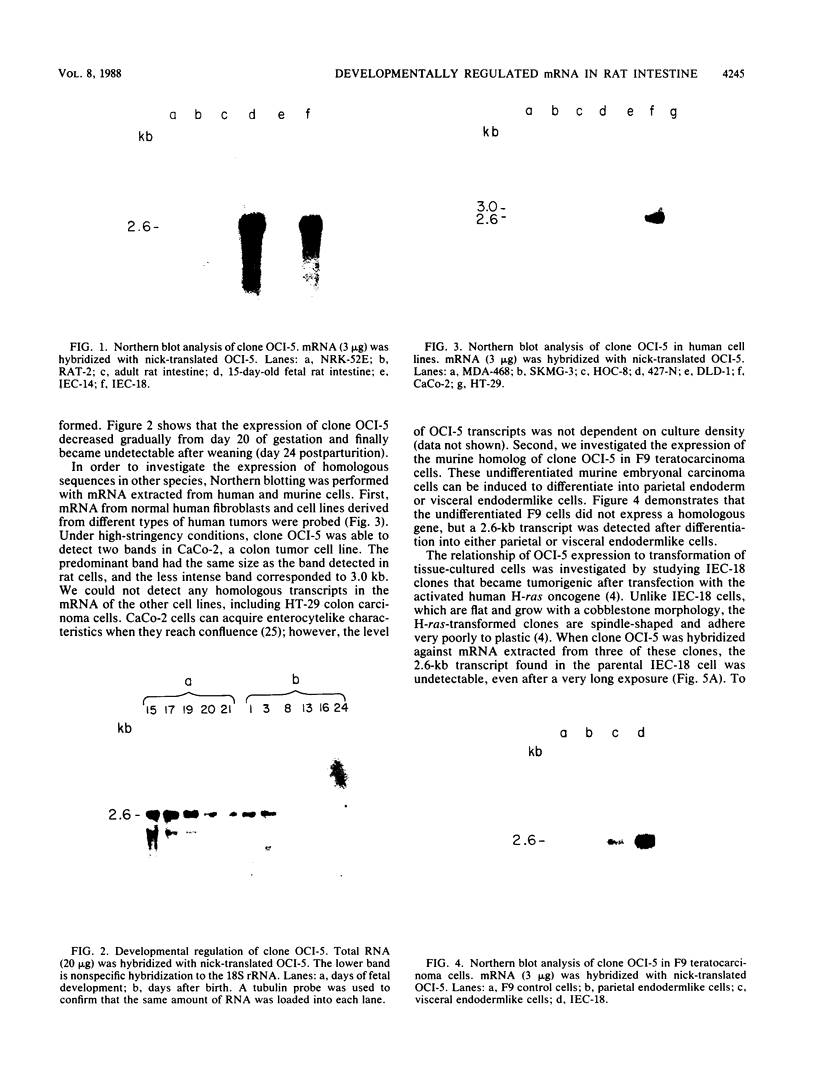
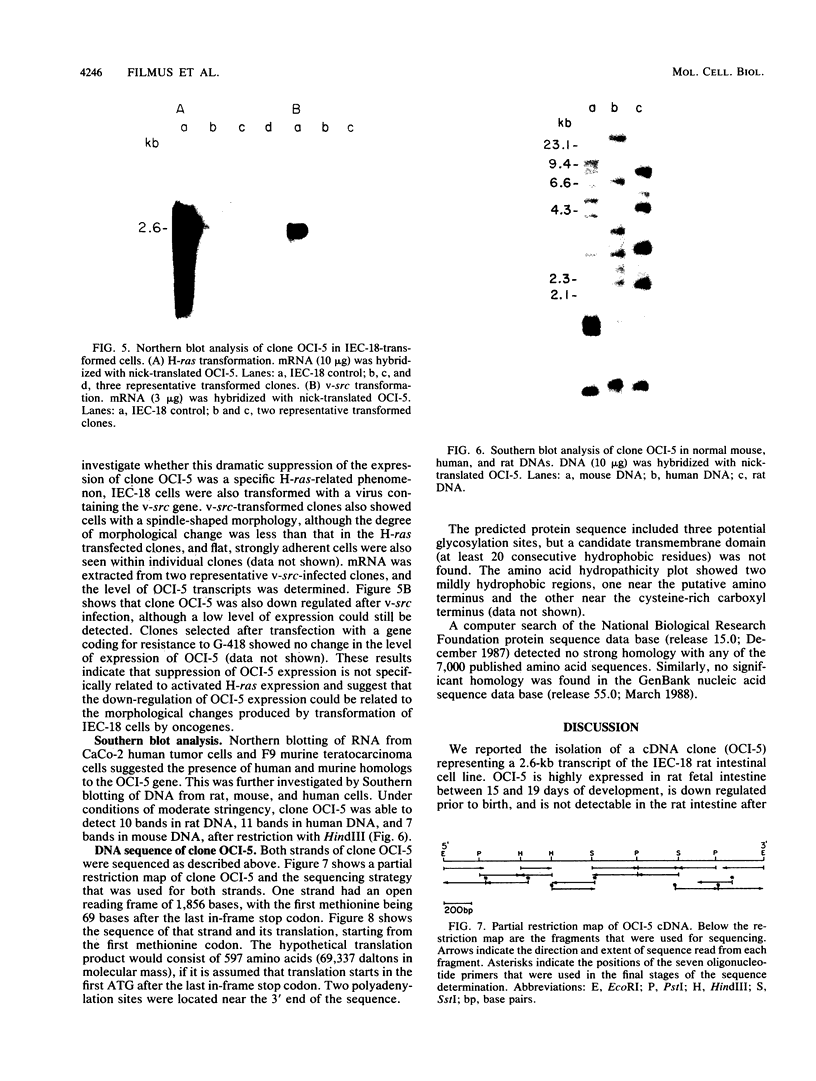
We report the isolation of a cDNA clone corresponding to a transcript that is accumulated differentially in rat intestine during development. Clone OCI-5 was selected from the rat intestinal cell line IEC-18, which represents primitive intestinal epithelial crypt cells. Expression was high in rat fetal intestine between 15 and 19 days of development and thereafter was progressively down regulated, becoming undetectable after weaning. Clone OCI-5 detected homologous sequences in human and murine cells. In particular, a high level of expression was detected in CaCo-2, a human colon carcinoma cell line, which is known to express molecules characteristic of fetal small intestinal cells. Expression of a homologous gene was also detected in F9 murine teratocarcinoma cells when they were induced to differentiate into parietal or visceral endodermlike cells. When IEC-18 cells were transformed by activated H-ras or v-src genes, expression of clone OCI-5 was suppressed; the degree of down-regulation correlated with the extent of morphological change induced in the transformed IEC-18 cells. The sequence of clone OCI-5 showed an open reading frame that was capable of encoding a protein of 597 amino acids, but no strong homology was found with any of the proteins registered in the protein sequence data base.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Alwine J. C., de Crombrugghe B., Pastan I. Use of recombinant plasmids to characterize collagen RNAs in normal and transformed chick embryo fibroblasts. J Biol Chem. 1979 Jun 25;254(12):4935–4938. [PubMed] [Google Scholar]
- Ben-Ze'ev A. The cytoskeleton in cancer cells. Biochim Biophys Acta. 1985;780(3):197–212. doi: 10.1016/0304-419x(85)90003-4. [DOI] [PubMed] [Google Scholar]
- Boulter C. A., Wagner E. F. The effects of v-src expression on the differentiation of embryonal carcinoma cells. Oncogene. 1988 Mar;2(3):207–214. [PubMed] [Google Scholar]
- Buick R. N., Filmus J., Quaroni A. Activated H-ras transforms rat intestinal epithelial cells with expression of alpha-TGF. Exp Cell Res. 1987 Jun;170(2):300–309. doi: 10.1016/0014-4827(87)90308-9. [DOI] [PubMed] [Google Scholar]
- Carlin B. E., Durkin M. E., Bender B., Jaffe R., Chung A. E. Synthesis of laminin and entactin by F9 cells induced with retinoic acid and dibutyryl cyclic AMP. J Biol Chem. 1983 Jun 25;258(12):7729–7737. [PubMed] [Google Scholar]
- Cheng H., Leblond C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat. 1974 Dec;141(4):461–479. doi: 10.1002/aja.1001410403. [DOI] [PubMed] [Google Scholar]
- Cooper H. L., Feuerstein N., Noda M., Bassin R. H. Suppression of tropomyosin synthesis, a common biochemical feature of oncogenesis by structurally diverse retroviral oncogenes. Mol Cell Biol. 1985 May;5(5):972–983. doi: 10.1128/mcb.5.5.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan J. B., Sobel M. E., Yamada K. M., de Crombrugghe B., Pastan I. Effects of transformation on fibronectin gene expression using cloned fibronectin cDNA. J Biol Chem. 1981 Jan 10;256(1):520–525. [PubMed] [Google Scholar]
- Filmus J., Trent J. M., Pullano R., Buick R. N. A cell line from a human ovarian carcinoma with amplification of the K-ras gene. Cancer Res. 1986 Oct;46(10):5179–5182. [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Hermos J. A., Mathan M., Trier J. S. DNA synthesis and proliferation by villous epithelial cells in fetal rats. J Cell Biol. 1971 Jul;50(1):255–258. doi: 10.1083/jcb.50.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedinger M., Simon-Assmann P. M., Lacroix B., Marxer A., Hauri H. P., Haffen K. Fetal gut mesenchyme induces differentiation of cultured intestinal endodermal and crypt cells. Dev Biol. 1986 Feb;113(2):474–483. doi: 10.1016/0012-1606(86)90183-1. [DOI] [PubMed] [Google Scholar]
- Kurokowa M., Lynch K., Podolsky D. K. Effects of growth factors on an intestinal epithelial cell line: transforming growth factor beta inhibits proliferation and stimulates differentiation. Biochem Biophys Res Commun. 1987 Feb 13;142(3):775–782. doi: 10.1016/0006-291x(87)91481-1. [DOI] [PubMed] [Google Scholar]
- Lampron C., Royal A. Expression of new proteins of the intermediate filament protein family in differentiating F9 embryonal carcinoma cell cytoskeleton. J Biol Chem. 1987 Apr 5;262(10):4893–4898. [PubMed] [Google Scholar]
- Leavitt J., Gunning P., Kedes L., Jariwalla R. Smooth muscle alpha-action is a transformation-sensitive marker for mouse NIH 3T3 and Rat-2 cells. 1985 Aug 29-Sep 4Nature. 316(6031):840–842. doi: 10.1038/316840a0. [DOI] [PubMed] [Google Scholar]
- Mathan M., Moxey P. C., Trier J. S. Morphogenesis of fetal rat duodenal villi. Am J Anat. 1976 May;146(1):73–92. doi: 10.1002/aja.1001460104. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Miyazaki K., Ashida Y., Kihira Y., Mashima K., Yamashita J., Horio T. Transformation of rat liver cell line by Rous sarcoma virus causes loss of cell surface fibronectin, accompanied with secretion of metallo-proteinase that preferentially digests the fibronectin. J Biochem. 1987 Sep;102(3):569–582. doi: 10.1093/oxfordjournals.jbchem.a122090. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak S., Siciliano M. J., Cailleau R., Wiseman C. L., Hsu T. C. A human breast adenocarcinoma with chromosome and isoenzyme markers similar to those of the HeLa line. J Natl Cancer Inst. 1979 Feb;62(2):263–271. [PubMed] [Google Scholar]
- Pfreundschuh M., Shiku H., Takahashi T., Ueda R., Ransohoff J., Oettgen H. F., Old L. J. Serological analysis of cell surface antigens of malignant human brain tumors. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5122–5126. doi: 10.1073/pnas.75.10.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A. Crypt cell antigen expression in human colon tumor cell lines: analysis with a panel of monoclonal antibodies to CaCo-2 luminal membrane components. J Natl Cancer Inst. 1986 Apr;76(4):571–585. doi: 10.1093/jnci/76.4.571. [DOI] [PubMed] [Google Scholar]
- Quaroni A. Crypt cell development in newborn rat small intestine. J Cell Biol. 1985 May;100(5):1601–1610. doi: 10.1083/jcb.100.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A. Development of fetal rat intestine in organ and monolayer culture. J Cell Biol. 1985 May;100(5):1611–1622. doi: 10.1083/jcb.100.5.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A. Fetal characteristics of small intestinal crypt cells. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1723–1727. doi: 10.1073/pnas.83.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A., Isselbacher K. J. Cytotoxic effects and metabolism of benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene in duodenal and ileal epithelial cell cultures. J Natl Cancer Inst. 1981 Dec;67(6):1353–1362. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Topp W. C. Normal rat cell lines deficient in nuclear thymidine kinase. Virology. 1981 Aug;113(1):408–411. doi: 10.1016/0042-6822(81)90168-9. [DOI] [PubMed] [Google Scholar]
- Warren S. L., Handel L. M., Nelson W. J. Elevated expression of pp60c-src alters a selective morphogenetic property of epithelial cells in vitro without a mitogenic effect. Mol Cell Biol. 1988 Feb;8(2):632–646. doi: 10.1128/mcb.8.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. II. Glycosyltransferases and endogenous acceptors of the undifferentiated cell surface membrane. J Biol Chem. 1973 Apr 10;248(7):2542–2548. [PubMed] [Google Scholar]