Abstract
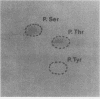
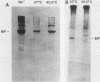
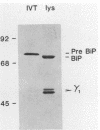
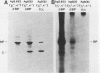
The 78,000-dalton glucose-regulated protein (GRP78) and the immunoglobulin heavy-chain-binding protein (BiP) were shown to be the same protein by NH2-terminal sequence comparison. Immunoprecipitation of GRP78-BiP induced by glucose starvation and a temperature-sensitive mutation in a hamster fibroblast cell line demonstrated the association of GRP78-BiP with other cellular proteins. In both fibroblasts and lymphoid cells, GRP78-BiP was found to label with 32Pi and [3H]adenosine. Phosphoamino acid analysis demonstrated that GRP78-BiP is phosphorylated on serine and threonine residues. Conditions which induce increased production of GRP78-BiP resulted in decreased incorporation of 32Pi and [3H]adenosine into GRP78-BiP. Furthermore, we report here that the phosphorylated form of BiP resides in the endoplasmic reticulum and that BiP which is associated with heavy chains is not phosphorylated or labeled with [3H]adenosine, whereas free BiP is. This suggests that posttranslational modifications may be important in regulating the synthesis and binding of BiP.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bairoch A., Claverie J. M. Sequence patterns in protein kinases. Nature. 1988 Jan 7;331(6151):22–22. doi: 10.1038/331022a0. [DOI] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Lazarides E. ADP-ribosylation of the Mr 83,000 stress-inducible and glucose-regulated protein in avian and mammalian cells: modulation by heat shock and glucose starvation. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4664–4668. doi: 10.1073/pnas.80.15.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. C., Wooden S. K., Nakaki T., Kim Y. K., Lin A. Y., Kung L., Attenello J. W., Lee A. S. Rat gene encoding the 78-kDa glucose-regulated protein GRP78: its regulatory sequences and the effect of protein glycosylation on its expression. Proc Natl Acad Sci U S A. 1987 Feb;84(3):680–684. doi: 10.1073/pnas.84.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Dorner A. J., Bole D. G., Kaufman R. J. The relationship of N-linked glycosylation and heavy chain-binding protein association with the secretion of glycoproteins. J Cell Biol. 1987 Dec;105(6 Pt 1):2665–2674. doi: 10.1083/jcb.105.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Haas I. G., Meo T. cDNA cloning of the immunoglobulin heavy chain binding protein. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2250–2254. doi: 10.1073/pnas.85.7.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas I. G., Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983 Nov 24;306(5941):387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Hendershot L., Bole D., Köhler G., Kearney J. F. Assembly and secretion of heavy chains that do not associate posttranslationally with immunoglobulin heavy chain-binding protein. J Cell Biol. 1987 Mar;104(3):761–767. doi: 10.1083/jcb.104.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Kamps M. P., Taylor S. S., Sefton B. M. Direct evidence that oncogenic tyrosine kinases and cyclic AMP-dependent protein kinase have homologous ATP-binding sites. Nature. 1984 Aug 16;310(5978):589–592. doi: 10.1038/310589a0. [DOI] [PubMed] [Google Scholar]
- Kassenbrock C. K., Garcia P. D., Walter P., Kelly R. B. Heavy-chain binding protein recognizes aberrant polypeptides translocated in vitro. Nature. 1988 May 5;333(6168):90–93. doi: 10.1038/333090a0. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kim Y. K., Kim K. S., Lee A. S. Regulation of the glucose-regulated protein genes by beta-mercaptoethanol requires de novo protein synthesis and correlates with inhibition of protein glycosylation. J Cell Physiol. 1987 Dec;133(3):553–559. doi: 10.1002/jcp.1041330317. [DOI] [PubMed] [Google Scholar]
- Lee A. S., Bell J., Ting J. Biochemical characterization of the 94- and 78-kilodalton glucose-regulated proteins in hamster fibroblasts. J Biol Chem. 1984 Apr 10;259(7):4616–4621. [PubMed] [Google Scholar]
- Lee A. S. The accumulation of three specific proteins related to glucose-regulated proteins in a temperature-sensitive hamster mutant cell line K12. J Cell Physiol. 1981 Jan;106(1):119–125. doi: 10.1002/jcp.1041060113. [DOI] [PubMed] [Google Scholar]
- Lee A. S., Wells S., Kim K. S., Scheffler I. E. Enhanced synthesis of the glucose/calcium-regulated proteins in a hamster cell mutant deficient in transfer of oligosaccharide core to polypeptides. J Cell Physiol. 1986 Dec;129(3):277–282. doi: 10.1002/jcp.1041290302. [DOI] [PubMed] [Google Scholar]
- Lizardi P. M. Methods for the preparation of messenger RNA. Methods Enzymol. 1983;96:24–38. doi: 10.1016/s0076-6879(83)96006-8. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Kong N., Hirani S., Rasmussen J. A vesicular intermediate in the transport of hepatoma secretory proteins from the rough endoplasmic reticulum to the Golgi complex. J Cell Biol. 1987 Feb;104(2):221–230. doi: 10.1083/jcb.104.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Ball E. H., Singer S. J. Vinculin: a cytoskeletal target of the transforming protein of Rous sarcoma virus. Cell. 1981 Apr;24(1):165–174. doi: 10.1016/0092-8674(81)90512-2. [DOI] [PubMed] [Google Scholar]
- Sood S. M., Chang P., Slattery C. W. Interactions in human casein systems: self-association of fully phosphorylated human beta-casein. Arch Biochem Biophys. 1985 Nov 1;242(2):355–364. doi: 10.1016/0003-9861(85)90220-6. [DOI] [PubMed] [Google Scholar]
- Ting J., Lee A. S. Human gene encoding the 78,000-dalton glucose-regulated protein and its pseudogene: structure, conservation, and regulation. DNA. 1988 May;7(4):275–286. doi: 10.1089/dna.1988.7.275. [DOI] [PubMed] [Google Scholar]
- Ueda K., Hayaishi O. ADP-ribosylation. Annu Rev Biochem. 1985;54:73–100. doi: 10.1146/annurev.bi.54.070185.000445. [DOI] [PubMed] [Google Scholar]
- Zala C. A., Salas-Prato M., Yan W. T., Banjo B., Perdue J. F. In cultured chick embryo fibroblasts the hexose transport components are not the 75 000 and 95 000 dalton polypeptides synthesized following glucose deprivation. Can J Biochem. 1980 Oct;58(10):1179–1188. doi: 10.1139/o80-158. [DOI] [PubMed] [Google Scholar]