Abstract
Background
Toxoplasmosis is a worldwide infection caused by the protozoan parasite Toxoplasma gondii, which causes chorioretinitis and neurological defects in congenitally infected newborns or immunodeficient patients. The efficacy of the current treatment is limited, primarily by serious host toxicity. In recent years, research has focused on the development of new drugs against T. gondii. β-Carbolines (βCs), such as harmane, norharmane and harmine, are a group of naturally occurring alkaloids that show microbicidal activity. In this work, harmane, norharmane and harmine were tested against T. gondii.
Findings
The treatment of extracellular tachyzoites with harmane, norharmane and harmine showed a 2.5 to 3.5-fold decrease in the invasion rates at doses of 40 μM (harmane and harmine) and 2.5 μM (norharmane) compared with the untreated parasites. Furthermore, an effect on the replication rate could also be observed with a decrease of 1 (harmane) and 2 (norharmane and harmine) division rounds at doses of 5 to 12.5 μM. In addition, the treated parasites presented either delayed or no monolayer lysis compared with the untreated parasites.
Conclusions
The three βC alkaloids studied (norharmane, harmane and harmine) exhibit anti-T. gondii effects as evidenced by the partial inhibition of parasite invasion and replication. A dose–response effect was observed at a relatively low drug concentration (< 40 μM), at which no cytotoxic effect was observed on the host cell line (Vero).
Keywords: Toxoplasma gondii, β-carbolines, Drug, Invasion, Cell cycle
Findings
The protozoan parasite Toxoplasma gondii is the etiologic agent of toxoplasmosis, a worldwide infection affecting 500 million to 1 billion people [1]. Toxoplasmosis occurs as an asymptomatic chronic (latent) infection. However, it is of medical health importance because toxoplasmosis can be dangerous or even fatal in immunocompromised individuals because of the reactivation of a latent infection. Congenital infection with Toxoplasma can also cause either spontaneous abortion or birth defects [2]. In the latter case, the active form of the parasite can cause encephalitis and neurologic diseases and can affect the heart, liver, inner ears, and eyes (chorioretinitis). Recently, chronic toxoplasmosis has been linked with brain cancer, attention deficit hyperactivity disorder, obsessive-compulsive disorder and schizophrenia [3-6].
There are effective drug regimens for toxoplasmosis based on a combination of pyrimethamine and sulfadiazine, but in some cases the efficacy of the current treatment is limited, primarily by serious host toxicity and/or development of drug-resistant parasites. In patients under immunosuppressive therapies and particularly in those with AIDS, treatment with sulfonamides and inhibitors of dihydrofolate reductase (DHFR) can produce side effects despite the preventive administration of folinic acid [7,8]. Moreover, the current therapy is ineffective against tissue cysts [9]. Other therapies, based on other types of drugs (clindamycin, spiramycin or atovaquone), have been used with limited success particularly in long-term patient management.
In recent years, research has focused on the development of new drugs against T. gondii[10-12]. Nevertheless, the need to identify and evaluate new drugs based on new and innovative therapeutic strategies against T. gondii is still evident. In this regard, β-carbolines (βCs) represent an excellent alternative that should be thoroughly investigated. βCs comprise a class of alkaloids that are widely found in nature. These alkaloids normally occur in plants [13,14] and mammals [15-17]. βCs are a group of heterocyclic compounds with a 9H-pyrido[3,4-b]indole structural unit (Figure 1) [18].
Figure 1.
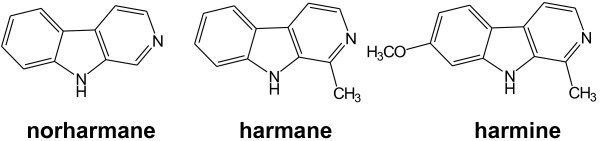
Structure of the different βCs studied: harmane, norharmane and harmine.
βCs were originally isolated from Peganumharmala (Zygophyllaceae, Syrian Rue), which is used as a traditional herbal drug [19,20]. Among them, harmane and tetrahydroharmane isolated from active extracts of different plants have been shown to have antimalarial activity and low cytotoxicity for human cells [21-23]. In addition, harmane and harmine have been shown a moderate effect on promastigotes of Leishmania infantum, mainly by accumulation of parasites arrested in the S-G2/M phases of the cell cycle, whereas harmaline has shown only anti-leishmanial activity against intracellular amastigotes by inhibiting the PKC enzyme [24]. Lala et al. [25] have shown that harmine is toxic for the promastigotes of Leishmania donovani, an effect attributed to necrosis due to non-specific membrane damage. The nifurtimox-resistant Trypanosoma cruzi LQ strain has shown a greater sensitivity to βCs, most likely due to inhibition of the respiratory chain of the parasite [26]. The chaperone HSP90 is an important drug target in protozoan parasites and new drugs are actively being sought [12,27]. In this regard, harmine was one of the three compounds selected among approximately 4,000 small molecules that inhibited P. falciparum HSP90 by specific competition with its ATP-binding domain. Interestingly, harmine has been shown to have anti-malarial effects in vitro and in vivo and acts synergistically with chloroquine and artemisinin [28,29]. El Sayed et al. [30] also reported that new enantiomers of 8-hydroxymanzamine A (ent-8-hydroxymanzamine A) and manzamine F (entmanzamine), isolated from Indo-Pacific sponge, together with manzamine A, exhibit significant activities against T. gondii and Plasmodium spp. There are also other studies in which βCs have shown parasiticidal and microbicidal effects that were further revised by Cao et al. [20].
The success of T. gondii infection relies on host cell recognition and attachment, invasion, replication and egress to spread throughout the host organism. Therefore, blocking any of these processes represents a target for new anti-Toxoplasma drugs/therapies. Our laboratory has gained experience in the analysis of harmane, norharmane and harmine for different purposes including their use as anti-viral drugs (Cabrerizo et al., unpublished results). The biological and pharmacological effects of these βCs are attributed in part to their ability to intercalate DNA and inhibit topoisomerase I and II, effects that result in alterations in DNA replication and, therefore, defects in cell cycle progress [20]. In addition, harmine has been found to be a potent and specific inhibitor of cyclin-dependent kinases (CDKs), showing a strong inhibitory effect on the growth and proliferation of carcinoma cells [31,32]. In this work, we evaluated these three βC alkaloids, harmane, norharmane and harmine, for their potential as new drugs against T. gondii, based on their ability to block invasion, replication and growth processes.
Materials and methods
Chemicals
Norharmane, harmane, harmine and sulfadiazine (Sigma-Aldrich Co., Buenos Aires, Argentina) were of the highest purity available (> 98%) and used without further purification.
Stock solutions preparation and pH adjustment
βC stock solutions (approximately 2 mM) were prepared as described elsewhere [33]. Briefly, each alkaloid was dissolved in acid-sterilized water. Once the alkaloid was fully dissolved, the pH of the aqueous solutions was adjusted to 7.4 by the addition of drops of HCl or NaOH solutions from a micropipette. The aliquots used in the in vitro experiments did not modify the pH of the cell culture. Sulfadiazine was dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich Co.) at 22 mM.
Parasite sources, culture and manipulation
Tachyzoites of the RH strain were cultured in standard tachyzoite conditions in vitro: Vero and human foreskin fibroblast (HFF) cell monolayers were infected with tachyzoites and incubated with Dulbecco’s modified Eagle medium (DMEM, Gibco BRL) supplemented with 10% fetal bovine serum (FBS), penicillin (100 UI/ml; Gibco BRL) and streptomycin (100 μg/ml; Gibco BRL) at 37°C and 5% CO2.
Invasion
Tachyzoites of the RH strain (2.5×106) were incubated for 1 h with different doses of βCs or water (untreated) in DMEM at 37°C. Treated and untreated tachyzoites were added to a Vero cell monolayer, incubated for 10 min on ice and then incubated at 37°C for 1 h, washed three times with PBS and fixed with 4% paraformaldehyde and 0.2% Triton X-100 and analyzed by immunofluorescence using murine anti-SAG1 antibody (SAG1: T. gondii surface antigen 1, 1:100 dilution)/Alexa Fluor 594-conjugated goat anti-mouse (1:4,000 dilution; Invitrogen, Argentina). In each experiment, 50 fields were analyzed and the number of tachyzoites per field was counted. Only fields with a similar number of host cells were considered for the experiments. The mean numbers of tachyzoites in each field plus the standard deviation were plotted using Prism GraphPad software.
Replication and growth analysis
The replication rate was determined by incubating the tachyzoites (2.5×106) with Vero cell monolayers, followed by 10 min of incubation on ice, then 1 h at 37°C and washing three times with PBS. Finally, the tachyzoites were incubated for an additional 24 h at 37°C, 5% CO2 with DMEM, 5% FBS and different doses of βCs or water (untreated). After the infected monolayers were fixed, they were immunolabeled with murine anti-SAG1 antibody/Alexa Fluor 594-conjugated goat anti-mouse antibody (Invitrogen, Argentina) and the number of tachyzoites per parasitophorous vacuole (PV) were counted. Fifty fields were counted in duplicate. Approximately 1000 PVs were counted at each dose. Data are presented as a percentage of PVs that contained a geometric progression (e.g. 1, 2, 4, 8 and so on) of tachyzoites per PV.
To analyze parasite growth, human foreskin fibroblast (HFF) monolayers were incubated with 2×104 tachyzoites as above mentioned. Because tachyzoite growth is destructive to cell monolayers, infected cultures were followed daily by inverted microscope visualization until complete monolayer lysis along with the presence of extracellular tachyzoites or complete (host cell/tachyzoites) destruction.
Results and discussion
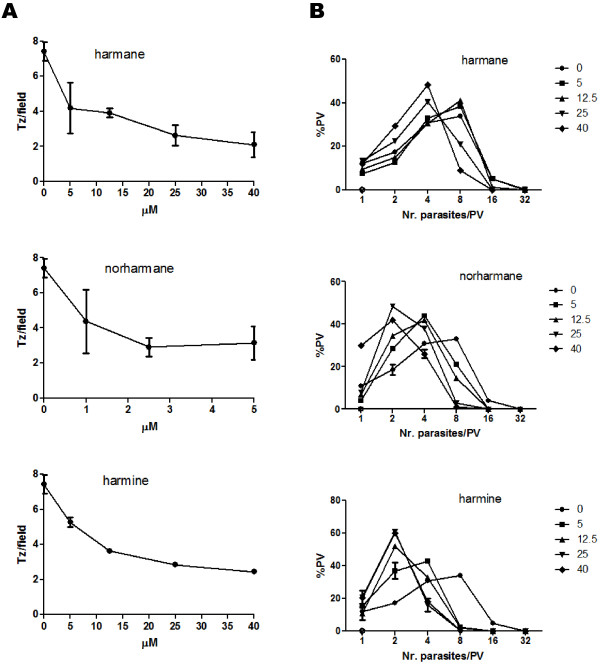
To analyze the effect of harmane, norharmane and harmine alkaloids on the ability of T. gondii to infect host cells, 2.5×106 extracellular tachyzoites (almost all comprising a G1 arrested form of the parasite) were incubated for 1 h with different doses (0 to 100 μM) of each βC. This dose range was chosen because it was previously shown that βCs and especially harmine induce apoptosis and necrosis, and inhibit proliferation of eukaryotic cells at concentrations higher than 40 μM [34]. Moreover, for some eukaryotic cell lines, such concentrations are cytotoxic [32]. Therefore, the selected βC-concentration range was chosen to avoid any secondary effect on the host cell line (Vero) that could interfere with the analysis of the invasion capability of the tachyzoites. The results plotted in Figure 2 (A) show that the three βCs studied have an inhibitory effect on parasite invasion in a dose-dependent manner. Norharmane exhibited its maximum effect at 2.5 μM whereas harmane and harmine had the highest parasiticidal effect at 40 μM (Figure 2A). The invasion rate of tachyzoites treated with βCs at the doses mentioned before showed a 3.5- (harmane), 2.5- (norharmane) and 3.1- (harmine) fold decrease compared with the untreated tachyzoites. Because this assay does not differentiate if the components affect the invasion process of the tachyzoites or the attachment process, we can only conclude that the decrease in the number of tachyzoites per field could be caused by an inhibitory effect on invasion, or attachment, or both.
Figure 2.
Effects of harmane, norharmane and harmine on parasite invasion and replication. A. Extracellular tachyzoites (2.5×106) were treated for 1 h with different doses of the βCs, washed and allowed to invade Vero cells monolayers for 1 h at 37°C, and then fixed and incubated with murine anti-SAG1 antibody. The secondary antibody used was Alexa Fluor 594 goat anti-rabbit. For each treatment, the number of parasitophorous vacuoles (PVs) per field was counted. Fifty fields selected at random were analyzed in duplicate. B. Infected monolayers were treated for 24 h with different doses of the βCs, fixed and immunostained with anti-SAG1 antibody. The number of tachyzoites inside the PV was counted. Fifty fields selected at random were counted in duplicate. In total, approximately 1000 PVs per dose were analyzed in A and B. These panels are representative of three independent experiments. All the experiments presented similar results.
To determine whether these βCs have any inhibitory effect on the parasite cycle process, we measured the ability of intracellular-treated parasites to replicate. Because tachyzoites replicate only within the host cell by a process called endodyogeny, every round of replication results in a geometric duplication of the parasite number per PV (e.g. 1, 2, 4, 8 and so on) [35]. Monolayers of Vero cells were infected with 2.5×106 of fresh tachyzoites to allow for the formation of PVs. Subsequently, the infected monolayers were washed and incubated at 37°C for 24 h in the presence of different doses of βCs (0 to 40 μM) (Figure 2B). Every PV was generated from one parasite; therefore, the number of tachyzoites inside the PV indicates the number of replication events. The panel shows the percentage of PVs that presented different numbers of tachyzoites. Harmane showed a slight inhibitory effect starting at 25 μM, resulting in a delay of one round of replication compared with the untreated parasites. Harmine and norharmane had a stronger inhibitory effect on parasite replication than harmane, because their effect was more pronounced at 25 μM, at which concentration the replication rate was delayed by two rounds (Figure 2B). Doses of norharmane lower than 5 μM did not show any effect on parasite replication (Additional file 1: Figure S1).
Toxoplasma gondii growth is the result of repeated cycles of host cell invasion, replication and egress, resulting in cell monolayer destruction in vitro. Because we observed a low rate of parasite entry and tachyzoite replication in the host cell, we expected a low rate of parasite growth. To analyze for this effect, HFF monolayers were infected with fresh tachyzoites, washed and incubated with the different doses of the βCs until the complete monolayer was lysed. HFF monolayers were completely lysed at day 3–4 after infection with the untreated parasites. For βC-treated monolayers, a delay in the time of complete lysis could be observed (Figure 3). As shown in Figure 3, harmine was shown to affect parasite growth at 40 μM. Infected monolayers treated with 5 mM of sulfadiazine did not show cell lysis during the 10 days of incubation (data not shown).
Figure 3.
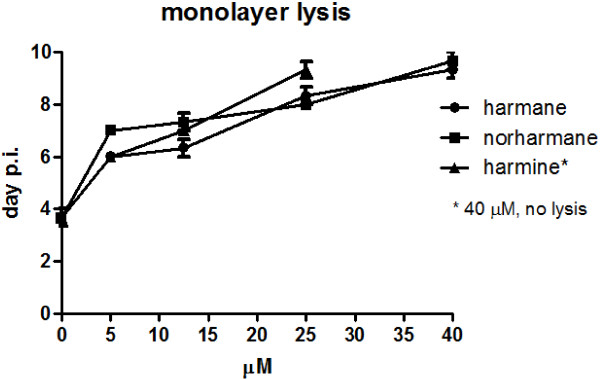
Analysis of the toxic effect of harmane, norharmane and harmine on T. gondii growth. Human foreskin fibroblast (HFF) monolayers were infected with fresh tachyzoites (2.5×104) for 1 h and incubated with different doses of βCs and followed by microscope observation until monolayer lysis (cell lysis in combination with extracellular tachyzoites). Data are presented as the day post-infection (p.i.) on which complete monolayer lysis was observed by microscope visualization. The panel is representative of three independent experiments performed in duplicate. All the experiments presented similar results. Sulfadiazine (5 mM) was used as a control of T. gondii growth inhibitory drugs and showed no monolayer lysis at day 10 p.i.
Our results demonstrate that the three βCs studied in this work have anti-T. gondii effects on both host cell invasion and replication processes and, consequently, on parasite growth. Particularly, harmine was shown to be the most active drug because it showed the strongest inhibitory effect on parasite replication and growth. In a recent study [25], it has been observed that harmine shows anti-leishmanial activity in part due to cell death attributed to non-specific membrane damage. In our hands, the highest dose (40 μM) of βCs affected neither the cell integrity nor the viability of the parasite. The entry of the tachyzoite into the host cell is an ATP-consuming process that includes the glideosome/myosin motor [36]. In this sense, some βCs have shown an inhibitory effect of the respiratory chain [26,37]. It is possible that parasite fitness is decreased because of a failure of mitochondrial functions. Our analysis did not allow us to distinguish which process (es) these drugs were affecting (attachment and/or invasion). Therefore, the βCs could be interfering with several metabolism pathways other than those related to attachment/invasion of the host cell, resulting in an impairment of these processes. Further analysis should be performed to elucidate which process/es is/are involved.
As mentioned above, βCs are considered to affect cell cycle. The tachyzoite is the highly replicative stage of T. gondii, a process that only occurs inside the host cell [38]. It is well known that these drugs can bind to DNA [39] and induce DNA damage [40] as well as inhibit topoisomerases I and II. These facts can contribute to the replication-associated DNA stress, affecting the cell replication rate [41,42]. Harmine also affects Plasmodium infection through its interaction with parasite HSP90, a recognized drug target against malaria [12,29]. The effect of this (or these) βC(s) on parasite replication could also involve the inhibition of Toxoplasma HSP90 functions. In fact, T. gondii HSP90 has been suggested as a key molecule in parasite replication [43]. Future studies should be performed to assess the interaction between these alkaloids and T. gondii topoisomerases and/or HSP90 protein and evaluate if these drugs can affect parasite cell cycle progression.
In conclusion, we have demonstrated that the three βC alkaloids studied (norharmane, harmane and harmine) exhibit anti-T. gondii effects. Interestingly, the effect on parasite invasion and replication occurred at low doses (below 40 μM), at which these alkaloids did not show any toxic effects on Vero and HFF cells used in our experiments [32,34]. However, βCs have been shown to inhibit some enzymes associated with mental disorders and could produce behavioral modifications, including hallucinogens in treated people [20]. This issue should be considered to determine in vivo doses [44]. Moreover, on the basis of the current knowledge [44], it would be possible to design novel harmine derivatives to avoid collateral effects without changing or even increasing the parasiticidal effect. Future studies should be performed to further elucidate the mechanism for their anti-T. gondii activity.
Competing interests
The authors declare that no conflicts of interest exist.
Authors’ contributions
MLA, conceived the study, participated in its design, performed the laboratory work and drafted the manuscript. FMC and SOA conceived the study, participated in its design and drafted the manuscript. FAORS collaborated with the cell viability and invasion assays. AG collaborated with the replication and growth assays. MVC participated in the experimental design and analysis of the data. All authors have read and approved the final manuscript.
Supplementary Material
Effect of norharmane on parasite invasion and replication. The analysis was similar to that mentioned in the Figure 3 legend, except that the drug doses were 0, 1, 2.5 and 5 μM.
Contributor Information
Maria L Alomar, Email: alomar.lis@gmail.com.
Federico AO Rasse-Suriani, Email: federasse@intech.gov.ar.
Agustina Ganuza, Email: agusganuza@intech.gov.ar.
Verónica M Cóceres, Email: coceres@intech.gov.ar.
Franco M Cabrerizo, Email: fcabrerizo@intech.gov.ar.
Sergio O Angel, Email: sangel@intech.gov.ar.
Acknowledgments
S. O. Angel (Researcher), F. M. Cabrerizo (Researcher), M. L. Alomar (Fellow), V. M. Cóceres (Fellow) and F. A. O. Rasse-Suriani (Fellow) are members of the National Research Council of Argentina (CONICET). A. Ganuza is a member of CIC (Province of Buenos Aires, Argentina). This work was supported by ANPCyT grant BID ‒ PICT 2011–0623 (to S. O. A.); NIH-NIAID 1R01AI083162-01 (to S. O. A.) and PIP-00400 (to F. M. C.). We are grateful to Alejandra Goldman and Valentina Martin for their critical readings of the manuscript.
References
- Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30(12–13):1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier Y, Truyens C, Deloron P, Peyron F. Congenital parasitic infections: a review. Acta Trop. 2012;121(2):55–70. doi: 10.1016/j.actatropica.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Brynska A, Tomaszewicz-Libudzic E, Wolanczyk T. Obsessive-compulsive disorder and acquired toxoplasmosis in two children. Eur Child Adolesc Psychiatry. 2001;10(3):200–204. doi: 10.1007/s007870170027. [DOI] [PubMed] [Google Scholar]
- Miman O, Mutlu EA, Ozcan O, Atambay M, Karlidag R, Unal S. Is there any role of Toxoplasma gondii in the etiology of obsessive-compulsive disorder? Psychiatry Res. 2010;177(1–2):263–265. doi: 10.1016/j.psychres.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Dickerson FB, Fuller Torrey E. Toxoplasma and schizophrenia. Parasite Immunol. 2009;31(11):706–715. doi: 10.1111/j.1365-3024.2009.01131.x. [DOI] [PubMed] [Google Scholar]
- Vittecoq M, Elguero E, Lafferty KD, Roche B, Brodeur J, Gauthier-Clerc M, Misse D, Thomas F. Brain cancer mortality rates increase with Toxoplasma gondii seroprevalence in France. Infect Genet Evol. 2012;12(2):496–498. doi: 10.1016/j.meegid.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15(2):211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- Wong SY, Remington JS. Biology of Toxoplasma gondii. AIDS. 1993;7(3):299–316. doi: 10.1097/00002030-199303000-00001. [DOI] [PubMed] [Google Scholar]
- Weiss LM, Kim K. The development and biology of bradyzoites of Toxoplasma gondii. Front Biosci. 2000;5:D391–405. doi: 10.2741/Weiss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JB, Szajnman SH. New antibacterials for the treatment of toxoplasmosis; a patent review. Expert Opin Ther Pat. 2012;22(3):311–333. doi: 10.1517/13543776.2012.668886. [DOI] [PubMed] [Google Scholar]
- Vanagas L, Jeffers V, Bogado SS, Dalmasso MC, Sullivan WJ Jr, Angel SO. Toxoplasma histone acetylation remodelers as novel drug targets. Expert Rev Anti Infect Ther. 2012;10(10):1189–1201. doi: 10.1586/eri.12.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel SO, Matrajt M, Echeverria PC. A review of recent patents on the protozoan parasite HSP90 as a drug target. Biotechnol: Recent Pat; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herraiz T, Gonzalez D, Ancin-Azpilicueta C, Aran VJ. Guillen H: beta-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO) Food Chem Toxicol. 2010;48(3):839–845. doi: 10.1016/j.fct.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Pfau W, Skog K. Exposure to beta-carbolines norharman and harman. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;802(1):115–126. doi: 10.1016/j.jchromb.2003.10.044. [DOI] [PubMed] [Google Scholar]
- Pari K, Sundari CS, Chandani S. Balasubramanian D: beta-carbolines that accumulate in human tissues may serve a protective role against oxidative stress. J Biol Chem. 2000;275(4):2455–2462. doi: 10.1074/jbc.275.4.2455. [DOI] [PubMed] [Google Scholar]
- Spijkerman R, van den Eijnden R, van de Mheen D, Bongers I, Fekkes D. The impact of smoking and drinking on plasma levels of norharman. Eur Neuropsychopharmacol. 2002;12(1):61–71. doi: 10.1016/S0924-977X(01)00143-2. [DOI] [PubMed] [Google Scholar]
- Torreilles J, Guerin MC, Previero A. Simple compounds with high pharmacologic potential: beta-carbolines. Origins, syntheses, biological properties. Biochimie. 1985;67(9):929–947. doi: 10.1016/S0300-9084(85)80289-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez MM, Arnbjerg J, Denofrio MP, Erra-Balsells R, Ogilby PR, Cabrerizo FM. One- and two-photon excitation of beta-carbolines in aqueous solution: pH-dependent spectroscopy, photochemistry, and photophysics. J Phys Chem A. 2009;113(24):6648–6656. doi: 10.1021/jp902105x. [DOI] [PubMed] [Google Scholar]
- Sobhani AM, Ebrahimi SA, Mahmoudian M. An in vitro evaluation of human DNA topoisomerase I inhibition by Peganum harmala L. seeds extract and its beta-carboline alkaloids. J Pharm Pharm Sci. 2002;5(1):19–23. [PubMed] [Google Scholar]
- Cao R, Peng W, Wang Z, Xu A. beta-Carboline alkaloids: biochemical and pharmacological functions. Curr Med Chem. 2007;14(4):479–500. doi: 10.2174/092986707779940998. [DOI] [PubMed] [Google Scholar]
- Azas N, Laurencin N, Delmas F, Di GC, Gasquet M, Laget M, Timon-David P. Synergistic in vitro antimalarial activity of plant extracts used as traditional herbal remedies in Mali. Parasitol Res. 2002;88(2):165–171. doi: 10.1007/s004360100454. [DOI] [PubMed] [Google Scholar]
- Ancolio C, Azas N, Mahiou V, Ollivier E, Di Giorgio C, Keita A, Timon-David P, Balansard G. Antimalarial activity of extracts and alkaloids isolated from six plants used in traditional medicine in Mali and Sao Tome. Phytother Res. 2002;16(7):646–649. doi: 10.1002/ptr.1025. [DOI] [PubMed] [Google Scholar]
- Fiot J, Jansen O, Akhmedjanova V, Angenot L, Balansard G, Ollivier E. HPLC quantification of alkaloids from Haplophyllum extracts and comparison with their cytotoxic properties. Phytochem Anal. 2006;17(5):365–369. doi: 10.1002/pca.927. [DOI] [PubMed] [Google Scholar]
- Di Giorgio C, Delmas F, Ollivier E, Elias R, Balansard G, Timon-David P. In vitro activity of the beta-carboline alkaloids harmane, harmine, and harmaline toward parasites of the species Leishmania infantum. Exp Parasitol. 2004;106(3–4):67–74. doi: 10.1016/j.exppara.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Lala S, Pramanick S, Mukhopadhyay S, Bandyopadhyay S, Basu MK. Harmine: evaluation of its antileishmanial properties in various vesicular delivery systems. J Drug Target. 2004;12(3):165–175. doi: 10.1080/10611860410001712696. [DOI] [PubMed] [Google Scholar]
- Rivas P, Cassels BK, Morello A, Repetto Y. Effects of some beta-carboline alkaloids on intact Trypanosoma cruzi epimastigotes. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999;122(1):27–31. doi: 10.1016/S0742-8413(98)10069-5. [DOI] [PubMed] [Google Scholar]
- Rochani AK, Singh M, Tatu U. Heat Shock Protein 90 Inhibitors as Broad Spectrum Anti-infectives. Curr Pharm Des. 2012. [DOI] [PubMed]
- Shahinas D, Liang M, Datti A, Pillai DR. A repurposing strategy identifies novel synergistic inhibitors of Plasmodium falciparum heat shock protein 90. J Med Chem. 2010;53(9):3552–3557. doi: 10.1021/jm901796s. [DOI] [PubMed] [Google Scholar]
- Shahinas D, Macmullin G, Benedict C, Crandall I, Pillai DR. Harmine is a potent antimalarial targeting Hsp90 and synergizes with chloroquine and artemisinin. Antimicrob Agents Chemother. 2012;56(8):4207–4213. doi: 10.1128/AAC.00328-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sayed KA, Kelly M, Kara UA, Ang KK, Katsuyama I, Dunbar DC, Khan AA, Hamann MT. New manzamine alkaloids with potent activity against infectious diseases. J Am Chem Soc. 2001;123(9):1804–1808. doi: 10.1021/ja002073o. [DOI] [PubMed] [Google Scholar]
- Song Y, Wang J, Teng SF, Kesuma D, Deng Y, Duan J, Wang JH, Qi RZ, Sim MM. β-Carbolines as specific inhibitors of cyclin-Dependent kinases. Bioorg Med Chem Lett. 2002;12(7):1129–1132. doi: 10.1016/S0960-894X(02)00094-X. [DOI] [PubMed] [Google Scholar]
- Song Y, Kesuma D, Wang J, Deng Y, Duan J, Wang JH, Qi RZ. Specific inhibition of cyclin-dependent kinases and cell proliferation by harmine. Biochem Biophys Res Commun. 2004;317(1):128–132. doi: 10.1016/j.bbrc.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Gonzalez MM, Salum ML, Gholipour Y, Cabrerizo FM, Erra-Balsells R. Photochemistry of norharmane in aqueous solution. Photochem Photobiol Sci. 2009;8(8):1139–1149. doi: 10.1039/b822173a. [DOI] [PubMed] [Google Scholar]
- Jimenez J, Riveron-Negrete L, Abdullaev F, Espinosa-Aguirre J, Rodriguez-Arnaiz R. Cytotoxicity of the beta-carboline alkaloids harmine and harmaline in human cell assays in vitro. Exp Toxicol Pathol. 2008;60(4–5):381–389. doi: 10.1016/j.etp.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Striepen B, Jordan CN, Reiff S, van Dooren GG. Building the perfect parasite: cell division in apicomplexa. PLoS Pathog. 2007;3(6):e78. doi: 10.1371/journal.ppat.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum J, Papenfuss AT, Baum B, Speed TP, Cowman AF. Regulation of apicomplexan actin-based motility. Nat Rev Microbiol. 2006;4(8):621–628. doi: 10.1038/nrmicro1465. [DOI] [PubMed] [Google Scholar]
- Bringmann G, Feineis D, Bruckner R, Blank M, Peters K, Peters EM, Reichmann H, Janetzky B, Grote C, Clement HW. Bromal-derived tetrahydro-beta-carbolines as neurotoxic agents: chemistry, impairment of the dopamine metabolism, and inhibitory effects on mitochondrial respiration. Bioorg Med Chem. 2000;8(6):1467–1478. doi: 10.1016/S0968-0896(00)00073-0. [DOI] [PubMed] [Google Scholar]
- Radke JR, Striepen B, Guerini MN, Jerome ME, Roos DS, White MW. Defining the cell cycle for the tachyzoite stage of Toxoplasma gondii. Mol Biochem Parasitol. 2001;115(2):165–175. doi: 10.1016/S0166-6851(01)00284-5. [DOI] [PubMed] [Google Scholar]
- Gonzalez MM, Vignoni M, Pellon-Maison M, Ales-Gandolfo MA, Gonzalez-Baro MR, Erra-Balsells R, Epe B, Cabrerizo FM. Photosensitization of DNA by beta-carbolines: kinetic analysis and photoproduct characterization. Org Biomol Chem. 2012;10(9):1807–1819. doi: 10.1039/c2ob06505c. [DOI] [PubMed] [Google Scholar]
- Gonzalez MM, Pellon-Maison M, Ales-Gandolfo MA, Gonzalez-Baro MR, Erra-Balsells R, Cabrerizo FM. Photosensitized cleavage of plasmidic DNA by norharmane, a naturally occurring beta-carboline. Org Biomol Chem. 2010;8(11):2543–2552. doi: 10.1039/c002235g. [DOI] [PubMed] [Google Scholar]
- Hsiang YH, Lihou MG, Liu LF. Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res. 1989;49(18):5077–5082. [PubMed] [Google Scholar]
- D'Arpa P, Beardmore C, Liu LF. Involvement of nucleic acid synthesis in cell killing mechanisms of topoisomerase poisons. Cancer Res. 1990;50(21):6919–6924. [PubMed] [Google Scholar]
- Echeverria PC, Matrajt M, Harb OS, Zappia MP, Costas MA, Roos DS, Dubremetz JF, Angel SO. Toxoplasma gondii Hsp90 is a potential drug target whose expression and subcellular localization are developmentally regulated. J Mol Biol. 2005;350(4):723–734. doi: 10.1016/j.jmb.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Reniers J, Robert S, Frederick R, Masereel B, Vincent S, Wouters J. Synthesis and evaluation of beta-carboline derivatives as potential monoamine oxidase inhibitors. Bioorg Med Chem. 2011;19(1):134–144. doi: 10.1016/j.bmc.2010.11.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of norharmane on parasite invasion and replication. The analysis was similar to that mentioned in the Figure 3 legend, except that the drug doses were 0, 1, 2.5 and 5 μM.