Abstract
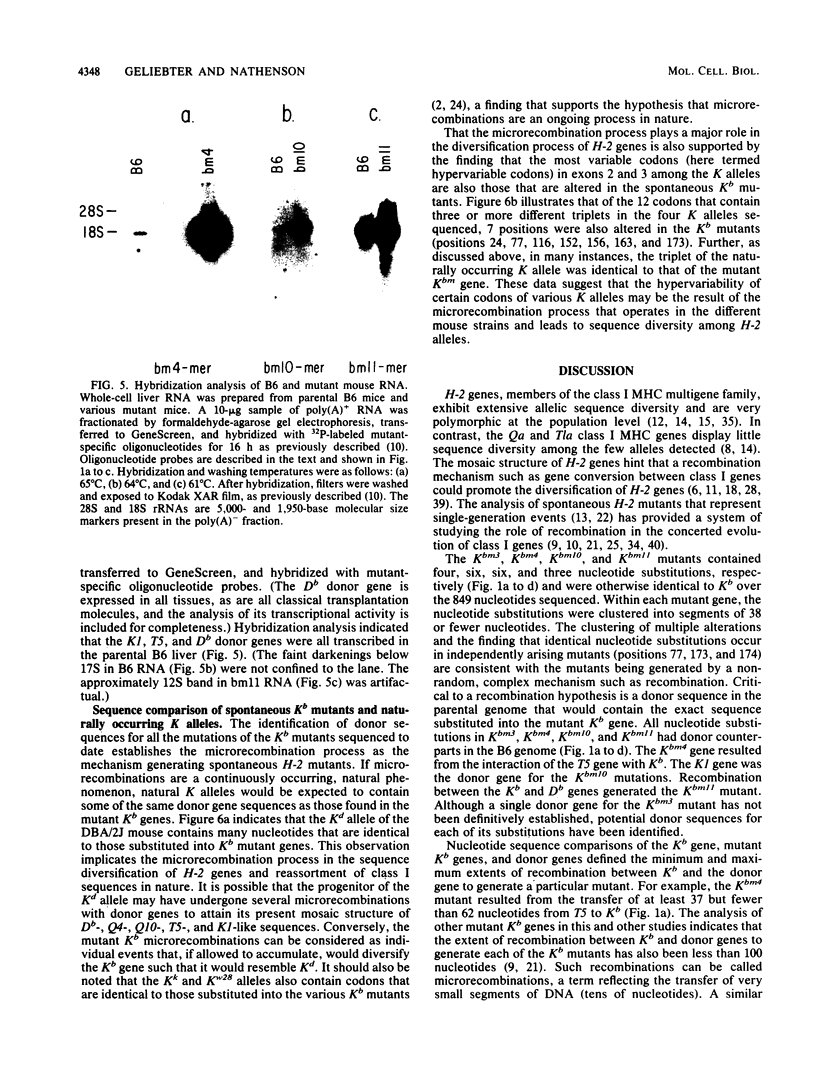
The mechanism that generates spontaneous mutants of the Kb histocompatibility gene was analyzed. Nucleotide sequence analysis of four mutant genes (Kbm3, Kbm4, Kbm10, and Kbm11) revealed that each mutant K gene contains clustered, multiple nucleotide substitutions. Hybridization analyses of parental B6 genomic DNA and cloned class I genes with mutant-specific oligonucleotide probes, followed by sequence analyses, have identified major histocompatibility complex class I genes in the K, D, and Tla regions (K1, Db, and T5, respectively) that contain the exact sequences as substituted into mutant Kb genes. These data provide evidence for the hypothesis that the mutant Kb genes are generated by a microrecombination (gene conversion) mechanism that results in the transfer of small DNA segments from class I genes of all four regions of the major histocompatibility complex (K, D, Qa, and Tla) to Kb. Many of the nucleotides substituted into the mutant Kb genes were identical to those found in other naturally occurring K alleles such as Kd. Thus, we propose that the accumulation of microrecombination products within the K genes of a mouse population is responsible for the high sequence diversity among H-2 alleles.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abastado J. P., Cami B., Dinh T. H., Igolen J., Kourilsky P. Processing of complex heteroduplexes in Escherichia coli and Cos-1 monkey cells. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5792–5796. doi: 10.1073/pnas.81.18.5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold B., Burgert H. G., Archibald A. L., Kvist S. Complete nucleotide sequence of the murine H-2Kk gene. Comparison of three H-2K locus alleles. Nucleic Acids Res. 1984 Dec 21;12(24):9473–9487. doi: 10.1093/nar/12.24.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. S., Geier S., Nathenson S. G., Forman J. Analysis of associative recognition determinants on class I H-2Kb mutant molecules. Immunogenetics. 1985;22(4):391–397. doi: 10.1007/BF00430922. [DOI] [PubMed] [Google Scholar]
- Devlin J. J., Lew A. M., Flavell R. A., Coligan J. E. Secretion of a soluble class I molecule encoded by the Q10 gene of the C57BL/10 mouse. EMBO J. 1985 Feb;4(2):369–374. doi: 10.1002/j.1460-2075.1985.tb03638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin J. J., Weiss E. H., Paulson M., Flavell R. A. Duplicated gene pairs and alleles of class I genes in the Qa2 region of the murine major histocompatibility complex: a comparison. EMBO J. 1985 Dec 1;4(12):3203–3207. doi: 10.1002/j.1460-2075.1985.tb04066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Camerini-Otero R. D., Ozato K., Seidman J. G. Structure and expression of a mouse major histocompatibility antigen gene, H-2Ld. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1994–1998. doi: 10.1073/pnas.79.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. A., Hunt S. W., 3rd, Hood L. Structure of a gene encoding a murine thymus leukemia antigen, and organization of Tla genes in the BALB/c mouse. J Exp Med. 1985 Aug 1;162(2):528–545. doi: 10.1084/jem.162.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. A., Allen H., Burkly L. C., Sherman D. H., Waneck G. L., Widera G. Molecular biology of the H-2 histocompatibility complex. Science. 1986 Jul 25;233(4762):437–443. doi: 10.1126/science.3726537. [DOI] [PubMed] [Google Scholar]
- Geliebter J., Zeff R. A., Melvold R. W., Nathenson S. G. Mitotic recombination in germ cells generated two major histocompatibility complex mutant genes shown to be identical by RNA sequence analysis: Kbm9 and Kbm6. Proc Natl Acad Sci U S A. 1986 May;83(10):3371–3375. doi: 10.1073/pnas.83.10.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geliebter J., Zeff R. A., Schulze D. H., Pease L. R., Weiss E. H., Mellor A. L., Flavell R. A., Nathenson S. G. Interaction between Kb and Q4 gene sequences generates the Kbm6 mutation. Mol Cell Biol. 1986 Feb;6(2):645–652. doi: 10.1128/mcb.6.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida H., Miyata T. Unusual evolutionary conservation and frequent DNA segment exchange in class I genes of the major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 May;80(9):2671–2675. doi: 10.1073/pnas.80.9.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball E. S., Coligan J. E. Structure of class I major histocompatibility antigens. Contemp Top Mol Immunol. 1983;9:1–63. doi: 10.1007/978-1-4684-4517-6_1. [DOI] [PubMed] [Google Scholar]
- Klein J., Figueroa F. Evolution of the major histocompatibility complex. Crit Rev Immunol. 1986;6(4):295–386. [PubMed] [Google Scholar]
- Klein J. H-2 mutations: their genetics and effect on immune functions. Adv Immunol. 1978;26:55–146. doi: 10.1016/s0065-2776(08)60229-1. [DOI] [PubMed] [Google Scholar]
- Kvist S., Roberts L., Dobberstein B. Mouse histocompatibility genes: structure and organisation of a Kd gene. EMBO J. 1983;2(2):245–254. doi: 10.1002/j.1460-2075.1983.tb01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalanne J. L., Bregegere F., Delarbre C., Abastado J. P., Gachelin G., Kourilsky P. Comparison of nucleotide sequences of mRNAs belonging to the mouse H-2 multigene family. Nucleic Acids Res. 1982 Feb 11;10(3):1039–1049. doi: 10.1093/nar/10.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh D. Y., Baltimore D. Sexual preference of apparent gene conversion events in MHC genes of mice. Nature. 1984 Jun 14;309(5969):639–640. doi: 10.1038/309639a0. [DOI] [PubMed] [Google Scholar]
- McIntyre K. R., Seidman J. G. Nucleotide sequence of mutant I-A beta bm12 gene is evidence for genetic exchange between mouse immune response genes. Nature. 1984 Apr 5;308(5959):551–553. doi: 10.1038/308551a0. [DOI] [PubMed] [Google Scholar]
- Mellor A. L., Weiss E. H., Ramachandran K., Flavell R. A. A potential donor gene for the bm1 gene conversion event in the C57BL mouse. Nature. 1983 Dec 22;306(5945):792–795. doi: 10.1038/306792a0. [DOI] [PubMed] [Google Scholar]
- Melvold R. W., Kohn H. I., Dunn G. R. History and genealogy of the H-2Kb mutants from the C57BL/6Kh colony. Immunogenetics. 1982;15(2):177–185. doi: 10.1007/BF00621950. [DOI] [PubMed] [Google Scholar]
- Michaelson J., Boyse E. A., Chorney M., Flaherty L., Fleissner E., Hämmerling U., Reinisch C., Rosenson R., Shen F. W. The biochemical genetics of the Qa-Tla region. Transplant Proc. 1983 Dec;15(4):2033–2038. [PubMed] [Google Scholar]
- Morita T., Delarbre C., Kress M., Kourilsky P., Gachelin G. An H-2K gene of the tw32 mutant at the T/t complex is a close parent of an H-2Kq gene. Immunogenetics. 1985;21(4):367–383. doi: 10.1007/BF00430802. [DOI] [PubMed] [Google Scholar]
- Nairn R., Yamaga K., Nathenson S. G. Biochemistry of the gene products from murine MHC mutants. Annu Rev Genet. 1980;14:241–277. doi: 10.1146/annurev.ge.14.120180.001325. [DOI] [PubMed] [Google Scholar]
- Nathenson S. G., Uehara H., Ewenstein B. M., Kindt T. J., Coligan J. E. Primary structural: analysis of the transplantation antigens of the murine H-2 major histocompatibility complex. Annu Rev Biochem. 1981;50:1025–1052. doi: 10.1146/annurev.bi.50.070181.005113. [DOI] [PubMed] [Google Scholar]
- Obata Y., Chen Y. T., Stockert E., Old L. J. Structural analysis of TL genes of the mouse. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5475–5479. doi: 10.1073/pnas.82.16.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T. Allelic and nonallelic homology of a supergene family. Proc Natl Acad Sci U S A. 1982 May;79(10):3251–3254. doi: 10.1073/pnas.79.10.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease L. R., Schulze D. H., Pfaffenbach G. M., Nathenson S. G. Spontaneous H-2 mutants provide evidence that a copy mechanism analogous to gene conversion generates polymorphism in the major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 Jan;80(1):242–246. doi: 10.1073/pnas.80.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontarotti P. A., Mashimo H., Zeff R. A., Fisher D. A., Hood L., Mellor A., Flavell R. A., Nathenson S. G. Conservation and diversity in the class I genes of the major histocompatibility complex: sequence analysis of a Tlab gene and comparison with a Tlac gene. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1782–1786. doi: 10.1073/pnas.83.6.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Grimal H., Weill J. C. A hyperconversion mechanism generates the chicken light chain preimmune repertoire. Cell. 1987 Feb 13;48(3):379–388. doi: 10.1016/0092-8674(87)90189-9. [DOI] [PubMed] [Google Scholar]
- Rogers J. H. Family organization of mouse H-2 class I genes. Immunogenetics. 1985;21(4):343–353. doi: 10.1007/BF00430800. [DOI] [PubMed] [Google Scholar]
- Rubocki R. J., Hansen T. H., Lee D. R. Molecular studies of murine mutant BALB/c-H-2dm2 define a deletion of several class I genes including the entire H-2Ld gene. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9606–9610. doi: 10.1073/pnas.83.24.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze D. H., Pease L. R., Geier S. S., Reyes A. A., Sarmiento L. A., Wallace R. B., Nathenson S. G. Comparison of the cloned H-2Kbm1 variant gene with the H-2Kb gene shows a cluster of seven nucleotide differences. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2007–2011. doi: 10.1073/pnas.80.7.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Hood L. Genes of the major histocompatibility complex in mouse and man. Science. 1983 Nov 18;222(4625):727–733. doi: 10.1126/science.6356354. [DOI] [PubMed] [Google Scholar]
- Sun Y. H., Goodenow R. S., Hood L. Molecular basis of the dm1 mutation in the major histocompatibility complex of the mouse: a D/L hybrid gene. J Exp Med. 1985 Nov 1;162(5):1588–1602. doi: 10.1084/jem.162.5.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkel-Meiman K., Keil R. L., Roeder G. S. Recombination-stimulating sequences in yeast ribosomal DNA correspond to sequences regulating transcription by RNA polymerase I. Cell. 1987 Mar 27;48(6):1071–1079. doi: 10.1016/0092-8674(87)90714-8. [DOI] [PubMed] [Google Scholar]
- Weiss E. H., Golden L., Fahrner K., Mellor A. L., Devlin J. J., Bullman H., Tiddens H., Bud H., Flavell R. A. Organization and evolution of the class I gene family in the major histocompatibility complex of the C57BL/10 mouse. Nature. 1984 Aug 23;310(5979):650–655. doi: 10.1038/310650a0. [DOI] [PubMed] [Google Scholar]
- Weiss E. H., Mellor A., Golden L., Fahrner K., Simpson E., Hurst J., Flavell R. A. The structure of a mutant H-2 gene suggests that the generation of polymorphism in H-2 genes may occur by gene conversion-like events. Nature. 1983 Feb 24;301(5902):671–674. doi: 10.1038/301671a0. [DOI] [PubMed] [Google Scholar]
- Weiss E., Golden L., Zakut R., Mellor A., Fahrner K., Kvist S., Flavell R. A. The DNA sequence of the H-2kb gene: evidence for gene conversion as a mechanism for the generation of polymorphism in histocompatibilty antigens. EMBO J. 1983;2(3):453–462. doi: 10.1002/j.1460-2075.1983.tb01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widera G., Flavell R. A. The nucleotide sequence of the murine I-E beta b immune response gene: evidence for gene conversion events in class II genes of the major histocompatibility complex. EMBO J. 1984 Jun;3(6):1221–1225. doi: 10.1002/j.1460-2075.1984.tb01956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willison K. R. Sex and frequency of gene conversions in meiosis. Nature. 1985 Feb 14;313(6003):604–604. doi: 10.1038/313604a0. [DOI] [PubMed] [Google Scholar]