Abstract
Cancer is not merely a cell-intrinsic genetic disease, but also the result of complex cell-extrinsic interactions with host components, including immune cells. For example, effector T lymphocytes and NK cells are thought to participate in an immunosurveillance process which eliminates neoplastic cells, whereas regulatory T lymphocytes and some myeloid cells, including macrophages, can create a milieu that prevents anti-tumor activity, supports tumor growth and reduces survival of the host. Increasing evidence supports the notion that carcinoma cells communicate with immune cells directly, both within and away from the tumor stroma, and that this process fosters suppression of immunosurveillance and promotes tumor outgrowth. An important mode of communication between carcinoma cells and immune cells may involve tumor-derived microvesicles (tMVs), also known as exosomes, ectosomes, or microparticles. These microvesicles carry lipids, proteins, mRNAs and miRNAs, and travel short or long distances to deliver undegraded and undiluted material to other cells. Here we consider the capacity of tMVs to control tumor-associated immune responses, and highlight the known and unknown tMV’s actions in vivo. We also discuss why microvesicles may play a role in cancer diagnostics and prognostics, and how they could be harnessed for anti-cancer therapy.
Background
A mode of communication between cells in the body is thought to involve extracellular microvesicles (MVs), which incorporate donor cell-derived material (membrane-bound and intracellular) and can be delivered to acceptor/recipient cells. This process, when altered or amplified, is thought to profoundly affect cell biological activities and, consequently, foster pathophysiological processes. Donor and recipient cells may reside in the same microenvironment, in which case MVs regulate local cell-to-cell communication. MVs may also be distributed systemically, for example via lymph and blood vessels (1), and operate as long-range communication signals between organs.
At present, pressing questions include: i) do tMVs target specific components of their immediate micro-environments and do some of these interactions control tumor progression?; ii) which distant organs come in contact with tMVs?; iii) what defines the ‘specificity’, if any, of tMVs’ recipient cells in vivo?; iv) do tMVs control host cells that are away from the tumor stroma ? v) what is the relative impact of tMVs on the host response when compared to all other modes of tumor cell/host cell communication?; and vi) can we exploit the accumulating knowledge on tMV biology to identify new vantage points for anti-cancer therapy? Some of these questions are being investigated experimentally and are discussed thereafter (see also Fig 1).
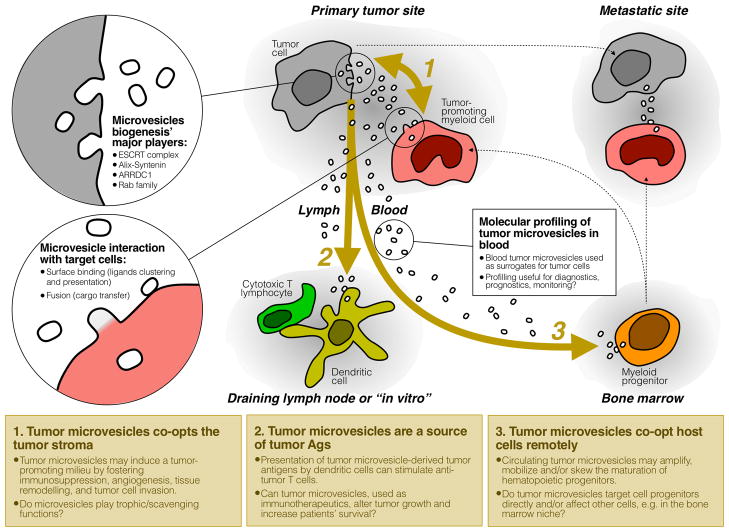
Fig. 1.
A sketch depicting MV tropism in vivo in cancer-bearing hosts. 1: Both tumor and host cells produce MVs that may affect other cell types locally, either by surface binding (MVs acting as ligand clustering agents) and/or by transferring bioactive material to target cells (horizontal transfer of proteins, RNAs and lipids). MVs from host cells may provide trophic functions by nurturing tumor cells; 2: tMVs can be drained into lymphatics and shape anti-tumor immune responses; 3: tMVs can circulate to distant organs like bone marrow and alter hematopoiesis. Blood MVs can be harnessed as surrogate tumor cells for diagnostic/prognostic purposes.
MV biogenesis
Extracellular MVs are cell-derived particles that contain a cytosol and are surrounded by a lipid bilayer. Donor cells and their MVs always share the same membrane orientation, though MVs can have different origins (endosomal versus plasma membranes) and vary largely in size (<100nm and >1um). Exosomes (2, 3), typically <100nm in diameter, are MVs that form inside endosomes following intraluminal budding of endosomal membranes. This process creates multi-vesicular bodies that must fuse with the cell surface to secrete their cargo in the extracellular space. Ectosomes (4), typically 100nm–1μm in diameter, are MVs that bud directly from the plasma membrane into the extracellular space. Other MVs have been characterized; they include exosome-like vesicles (5) (<100 nm), which may also bud from the plasma membrane (6), and apoptotic bodies which are produced following cell death (7).
Exosome biogenesis involves the Rab family of small GTPases, which recruit specific effector proteins onto endosomal membranes and drive vesicle docking and fusion (8). Instead, ectosome development depends on arrestins, which promote endocytosis of plasma membrane receptors (9). However, production of both ectosomes (9) and exosomes (10) is thought to require ESCRT (endosomal sorting complex required for transport), a machinery known to be required for sorting of cargo proteins into internal vesicles of multivesicular bodies. There is a stricking convergence between budding of enveloped viruses and MV biogenesis (11).
MV cargo is made of proteins, lipids, mRNAs and microRNAs. The mechanisms that control material inclusion (or exclusion) in MVs remain largely unknown, yet it is well established that different MVs can carry extensively different cargo repertoires. Consequently, MV preparations are often characterized based on the presence (or absence) of molecular pathway components that generate MVs (e.g., Rab27, Tsg101 and Alix) (2), factors produced by MV producing cells (e.g., MHC molecules, CD61 and CD14) (12), proteins involved in target cell selection (e.g., tetraspanins, integrins and selectins) (13), and molecules associated with MVs’ biological significance (e.g., Tissue Factor, matrix metalloproteinases, microRNAs) (14).
MV production and release require energy input, RNA synthesis and protein translation (15). The process can be enhanced by exogenous factors including ATP (16), phorbol ester-activated protein kinase C (17), low pH (18), and hypoxic conditions (19), which are all commonly altered in the stroma of growing tumors. However, it remains to be determined whether exosomes and ectosomes have either distinct or overlapping effects on host cell components and tumor development.
tMVs’ biological relevance: in vitro findings
MV transfer into, and impact on, recipient cells has been mostly analyzed in co-culture systems. These studies have shown that MVs can engage specific receptor/ligand interactions with recipient cells (20–23). MVs can further transfer cell surface receptors (24) and deliver intracellular proteins (25), mRNAs, miRNAs (14, 26, 27) and reporter genes (28, 29) into cells. MVs are thought to change the recipient cells’ makeup and thus to influence cellular functions and fate.
The motivation to address whether tMVs affect the immune system comes from experimental and clinical evidence that neoplastic diseases control various immune cell types (30). Evidence exists that effector T lymphocytes and NK cells can exhibit anti-tumor activity in the tumor stroma; that the presence of tumor-infiltrated T cells increase patients’ survival (31); and that regulatory T lymphocytes (Tregs) (32) and myeloid cells, including macrophages (33), however, can generate an immunosuppressive milieu that counteracts anti-tumor immunity, promotes tumor progression and decreases patients’ survival. The precise mechanisms of interactions that occur between tumor and immune cells remain largely unknown; nevertheless, recent data suggest that tMVs are involved in promoting tumor outgrowth by controlling the fate of all the immune cell types mentioned above. MVs may induce apoptosis of effector T cells (34–39); switch off NK cell mediated cytotoxicity (40, 41); activate immunosuppressive functions within myeloid cells (21, 42–44); impair dendritic cell production (45); and induce Treg responses (46, 47). Local immunosuppression may also be promoted by extracellular adenosine, which can be released from MVs (48).
In addition to their impact on immune cells MVs may promote tumor outgrowth through other mechanisms, which include degradation of extracellular matrix components (49), acceleration of tumor angiogenesis (29, 50), modulation of stromal cell differentiation (51), transfer of oncogenic activity to other cancer cells (52) and resistance to therapy via sequestration and expulsion of drugs out of tumor cells (53, 54). However, conclusions derived from in vitro data alone should be considered with some caution because contacts between MVs and recipient cells in these studies were artificially enforced and the amount of MVs used in vitro may be higher than that found in vivo (55). The fate of recipient cells in vivo may also be dictated by local factors (anatomical features, pH, oxygenation, forces of fluid flow, various cell types and cytokines), which often cannot be reproduced fully in vitro (56).
tMVs’ biological relevance: analysis in context
Human and mouse carcinomas can produce elevated amounts of MVs. At least some of these vesicles enter circulation (57) and may have biological effects far away from their production sites. Remarkably, Peinado et al. recently reported that mouse bone marrow (BM), which was pre-conditioned with tMVs derived from highly metastatic B16-F10 melanoma cells and then used to reconstitute lethally irradiated subjets, not only promoted tumor infiltration by BM cells, but also accelerated primary and metastatic cancer growth (58). Adoptive tMV transfer experiments further indicated that tMVs could increase vascular permeability at pre-metastatic sites and expand BM progenitors expressing c-Kit, Tie2 and Met. The phenotype of these cells may be functionally relevant because Tie2 can promote tumor angiogenic activity (59), whereas MET is associated with tumor cell invasion (60) and BM cell mobilization (61). Co-culture of tMVs with recipient cells suggested that MET was transferred from tumor cells to bone marrow progenitors via exosomes. Also, reduction of tMV production in vivo, through inhibition of Rab27a in tumor cells, reduced BM cell recruitment to tumors and delayed tumor outgrowth.
This in vivo investigation suggests that tMVs can enhance tumor outgrowth in mice by programming bone marrow progenitor cells with tumor-promoting functions. Nevertheless, the capacity of tMVs to educate BM cells permanently will require further study. It is formally possible that the BM pre-conditioning protocol employed in this study did only skew the hematopoietic repertoire toward the myeloid lineage, which is a process that favors primary and metastatic cancer growth (62, 63). It will also be interesting to define whether tMVs communicate with BM cells through horizontal transfer of information or more simply by surface binding. Finally, Rab27a knockdown–mediated inhibition of tMV production also reduced secretion of soluble factors that were previously shown to elicit tumor-promoting host responses (e.g. Ospeopontin (64), PlGF-2 (65, 66) and PDGF-AA (67)). In general, identifying the relative impact of tMVs and soluble factors (68) as long range signals between tumor cells and BM progenitor cells will require more examination.
A role for tMVs in regulating immune suppression has also been proposed by Chalmin et al. using in vitro and in vivo approaches (21). In this study, tMVs isolated from different mouse cell lines were shown to enhance the immunosuppressive activity of myeloid cells. The process did not involve horizontal material transfer but instead required direct surface receptor binding between HSP72 on tMVs and TLR2 on myeloid cells. Inhibition of HSP72 expression in tMVs reduced the myeloid cells’ capacity to foster metastatic progression. Injections of dimethyl amiloride—used to interfere with tMV secretion in vivo—also delayed tumor outgrowth and further enhanced the efficacy of cyclophosphamide therapy in various mouse models (21).
The authors went on to measure the effects of amiloride (an analogue of dimethyl amiloride that is used for the treatment of edema and high blood pressure) in patients suffering from colorectal invasive cancer. Myloid cells prepared from the patients’ peripheral blood showed that amiloride treatment decreased suppressor activity (21). These data suggest that interfering with tMV secretion may serve to enhance the efficacy of chemotherapies.
The same study identified that tMV-myeloid cell interaction controlled STAT3 activation and downstream suppressive activities within the sensitized cells. tMVs did not control myeloid cell expansion; this process was instead selectively controlled by tumor-derived soluble factors. Thus MVs and soluble factors may differentially regulate immune cell function and proliferation during tumor progression. Nevertheless adoptive tMV transfer was shown to induce myeloid cell accumulation in the spleen in another study (45), suggesting that tMV’s actions may be context-dependent.
It should also be noted that experimental approaches used for in vivo studies have limitations. First, the capacity to interfere selectively with tMV production and/or transfer in vivo is an unmet need. Diannexin (50), neutral sphingomyelinase 2 inhibitors (69), the H+/Na+ and Na+/Ca2+ channel inhibitor dimethyl amiloride (21), the K+/H+ ATPase inhibitor Omeprazole (70), and the Na(+)/K(+)-ATPase inhibitor Ouabain (71) have been used to control MV biogenesis or binding; however, these agents may also affect non-neoplastic cells. Another challenge imposed by in vivo studies is related to difficulties in achieving selective modulation of tMV production or transfer without compromising tumor cell viability. RNAi technology may be used to selectively target tMVs and thus represents a potentially useful tool to establish causal relationships between tMVs and host responses, when properly employed (72). This type of approach should benefit from a better understanding of the molecular players involved in MV biogenesis.
Second, fluorescently labeled tMVs used in adoptive transfer experiments may not fully recapitulate the tropism and impact of endogenous tMVs. Limitations include the existence of various tMV isolation protocols that may enrich vesicles with distinct functions (73); the necessity to transfer MVs as a bolus, which is distinct from uninterrupted tMV production in vivo; the fact that MV concentrations observed immediately after transfer may be non-physiological; and the choice of the MV labeling agent. Reagents commonly used to mark MVs are highly lipophilic membrane dyes such as PKH26 (45, 58); these molecules tend to aggregate in micelles, which co-purify with MVs by membrane filtration (100kDa cut off) and ultracentrifugation (unpublished observations) and can contaminate MV preparations. Thus, proper controls should be performed when using membrane dyes in vivo. MV marking with membrane-bound fluorescent proteins (e.g., CD63-EGFP (57)), rather than membrane dyes, may allow one to prevent the contamination of MV preparations with unbound fluorescent material, even though the fusion protein may not be present in all MV types (74). Finally, detection of membrane dyes on recipient cells, either by conventional flow cytometry or immunofluorescence, should not be used to prove transfer of intracellular molecules because MVs may only bind the surface of recipient cells (75). Discrimination between MV surface binding and fusion requires specific experimental settings (76). New technological advances in flow cytometry allow real time imaging at subcellular resolution and may help to discriminate between these possibilities (77). As the details of MV biogenesis become unravelled, new genetic approaches may permit more selective targeting of MV cargo and/or marking of distinct MV types.
Clinical-Translational advances
Role in diagnostics?
Notwithstanding their capacity to control the host response, tMVs may also be relevant for screening asymptomatic patients, diagnosing and profiling disease, and predicting treatment efficacy. At least, initial studies suggest that cancer patients may carry unique circulating MV signatures that reflect the genetic status of the tumor (78). One analysis reported significantly increased exosome levels in lung adenocarcinoma patients when compared to control individuals (79). Another study concluded that circulating tumor-derived (EpCAM+) exosomes in ovarian cancer patients could potentially be used as surrogate diagnostic markers for biopsy profiling (80). Also, some glioblastoma patients were identified with detectable amounts of circulating MVs incorporating a tumor-specific mRNA variant (EGFRvIII) (29), and thus could be diagnosed noninvasively. Interestingly, EGFRvIII mRNA was not detected in serum samples drawn two weeks after resection of the tumor, consistent with this tumor being the source of MVs (29). The diagnostic value of MVs has been investigated in patients with other cancer types, including bladder cancer (81), prostate cancer (82), and colorectal cancer (83). Circulating tumor cells (CTCs) are other relevant candidates for cancer diagnostics, though their low abundance—typically less than one per ml of blood (84)—may render their analysis more challenging.
In some cases MVs may have a prognostic value. A retrospective analysis of stage IV melanoma patients suggested a decreased mortality for those patients who contained protein-poor exosomes in circulation (58). More recently, an analytical technology was reported for MV quantification and protein profiling directly from blood samples (75). The approach consists to introduce MVs onto a portable microfluidic chip for labeling with target-specific magnetic nanoparticles and detection by a miniaturized nuclear magnetic resonance system. The technology was used to screen MVs from glioblastoma patients and thereby predicted which patients would clinically respond to treatment with temozolomide (75). Multiparameter molecular evaluation of MVs should become instrumental in clinical care. Longitudinal analysis makes it possible to monitor tumor molecular responses to therapeutic agents, to determine the emergence of drug resistant tumor variants, and to rapidly phenotype the molecular profile of the emerging cells for adjustment of targeted therapy.
Role in therapy?
More than 10 years ago, MVs isolated from tumor-peptides pulsed, in vitro generated dendritic cells were shown to elicit a tumor-specific cytotoxic T cell response that eradicated established, transplanted tumors in mice (12). The same group has reported that vaccination with dendritic cell-derived MVs is a safe approach for cancer patients (85) and new combinations are being tested in clinical trials. In vitro manipulation of patient-derived tumor cells could also be employed to load genetically-encoded adjuvants into tMVs, which may then be used for reinfusion into the patient as an anti-tumor vaccine. The presence of bacterial adjuvants, such as flagellin (86), may improve vaccination efficacy. MV removal from the circulation of cancer patients has also been proposed as a therapeutic intervention (87). Finally, injection of MV biogenesis inhibitors, before or concomitantly with cytotoxic drugs, may increase, at least temporarily, the drug’s concentration inside tumor cells. Limiting tMV secretion may also serve to improve anti-tumor immune activity.
Conclusions
Several studies suggest that tMVs control tumor-associated immune responses. The reported presence of circulating tMVs in both humans and mouse models also hints toward an endocrine function for these vesicles, although additional investigation is needed to define their in vivo contributions more precisely. tMVs represent interesting vantage points not only for uncovering mechanisms of tumor-host cells interactions but also for developing less invasive diagnostic and prognostic clinical readouts.
Acknowledgments
We apologize to the authors whose work we could not cite because of the limit on the number of references. This work was supported in part by US National Institutes of Health (NIH) grants R01-AI084880 and P50-CA086355 (to M.J.P.) and by EMBO long-term fellowship program (to F.P.).
Footnotes
The authors do not have conflicts of interest.
References
- 1.Swartz MA, Lund AW. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat Rev Cancer. 2012;12:210–9. doi: 10.1038/nrc3186. [DOI] [PubMed] [Google Scholar]
- 2.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 3.Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–81. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Cocucci E, Meldolesi J. Ectosomes. Curr Biol. 2011;21:R940–1. doi: 10.1016/j.cub.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Hawari FI, Rouhani FN, Cui X, Yu ZX, Buckley C, Kaler M, et al. Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc Natl Acad Sci U S A. 2004;101:1297–302. doi: 10.1073/pnas.0307981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172:923–35. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–18. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 8.Bobrie A, Colombo M, Raposo G, Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–68. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 9.Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles {(ARMMs)} at plasma membrane by recruitment of {TSG101} protein. Proceedings of the National Academy of Sciences. 2012;109:4146–51. doi: 10.1073/pnas.1200448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, et al. Syndecan-syntenin-{ALIX} regulates the biogenesis of exosomes. Nature Cell Biology. 2012;14:677–85. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 11.Wurdinger T, Gatson NN, Balaj L, Kaur B, Breakefield XO, Pegtel DM. Extracellular vesicles and their convergence with viral pathways. Adv Virol. 2012;2012:767694. doi: 10.1155/2012/767694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 13.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–9. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 14.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan MLG, Karlsson JM, et al. Mechanism of transfer of functional {microRNAs} between mouse dendritic cells via exosomes. Blood. 2012;119:756–66. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dainiak N, Sorba S. Intracellular regulation of the production and release of human erythroid-directed lymphokines. The Journal of clinical investigation. 1991;87:213–20. doi: 10.1172/JCI114974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001;15:825–35. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- 17.Sidhu SS, Mengistab AT, Tauscher AN, LaVail J, Basbaum C. The microvesicle as a vehicle for EMMPRIN in tumor-stromal interactions. Oncogene. 2004;23:956–63. doi: 10.1038/sj.onc.1207070. [DOI] [PubMed] [Google Scholar]
- 18.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De M, Angelo, et al. Microenvironmental {pH} is a key factor for exosome traffic in tumor cells. The Journal of biological chemistry. 2009;284:34211–22. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svensson KJ, Kucharzewska P, Christianson HC, Sköld S, Löfstedt T, Johansson MC, et al. Hypoxia Triggers a Proangiogenic Pathway Involving Cancer Cell Microvesicles and PAR-2 Mediated Heparin-Binding {EGF} Signaling in Endothelial Cells. Proceedings of the National Academy of Sciences. 2011;108:13147–52. doi: 10.1073/pnas.1104261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood. 2004;104:2543–8. doi: 10.1182/blood-2004-01-0361. [DOI] [PubMed] [Google Scholar]
- 21.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–71. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segura E, Guerin C, Hogg N, Amigorena S, Thery C. CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. J Immunol. 2007;179:1489–96. doi: 10.4049/jimmunol.179.3.1489. [DOI] [PubMed] [Google Scholar]
- 23.Nolte-’t Hoen EN, Buschow SI, Anderton SM, Stoorvogel W, Wauben MH. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113:1977–81. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- 24.Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Baran J, Urbanowicz B, Branski P, et al. Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol Immunother. 2006;55:808–18. doi: 10.1007/s00262-005-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Putz U, Howitt J, Doan A, Goh C-P, Low L-H, Silke J, et al. The Tumor Suppressor {PTEN} Is Exported in Exosomes and Has Phosphatase Activity in Recipient Cells. Science Signaling. 2012;5:ra70. doi: 10.1126/scisignal.2003084. [DOI] [PubMed] [Google Scholar]
- 26.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133–44. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Russell TB, Skinner AM, Kurre P. Programmed vesicle transfer of green fluorescent protein from a stably transduced cell line to primary hematopoietic cells. Blood. 2012;119:5330–2. doi: 10.1182/blood-2012-04-422477. [DOI] [PubMed] [Google Scholar]
- 29.Skog J, Wurdinger T, Rijn Sv, Meijer DH, Gainche L, Curry WT, et al. Glioblastoma microvesicles transport {RNA} and proteins that promote tumour growth and provide diagnostic biomarkers. Nature Cell Biology. 2008;10:1470. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 31.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 32.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 33.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albanese J, Meterissian S, Kontogiannea M, Dubreuil C, Hand A, Sorba S, et al. Biologically active Fas antigen and its cognate ligand are expressed on plasma membrane-derived extracellular vesicles. Blood. 1998;91:3862–74. [PubMed] [Google Scholar]
- 35.Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195:1303–16. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res. 2005;11:1010–20. [PubMed] [Google Scholar]
- 37.Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, et al. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis. 2005;35:169–73. doi: 10.1016/j.bcmd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P, et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005;128:1796–804. doi: 10.1053/j.gastro.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 39.Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol. 2006;176:1534–42. doi: 10.4049/jimmunol.176.3.1534. [DOI] [PubMed] [Google Scholar]
- 40.Clayton A, Tabi Z. Exosomes and the MICA-NKG2D system in cancer. Blood Cells Mol Dis. 2005;34:206–13. doi: 10.1016/j.bcmd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176:1375–85. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Xiang X, Zhuang X, Zhang S, Liu C, Cheng Z, et al. Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am J Pathol. 2010;176:2490–9. doi: 10.2353/ajpath.2010.090777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, et al. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66:9290–8. doi: 10.1158/0008-5472.CAN-06-1819. [DOI] [PubMed] [Google Scholar]
- 44.Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124:2621–33. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178:6867–75. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- 46.Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg) PLoS One. 2010;5:e11469. doi: 10.1371/journal.pone.0011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang GJ, Liu Y, Qin A, Shah SV, Deng ZB, Xiang X, et al. Thymus exosomes-like particles induce regulatory T cells. J Immunol. 2008;181:5242–8. doi: 10.4049/jimmunol.181.8.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187:676–83. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 49.Hakulinen J, Sankkila L, Sugiyama N, Lehti K, Keski-Oja J. Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. J Cell Biochem. 2008;105:1211–8. doi: 10.1002/jcb.21923. [DOI] [PubMed] [Google Scholar]
- 50.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A. 2009;106:3794–9. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70:9621–30. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 52.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–24. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 53.Filipazzi P, Burdek M, Villa A, Rivoltini L, Huber V. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin Cancer Biol. 2012;22:342–9. doi: 10.1016/j.semcancer.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 54.Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63:4331–7. [PubMed] [Google Scholar]
- 55.Sverdlov ED. Amedeo Avogadro’s cry: what is 1 microg of exosomes? Bioessays. 2012;34:873–5. doi: 10.1002/bies.201200045. [DOI] [PubMed] [Google Scholar]
- 56.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–9. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suetsugu A, Honma K, Saji S, Moriwaki H, Ochiya T, Hoffman RM. Imaging exosome transfer from breast cancer cells to stroma at metastatic sites in orthotopic nude-mouse models. Adv Drug Deliv Rev. 2012 doi: 10.1016/j.addr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 58.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–91. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512–26. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 60.Boccaccio C, Comoglio PM. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer. 2006;6:637–45. doi: 10.1038/nrc1912. [DOI] [PubMed] [Google Scholar]
- 61.Tesio M, Golan K, Corso S, Giordano S, Schajnovitz A, Vagima Y, et al. Enhanced c-Met activity promotes G-CSF-induced mobilization of hematopoietic progenitor cells via ROS signaling. Blood. 2011;117:419–28. doi: 10.1182/blood-2009-06-230359. [DOI] [PubMed] [Google Scholar]
- 62.Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–78. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cortez-Retamozo V, Etzrodt M, Newton A, Rauch PJ, Chudnovskiy A, Berger C, et al. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci U S A. 2012;109:2491–6. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marrony S, Bassilana F, Seuwen K, Keller H. Bone morphogenetic protein 2 induces placental growth factor in mesenchymal stem cells. Bone. 2003;33:426–33. doi: 10.1016/s8756-3282(03)00195-9. [DOI] [PubMed] [Google Scholar]
- 66.Ding Y, Huang Y, Song N, Gao X, Yuan S, Wang X, et al. NFAT1 mediates placental growth factor-induced myelomonocytic cell recruitment via the induction of TNF-alpha. J Immunol. 2010;184:2593–601. doi: 10.4049/jimmunol.0902378. [DOI] [PubMed] [Google Scholar]
- 67.Martelli F, Verrucci M, Migliaccio G, Zingariello M, Rana RA, Vannucchi AM, et al. Removal of the spleen in mice alters the cytokine expression profile of the marrow micro-environment and increases bone formation. Ann N Y Acad Sci. 2009;1176:77–86. doi: 10.1111/j.1749-6632.2009.04968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cortez-Retamozo V, Etzrodt M, Newton A, Ryan R, Pucci F, Sio SW, et al. Angiotensin II Drives the Production of Tumor-Promoting Macrophages. Immunity. 2013 doi: 10.1016/j.immuni.2012.10.015. In press: http://dx.doi.org/10.1016/j.immuni.2012.10.015. [DOI] [PMC free article] [PubMed]
- 69.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–7. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 70.Savina A, Furlan M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278:20083–90. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 71.Ceccarelli S, Visco V, Raffa S, Wakisaka N, Pagano JS, Torrisi MR. Epstein-Barr virus latent membrane protein 1 promotes concentration in multivesicular bodies of fibroblast growth factor 2 and its release through exosomes. Int J Cancer. 2007;121:1494–506. doi: 10.1002/ijc.22844. [DOI] [PubMed] [Google Scholar]
- 72.Cullen BR. Enhancing and confirming the specificity of RNAi experiments. Nat Methods. 2006;3:677–81. doi: 10.1038/nmeth913. [DOI] [PubMed] [Google Scholar]
- 73.Mignot G, Chalmin F, Ladoire S, Rebe C, Ghiringhelli F, Xiang X, et al. Tumor Exosome-Mediated {MDSC} Activation. The American Journal of Pathology. 2011;178:1403–5. doi: 10.1016/j.ajpath.2010.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sadallah S, Eken C, Martin PJ, Schifferli JA. Microparticles (ectosomes) shed by stored human platelets downregulate macrophages and modify the development of dendritic cells. J Immunol. 2011;186:6543–52. doi: 10.4049/jimmunol.1002788. [DOI] [PubMed] [Google Scholar]
- 75.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of {mRNA} and protein delivery. Leukemia. 2006;20:847–56. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 76.Hoekstra D, de BT, Klappe K, Wilschut J. Fluorescence method for measuring the kinetics of fusion between biological membranes. Biochemistry. 1984;23:5675–81. doi: 10.1021/bi00319a002. [DOI] [PubMed] [Google Scholar]
- 77.Vallhov H, Gutzeit C, Johansson SM, Nagy N, Paul M, Li Q, et al. Exosomes containing glycoprotein 350 released by EBV-transformed B cells selectively target B cells through CD21 and block EBV infection in vitro. J Immunol. 2011;186:73–82. doi: 10.4049/jimmunol.1001145. [DOI] [PubMed] [Google Scholar]
- 78.Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rabinowits G, Gercel-Taylor C, Day JM, Taylor DD, Kloecker GH. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer. 2009;10:42–6. doi: 10.3816/CLC.2009.n.006. [DOI] [PubMed] [Google Scholar]
- 80.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 81.Chen CL, Lai YF, Tang P, Chien KY, Yu JS, Tsai CH, et al. Comparative and Targeted Proteomic Analyses of Urinary Microparticles from Bladder Cancer and Hernia Patients. J Proteome Res. 2012 doi: 10.1021/pr3008732. [DOI] [PubMed] [Google Scholar]
- 82.Bryant RJ, Pawlowski T, Catto JW, Marsden G, Vessella RL, Rhees B, et al. Changes in circulating microRNA levels associated with prostate cancer. Br J Cancer. 2012;106:768–74. doi: 10.1038/bjc.2011.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choi DS, Park JO, Jang SC, Yoon YJ, Jung JW, Choi DY, et al. Proteomic analysis of microvesicles derived from human colorectal cancer ascites. Proteomics. 2011;11:2745–51. doi: 10.1002/pmic.201100022. [DOI] [PubMed] [Google Scholar]
- 84.Balic M, Williams A, Lin H, Datar R, Cote RJ. Circulating Tumor Cells: From Bench to Bedside. Annu Rev Med. 2012 doi: 10.1146/annurev-med-050311-163404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garaude J, Kent A, van Rooijen N, Blander JM. Simultaneous targeting of toll- and nod-like receptors induces effective tumor-specific immune responses. Sci Transl Med. 2012;4:120ra16. doi: 10.1126/scitranslmed.3002868. [DOI] [PubMed] [Google Scholar]
- 87.Marleau AM, Chen CS, Joyce JA, Tullis RH. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med. 2012;10:134. doi: 10.1186/1479-5876-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]