Abstract
Background
Protamine is widely used to reverse the anticoagulant effects of heparin. Although mild thrombocytopenia is common in patients given protamine after cardiac procedures, acute severe thrombocytopenia has not been described. We encountered a patient who experienced profound thrombocytopenia and bleeding shortly after administration of protamine and performed studies to characterize the responsible mechanism.
Study design and methods
Patient serum was studied for antibodies that recognize protamine, heparin/protamine complexes and platelets pre-treated with protamine using flow cytometry, ELISA and serotonin release from labeled platelets.
Results
A high titer IgG antibody was detected in patient serum that recognizes protamine in a complex with heparin or platelet surface glycosaminoglycans (GAG) and activates platelets pre-treated with protamine at concentrations achieved in vivo following protamine infusion. The antibody is distinctly different from those found in patients with heparin-induced thrombocytopenia on the basis of its failure to recognize heparin in a complex with platelet factor 4 (PF4) and to release serotonin from labeled platelets in the absence of protamine.
Conclusions
Findings made suggest that the patient’s antibody is specific for conformational changes induced in protamine when it reacts with heparin or a platelet surface GAG. Development of severe thrombocytopenia following treatment of this patient with protamine defines a previously undescribed mechanism of drug-induced immune thrombocytopenia. Patients given protamine who produce this type of antibody may be at risk to experience thrombocytopenia if given the drug a second time while antibody is still present.
Keywords: heparin, protamine, thrombocytopenia
INTRODUCTION
Protamine sulfate, a mixture of 5–10 kD cationic DNA binding-proteins derived from salmon sperm (1, 2) is commonly used to reverse the effects of heparin following cardiac surgery involving cardiopulmonary bypass (CPB). A modest drop in platelet levels almost invariably follows CPB (3, 4). Various studies have suggested that platelet levels sometimes drop further when protamine is given (3), possibly because protamine-heparin complexes bind to platelets and cause them to be sequestered transiently in the lungs (3, 5). Infusion of protamine alone to normal subjects caused a 50% decrease in platelet levels lasting about 30 minutes in one study (5). However, severe, sustained thrombocytopenia following protamine infusion has not been reported. Here, we describe a patient who experienced profound thrombocytopenia and bleeding symptoms shortly after protamine was given to counteract heparin. Laboratory studies revealed a high-titer antibody that reacted with protamine-coated platelets and with heparin-protamine complexes. Thrombocytopenia in this case appears to be mediated by a previously undescribed mechanism involving antibody recognition of neoepitopes induced in the positively charged protamine molecule when it binds to negatively charged glycosaminoaminoglycans (GAG) expressed on the platelet surface.
MATERIAL AND METHODS
Flow cytometry
The method has been described in detail previously (6). In brief, 1 × 10−7 washed group O platelets were incubated with 40 µl of test serum and protamine sulfate (Sigma–Aldrich, St Louis, MO) at various concentrations in a total volume of 75 µl. After washing in buffer containing protamine at the same concentration as in the primary mixture, platelet-associated immunoglobulins were detected by flow cytometry (FACSCalibur, Becton Dickinson, San Jose, CA) using fluorescein isothiocyanate (FITC)-tagged anti-human IgG (Fc-specific) (Jackson ImmunoResearch, West Grove PA). A positive reaction was defined as one in which median platelet fluorescence intensity (MFI) was at least twice that obtained with the same serum sample in the absence of drug. Reactions of this strength always exceeded control values by at least three standard deviations.
14C - serotonin release assay
The 14C - serotonin release assay (SRA) was performed with slight modifications according to the procedure described by Sheridan, et al. (7).
Detection of antibodies recognizing heparin/protamine complexes
Heparin was incubated with protamine at various ratios of the two substances for one hour and aliquots of the resulting complexes were plated in the wells of a microtiter plate as described previously for complexes of heparin and platelet factor 4 (PF4) used to detect antibodies found in patients with heparin-induced thrombocytopenia (8, 9). Patient or normal control serum (50 µl) diluted 1:50 in phosphate-buffered saline (PBS) was incubated in the wells for 1 hour at room temperature followed by washing. Bound antibodies were detected by adding 100 µl of a 1:8,000 dilution of horseradish peroxidase (HRP) labeled goat anti-human IgG Fc (Jackson Immunoresearch, West Grove PA) and incubating for 1 hour at room temperature, followed by washing and addition of substrate. Optical density (490nm) was measured in each well using an ELISA plate reader.
Case report
A 75 year-old woman was admitted to the Cleveland Clinic in 2011 because of shortness of breath and chest discomfort. She had been taking aspirin and clopidogrel, but no other antithrombotic agents. Past medical history included a diagnosis of scleroderma with pulmonary fibrosis. She had undergone coronary artery bypass grafting (CABG) in 1994, stent grafting and angioplasty of the thoracic aorta in 2005 and CABG and homograft replacement of the aortic valve in 2006. Complete blood count (CBC) performed at the time of this hospital admission (2011) showed hemoglobin 10.7 g/dL, WBC 7,800/ul and platelets 201,000/ul. Cardiac catheterization revealed severe stenosis of the subclavian artery and the origin of the left internal mammary artery at its origin. Stenting of the subclavian and the origin of left internal mammary artery was successfully performed. During the procedure, a bolus of 5,000 IU unfractionated heparin was administered. After the procedure, she was given 20 mg protamine (APP pharmaceuticals) intravenously. A blood count done two hours later showed Hgb was 10.6 g/dL and platelets had dropped to 23,000/µL. She was transferred to intensive care unit. The next morning the platelet count was 3,000/µl and petechial hemorrhages were noted in the skin and buccal mucosa. A fresh hematoma was noted in the left shoulder area. A solid phase assay for heparin-dependent antibodies (PF4 ELISA) was negative. Repeat blood count in citrate again revealed a platelet level of 3,000/µL and no platelet clumps were observed on the blood film, ruling out pseudo-thrombocytopenia. Transfusion of 5 units of pooled platelets raised the platelet count to 50,000/µL. She was discharged two days later. The platelet count had stabilized in the normal range and the shoulder hematoma had resolved 6 days later when she was seen as an outpatient.
RESULTS
A protamine-dependent, platelet-binding antibody was identified in the patient’s serum
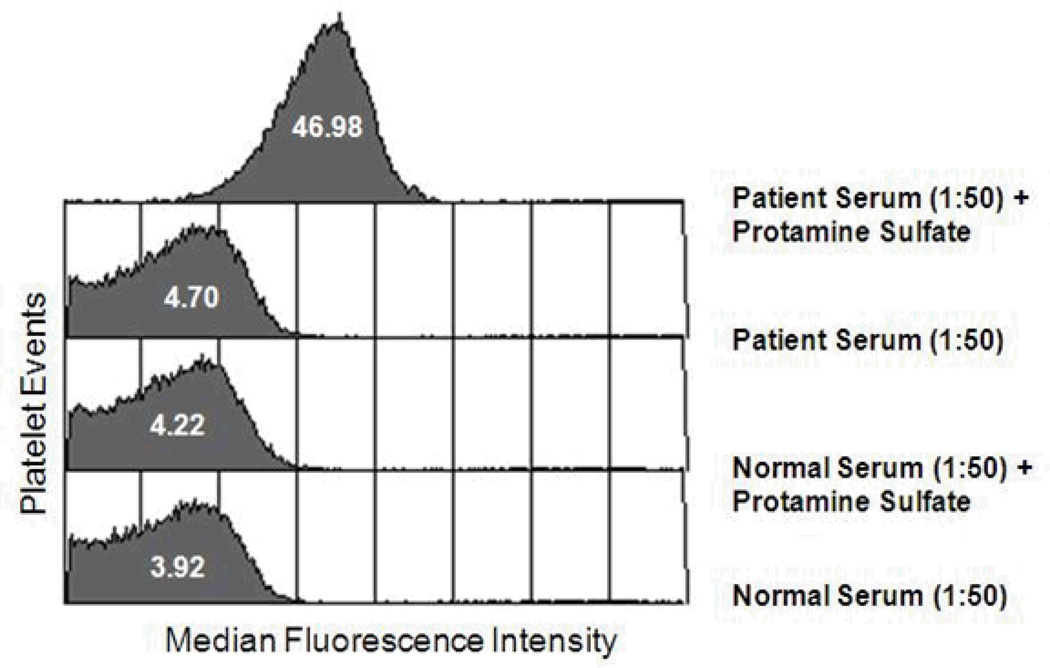
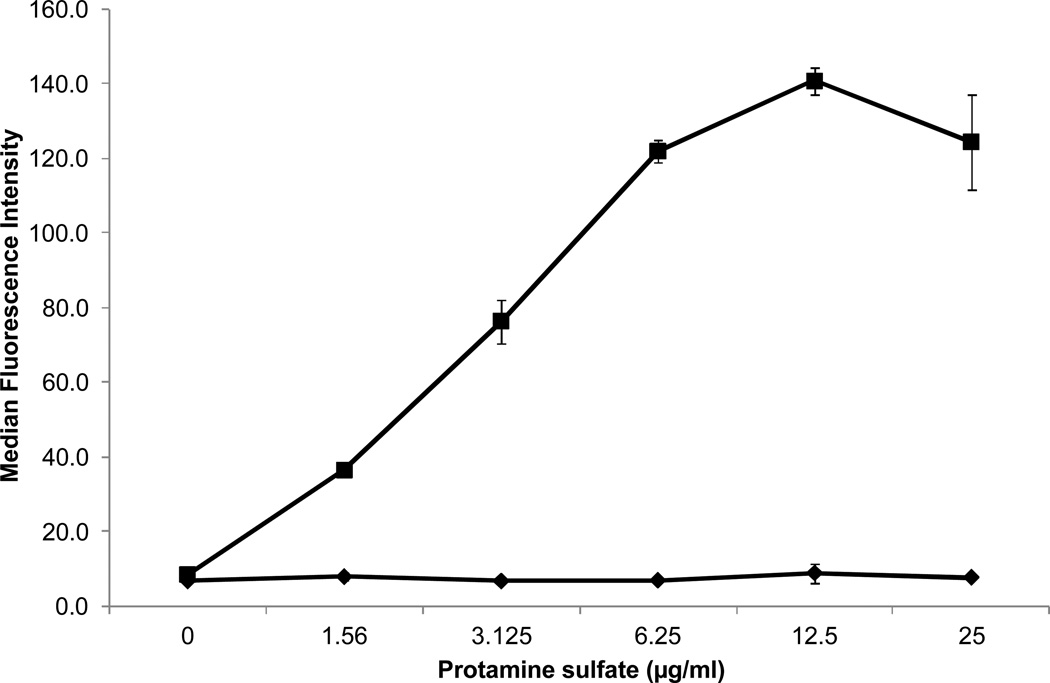
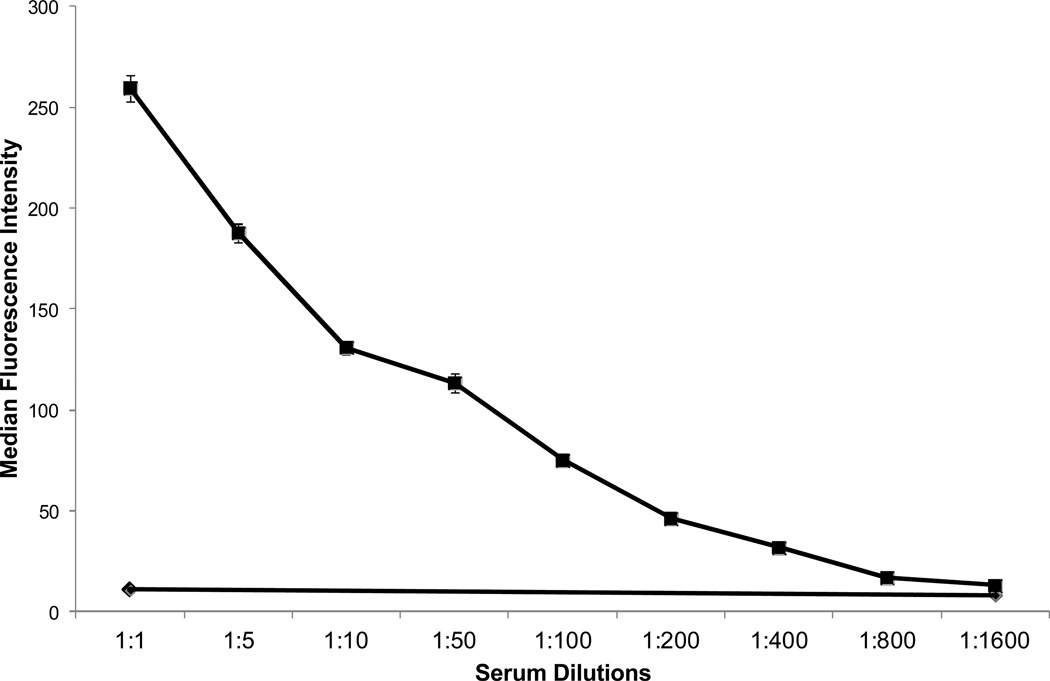
When normal platelets were incubated with patient serum and protamine (12.5 µg/ml) and then washed in buffer containing protamine at the same concentration, a strong IgG, platelet-reactive antibody was demonstrated using flow cytometry (Figure 1). No reaction was obtained with patient serum alone or with normal serum plus protamine. Weaker reactions were obtained when protamine was used at concentrations higher or lower than 12.5 µg/ml (Figure 2). When protamine was omitted from the buffer in which sensitized platelets were washed, positive IgG reactivity was no longer detected (data not shown). Testing of patient serum at serial dilutions showed that protamine-dependent reactions were obtained with dilutions as high as 1:800 (Figure 3). No protamine-dependent, platelet-reactive antibodies were identified in serum from any of 50 unselected normal subjects (data not shown).
Figure 1. An IgG antibody present in the patient’s serum reacted with normal Group O platelets when protamine is present.
Results shown (flow cytometry) are typical of four independent studies that gave comparable results. Protamine sulfate was used at 12.5 µg/ml in both the primary reaction and in buffer used for washing prior to adding FITC-labeled secondary antibody.
Figure 2. A protamine concentration of about 12.5 µg/ml was optimal for protamine-dependent binding of patient’s antibody to platelets (filled squares).
Results with normal serum and protamine were negative (filled squares). Values shown (flow cytometry) are averages of triplicate determinations. Brackets denote 1.0 S.D.
Figure 3. The protamine-dependent patient antibody reacted with platelets at dilutions up to 1:800.
Filled squares indicate reactions (flow cytometry) obtained with protamine at 12.5 µg/ml; filled diamonds indicate reaction in the absence of protamine.
The patient’s antibody reacted with protamine/heparin complexes in a solid phase assay and induced platelet activation via FcγRIIa when incubated with platelets in the presence of protamine
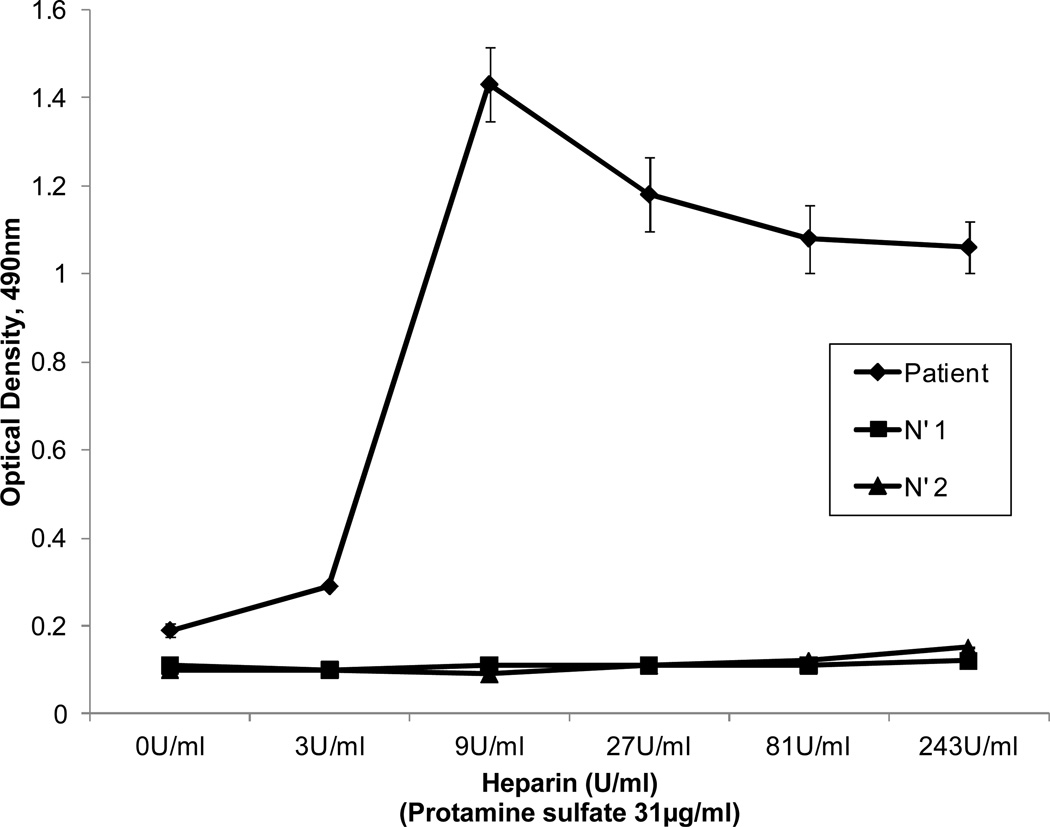
Patients with heparin-induced thrombocytopenia (HIT) have antibodies that recognize the basic platelet alpha granule protein platelet factor four (PF4) in a complex with heparin (10, 11). In preliminary studies using an in-house assay, we confirmed that this type of antibody was not present in patient serum. However, findings shown in Figures 1–3 suggested that the patient might have an antibody analogous to those found in HIT that was specific not for PF4, but for the highly basic protamine molecule in a complex with heparin or another glycosaminoglycan (GAG). To test this possibility, we prepared complexes of heparin and protamine sulfate at different ratios, fixed them by passive absorption to wells of a microtiter plate (8), and studied their reactions with the patient’s antibody. As shown in Figure 4, strong reactions were obtained with complexes produced in a mixture containing protamine 31 µg/ml and heparin 9 units/ml and weaker binding was seen at other ratios. Assuming an average molecular weight for protamine of 5,000 Da (manufacturer specifications), heparin specific activity of 175 units per mg and an average molecular weight of 12,000 Da for unfractionated heparin (8), the molar ratio of protamine to heparin in complexes that were optimal for antibody detection was about 1:1.
Figure 4. Reactions of patient’s antibody (diluted 1:50) with complexes of heparin:protamine prepared at various ratios.
The strongest reactions were obtained with complexes prepared using protamine sulfate 31 µg/ml and heparin 9 international units/ml (filled diamonds). No reactions were obtained with either of two normal sera (filled squares and triangles). Values shown are averages of triplicate determinations. Brackets denote 1.0 S.D.
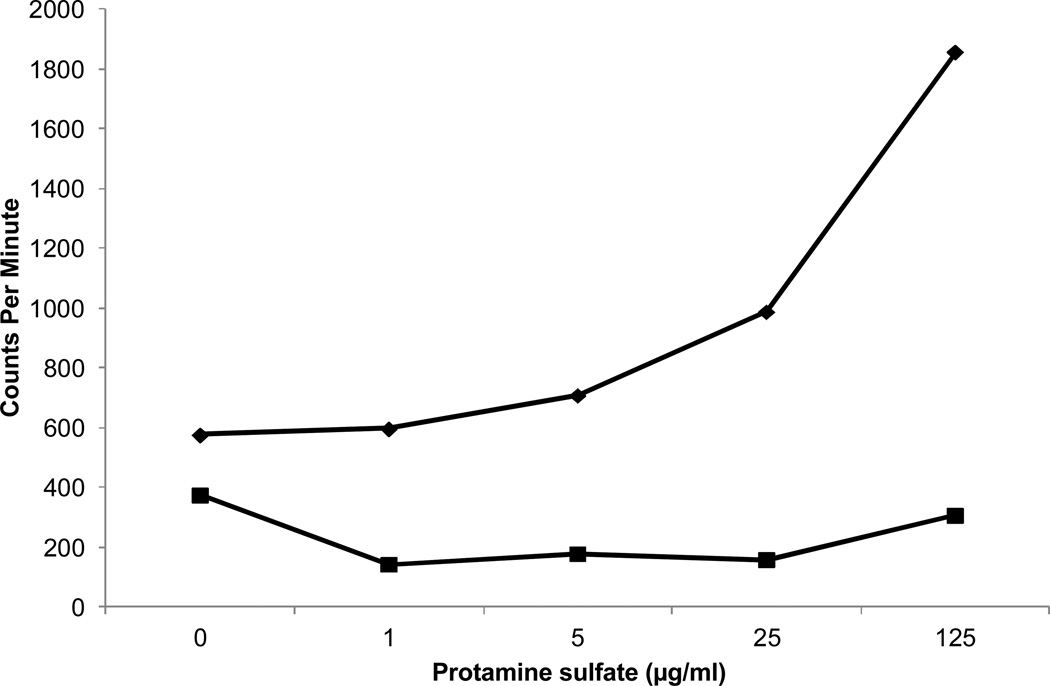
A second property of HIT antibodies is that they activate washed platelets in the presence of low concentrations of heparin (0.1–0.3 units per ml), leading to release of serotonin from platelets labeled with 14-C serotonin (7, 11). The patient’s serum tested negative in the standard (7) serotonin release assay using heparin at a 0.1 and 100 units per ml. However, when labeled platelets were incubated with patient serum and protamine at various concentrations, dose-dependent release of serotonin occurred (Figure 5). No release was obtained with normal serum tested similarly. Protamine-dependent release of serotonin by patient antibody was completely inhibited when platelets were pre-treated with 1.0 µg of monoclonal IV.3 specific for the FcγRIIa receptor (data not shown), indicating that the patient’s antibody, like those from patients with HIT (7), induces release by activating platelets via FcγRIIa.
Figure 5. The patient’s antibody caused release of serotonin from platelets labeled with 14C-serotonin when protamine was present at 5–125 µg/ml.
14C-release is shown as counts/minute (CPM), and the equivalent percent release obtained with the normal serum (filled squares) was 0%. The equivalent percent release obtained with the patient’s serum (filled diamonds) ranged from 6% (0 µg/ml protamine) to 43% (125 µg/ml protamine). Results shown are typical of two independent studies.
DISCUSSION
Our findings show that the patient studied had a high-titer IgG antibody that reacted with normal platelets only when protamine was present. Acute, severe thrombocytopenia occurred shortly after an intravenous infusion of 20 mg protamine. Assuming uniform distribution of the drug throughout a plasma volume of about 3000 ml, the peak concentration of protamine after injection could have been as high as 7 µg/ml. As shown in Figure 3, binding of antibody to platelets was detectable at protamine concentrations as low as 1.5 µg/ml even when patient serum was used at a dilution of 1:50. Assuming the antibody level in the test sample obtained several days after the thrombocytopenic episode is representative of the level when protamine was administered, it is clear that the amount of protamine infused would have been sufficient to promote antibody binding to the patient’s platelets in vivo. Together, these observations strongly suggest that acute thrombocytopenia was caused by a protamine-dependent, platelet-reactive antibody. Our patient had a likely exposure to protamine when she underwent cardiac surgery several years prior to her thrombocytopenic episode and it seems possible that her antibody persisted from that time, explaining why thrombocytopenia developed acutely following protamine administration.
Findings shown in Figures 2, 4 and 5 show that protamine-dependent reactions of the patient’s antibody resembled PF4-dependent reactions of a typical HIT antibody in several respects. Figure 2 shows that the antibody binds to platelets only in the presence of protamine and that binding is optimal at protamine concentrations ranging from 6 to 25 µg/ml. Rauova et al showed that HIT antibodies and an HIT-like monoclonal antibody KKO react with platelets pre-treated with PF4 at concentrations ranging from 50–100 µg/ml and that binding is decreased at higher or lower PF4 concentrations and following enzymatic degradation of the major platelet surface glycosaminoglycan (GAG) chondroitin sulfate (CS) (12). Their findings indicate that at certain molar ratios of PF4:CS, PF4 is structurally modified to create epitopes for which HIT antibodies are specific. Findings shown in Figure 2 suggest that when protamine binds to CS, it is similarly modified to create an epitope or epitopes recognized by the patient’s antibody. A second characteristic of HIT antibodies is that they recognize complexes of heparin and PF4 produced at PF4/heparin molar ratios about 2:1 but react less well with complexes formed at higher or lower ratios (8). Figure 4 illustrates that protamine-heparin complexes formed at a molar ratio about 1:1 were optimal for detection of the patient’s antibody. Finally, HIT antibodies release serotonin from platelets by a mechanism dependent on the FcΥRIIa receptor (7). Although the serotonin release assay is usually performed by adding low dose heparin (0.1 U/ml), strong HIT antibodies often cause release without added heparin, apparently because they recognize small quantities of PF4 in a complex with CS on the platelet surface (13–15). Protamine-dependent release of serotonin by the patient’s antibody (Figure 5) that was blocked by the FcRΥIIa-specific monoclonal IV.3 closely resembles the behavior of this type of HIT antibody, the difference being recognition of protamine on the platelet surface rather than PF4. Together, these considerations make it likely that the antibody identified in this patient is specific for structural changes induced in protamine when it binds to chondroitin sulfate or to another GAG. One major difference is that after platelets are washed in buffer, the patient’s antibody remains bound to protamine-treated platelets in quantities readily detected by flow cytometry, provided protamine is kept present in the wash solutions (Figures 1, 2).
To the best of our knowledge, thrombocytopenia caused by a protamine-dependent, platelet-reactive antibody of the type described here has not been described previously. However, in a recent report, Chudasama and colleagues showed that mice immunized with protamine-heparin complexes produce antibodies that recognize complexes formed in a mixture of protamine 31 µg/ml and heparin 4 units per ml (16). This ratio is similar to the one we found to be optimal for detecting our patient’s antibody in a solid phase assay (31 µg protamine, 9 units heparin per ml) (Figure 4). Moreover, Lee et al recently studied a large series of patients given protamine following cardiac surgery and found that, at 30 days, 29% have IgG antibodies specific for protamine-heparin complexes (17). Studies performed with a subset of these antibodies showed that they induce protamine-dependent release of serotonin from platelets. The findings of Chudusama et al (16) and Lee et al (17) show that heparin-protamine complexes are immunogenic in mice and humans and that the resulting antibodies are capable of activating platelets in the presence of protamine. Findings made in our patient suggest that re-exposure of a patient who has this type of antibody to protamine can cause thrombocytopenia. Studies are therefore indicated to determine how long protamine-dependent, platelet-reactive antibodies persist in patients given protamine in order to define the window period during which they may be at risk for thrombocytopenia if the drug is administered a second time.
ACKNOWLEDGEMENT
We are indebted to Gowthami Arepally, M.D., Department of Hematology, Duke University Medical Center, for review of the manuscript and for permission to comment on studies described in an abstract now under review (17).
Supported by Grant 13629 from the National Heart Lung and Blood Institute
Footnotes
The authors declare no conflict of interest in connection with this publication.
Author contributions: Drs. Singla, Bartholomew and Kapadia cared for the patient, obtained blood samples, prepared the case report and critiqued the manuscript. Ms. Sullivan performed laboratory studies, prepared figures and critiqued the manuscript. Dr. Lee performed serologic studies of patients given protamine following cardiac surgery. Drs. Aster and Curtis provided oversight to the other laboratory studies, prepared the manuscript and contributed equally to this work.
REFERENCES
- 1.Eirin-Lopez JM, Frehlick LJ, Ausio J. Protamines, in the footsteps of linker histone evolution. The Journal of biological chemistry. 2006;281(1):1–4. doi: 10.1074/jbc.R500018200. [DOI] [PubMed] [Google Scholar]
- 2.Balhorn R. The protamine family of sperm nuclear proteins. Genome biology. 2007;8(9):227. doi: 10.1186/gb-2007-8-9-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Mondhiry H, Pierce WS, Basarab RM. Protamine-induced thrombocytopenia and leukopenia. Thromb. Haemost. 1985;53(1):60–64. [PubMed] [Google Scholar]
- 4.Nathan N, Mercury P, Denizot Y, Cornu E, Laskar M, Arnoux B, et al. Effects of the platelet-activating factor receptor antagonist BN 52021 on hematologic variables and blood loss during and after cardiopulmonary bypass. Anesthesia and analgesia. 1994;79(2):205–211. doi: 10.1213/00000539-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Heyns AD, Lotter MG, Badenhorst PN, Kotze H, Killian FC, Herbst C, et al. Kinetics and in vivo redistribution of (111)Indium-labelled human platelets after intravenous protamine sulphate. Thrombosis and Haemostasis. 1980;44(2):65–68. [PubMed] [Google Scholar]
- 6.Curtis BR, McFarland JG. Detection and identification of platelet antibodies and antigens in the clinical laboratory. Immunohematology. 2009;25(3):125–135. [PubMed] [Google Scholar]
- 7.Sheridan D, Carter C, Kelton JG. A diagnostic test for heparin-induced thrombocytopenia. Blood. 1986;67(1):27–30. [PubMed] [Google Scholar]
- 8.Visentin GP, Ford SE, Scott JP, Aster RH. Antibodies from patients with heparin-induced thrombocytopenia/thrombosis are specific for platelet factor 4 complexed with heparin or bound to endothelial cells. J Clin Invest. 1994;93(1):81–88. doi: 10.1172/JCI116987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suh JS, Malik MI, Aster RH, Visentin GP. Characterization of the humoral immune response in heparin-induced thrombocytopenia. Am J Hematol. 1997;54(3):196–201. doi: 10.1002/(sici)1096-8652(199703)54:3<196::aid-ajh4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 10.Davoren A, Aster RH. Heparin-induced thrombocytopenia and thrombosis. Am J Hematol. 2006;81(1):36–44. doi: 10.1002/ajh.20490. [DOI] [PubMed] [Google Scholar]
- 11.Warkentin TE, Sheppard JA. Testing for heparin-induced thrombocytopenia antibodies. Transfusion Medicine Reviews. 2006;20(4):259–272. doi: 10.1016/j.tmrv.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Rauova L, Zhai L, Kowalska MA, Arepally GM, Cines DB, Poncz M. Role of platelet surface PF4 antigenic complexes in heparin-induced thrombocytopenia pathogenesis: diagnostic and therapeutic implications. Blood. 2006;107(6):2346–2353. doi: 10.1182/blood-2005-08-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warkentin TE, Kelton JG. Delayed-onset heparin-induced thrombocytopenia and thrombosis. Annals of Internal Medicine. 2001;135(7):502–506. doi: 10.7326/0003-4819-135-7-200110020-00009. [DOI] [PubMed] [Google Scholar]
- 14.Prechel MM, McDonald MK, Jeske WP, Messmore HL, Walenga JM. Activation of platelets by heparin-induced thrombocytopenia antibodies in the serotonin release assay is not dependent on the presence of heparin. Journal of thrombosis and haemostasis : JTH. 2005;3(10):2168–2175. doi: 10.1111/j.1538-7836.2005.01560.x. [DOI] [PubMed] [Google Scholar]
- 15.Aster RH. Heparin-independent activation of platelets by HIT antibodies: a clue to the etiology of delayed thrombocytopenia/thrombosis in patients given heparin? Journal of thrombosis and haemostasis : JTH. 2005;3(10):2166–2167. doi: 10.1111/j.1538-7836.2005.01595.x. [DOI] [PubMed] [Google Scholar]
- 16.Chudasama SL, Espinasse B, Hwang F, Qi R, Joglekar M, Afonina G, et al. Heparin modifies the immunogenicity of positively charged proteins. Blood. 2010;116(26):6046–6053. doi: 10.1182/blood-2010-06-292938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee GMWJ, Bute BP, Ortel TL, Arepally GM. High Incidence of Antibodies to Protamine and Protamine/Heparin Complexes in Patients Undergoing Cardiopulmonary Bypass. Blood. 2012;120 doi: 10.1182/blood-2012-11-469130. In Press (Abstract submitted to Annual meeting of the American Society of Hematology) [DOI] [PMC free article] [PubMed] [Google Scholar]