Abstract
Germline mutations in PTEN have been described in a spectrum of syndromes that are collectively known as PTEN hamartoma tumor syndrome (PHTS). In addition to being mutated in the germline in PHTS, somatic loss-of-function PTEN mutations are seen in a wide range of sporadic human tumors. Here, we show evidence of upregulated proteasome activity in PHTS-derived lymphoblasts, Pten knock-in mice and cell lines expressing missense and nonsense PTEN mutations. Notably, elevated nuclear proteasome activity occurred in cells expressing the nuclear mislocalized PTEN-K62R mutant, whereas elevated cytosolic proteasome activity was observed in cells expressing the cytosolic-predominant mutant PTEN (M3M4 and C136R). Treatment with proteasome inhibitor MG-132 was able to restore both nonsense and missense mutant PTEN protein levels in vitro. PHTS patients with destabilizing PTEN mutations and proteasome hyperactivity are more susceptible to develop neurological symptoms such as mental retardation and autism than mutation-positive patients with normal proteasome activity. A detailed molecular and functional analysis shows that PTEN mutants most likely cause proteasome hyperactivity via two different mechanisms, namely, induction of proteotoxic stress and loss of protein phosphatase activity. These results provide novel insights into the cellular functions of PTEN and reveal molecular mechanisms whereby PTEN mutations increase proteasome activity and lead to neurological phenotypes.
Keywords: Cowden syndrome, PHTS, PTEN instability, localization, germline mutation
Introduction
PTEN is regarded as one of the most integral tumor suppressors due to its regulation of cellular proliferation, differentiation, and survival between multiple signaling pathways (1). Located at 10q23, PTEN encodes a protein that functions as a dual lipid and protein phosphatase (2, 3). Germline mutations in PTEN occur in subsets of several clinically distinct inherited disorders, such as Cowden syndrome (CS), Bannayan-Riley-Ruvalcaba syndrome and autism spectrum disorders (4), collectively termed PTEN hamartoma tumor syndrome (PHTS). The most common PHTS is CS which is a multiple hamartoma syndrome associated with a high risk of benign and malignant tumors of the thyroid, breast, and endometrium, and megencephaly (5, 6). Recently, our group reported that approximately 25% of individuals who meet the strict diagnostic criteria for CS, who were accrued from the community, have a germline pathogenic PTEN mutation (7). In a wide variety of sporadic tumors, especially glioblastoma multiforme (8) and endometrial carcinoma (9), high frequencies of somatic PTEN mutations are well documented.
Certain mutations in DNA can result in misfolded or truncated proteins. Ubiquitin-dependent protein degradation is an essential mechanism of cellular clearance of such misfolded proteins. Following multiple cycles of misfolding in the endoplasmic reticulum, proteins are retro-translocated to the cytosol and conjugated with ubiquitin. Polyubiquitinated proteins are targeted for degradation by an ATP-dependent process in proteasomes, which are located in the cytosol and nucleus (10). Using a cohort of 3042 CS patients, we have shown that decreased peripheral blood PTEN protein levels correlate with individuals harboring germline PTEN mutations (7). More interestingly, decreasing PTEN protein levels roughly correlate with increasing, so-called, PTEN-CC score, which is a measure of increasing phenotypic load (7). These observations may suggest that proteasome hyperactivity may play a role in the resulting CS phenotypes if a subset of PTEN mutations diminish PTEN’s protein stability by whatever mechanism.
Proteotoxic stress is a cellular stress that is induced by proteins that fail to fold properly. Several lines of evidence suggest that proteotoxic stress and proteasome hyperactivity may be a hallmark of human cancers (11). Indirect evidence for this type of “gain-of-function” of proteasomes in cancers is demonstrated by the increased sensitivity of cancer cells to proteasome inhibitors such as bortezomib (12). We therefore hypothesized that proteasome hyperactivity is a common phenomenon in cells expressing misfolded PTEN proteins encoded by mutant PTEN gene, germane to PHTS. We sought to address our hypothesis by interrogating proteasome activity in a mouse model, PHTS-derived lymphoblastoid cells and cancer cell lines expressing PTEN mutations.
Materials and methods
Reagents
MG-132 (>99% pure) was purchased from LC Laboratories (Woburn, MA. Cat# B-1408). Cycloheximide was purchased from Sigma Aldrich (St. Louis, MO). The Mitogen-Activated Protein Kinase (MAPK)/ERK Kinase (MEK) inhibitor, PD98059, was purchased from Calbiochem (La Jolla, CA). The AKT inhibitor, Perifosine (>99% pure), was purchased from LC laboratories (Woburn, MA)
Cell culture
MCF-7 and HEK-293 cells were grown in DMEM supplemented with 10% fetal bovine serum (FBS). Immortalized lymphoblast cells obtained from PHTS patients or normal controls were grown in RPMI 1640 supplemented with 20% fetal bovine serum.
Patients
We utilized peripheral blood samples and lymphoblastoid lines derived from PHTS patients with a missense mutation (C136R) and 2 common nonsense mutations (R233X and R335X) and from normal PTEN wild-type (WT) controls. Informed consent was obtained for all research participants (PHTS individuals and controls) in accordance with procedures and protocols approved by the respective Human Subjects Protection Committee of each participating institution. All research participants, whether PHTS patients or controls, participated on a voluntary (unpaid) basis.
Mutation analysis
Genomic DNA extracted from peripheral leukocytes, obtained from both PHTS patients and controls, were amplified by PCR and subjected to direct Sanger sequencing (ABI3730xl) of all PTEN exons and flanking introns. All controls had no detectable PTEN sequence alterations.
Mutagenesis
Each PTEN mutant was engineered using the QuikChange in vitro site-directed mutagenesis system according to the manufacturer’s instructions (Stratagene, La Jolla, CA). The accurate construction of mutant PTEN plasmids was verified by sequence analysis. All plasmids generated contain a FLAG-epitope at the C-terminus such that the expressed PTEN contains a C-terminal FLAG fusion.
Plasmid transfection
Cells were either grown on coverslips in 6-well plates (for confocal microscopy assays) or cultured in 60 cm2 dishes (for Western analysis) and were transfected with plasmid DNA (PTEN-WT or PTEN-mutant) using FuGENE 6 (Roche Applied Science, Indianapolis, IN).
Protein isolation, SDS-PAGE, and Western blot analyses
For whole cell protein lysates, cells were washed twice with ice cold PBS and harvested in M-PER buffer (Thermo Scientific, Waltham, MA) with protease inhibitors and phosphatase inhibitors. After quantification, proteins were run on 4–15% SDS –PAGE gels and transferred to nitrocellulose. Blots were probed with primary antibodies and followed by incubation with secondary antibody and then visualized using enhanced chemiluminescence detection. FLAG M2 monoclonal antibody was purchased from Sigma Aldrich (St. Louis, MO). PTEN antibody (Clone 6H2.1) was from Cascade Biosciences (Winchester, MA).
Cyclohexmide (CHX)-chase study
MCF-7 cells stably expressing FLAG-tagged WT or mutant PTEN were incubated with CHX (50µg/mL) and were harvested at the indicated time points. Whole protein lysates were extracted and ran for Western blots using anti-FLAG antibody for transfectant PTEN and GAPDH antibody as a loading control.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
PTEN mRNA expression was measured by qRT-PCR to quantitate as described previously (13) using SYBR Green (Applied Biosystem, Foster City, CA). Expression of GAPDH was used as the internal control. PTEN Primers are as following: F: AAAGGCACAAGAGGCCCTAGAT. R: CAAGTTCCGCCACTGAACATTGGAA.
Proteasome activity assay
Cells or brain tissues were homogenized in lysis buffer (0.05M HEPES, 0.005M EDTA, 0.15M NaCl, 1% Triton X-100) and 3 µg whole cell lysates (for fractionation study, 3 µg cytosolic-fraction, 10 µg nuclear-fraction), were mixed with the fluorogenic proteasome substrates Suc-LLVY-AMC (Enzo Life Sciences, Farmingdale, NY) and incubated in 37°C for 2.5 hours. Fluorescent values were read with excitation and emission wavelengths of 355 and 460 nm, respectively.
Subcellular fractionation
For the nuclear and cytosolic fractionation, cells were harvested by trypsinization and then were incubated with buffer A (10 mM MOPS, 1.5 mM MgCl2, 10 mM KCl and 1% Triton X-100) for 15 min on ice with vortex for every 5 min. Cells were then centrifuged at 13,000 rpm for 5 min at 4°C and the supernatant was carefully collected as the cytosolic fraction. The pellets were then incubated with buffer A for another 15 min and separated by centrifugation at 13,000 rpm for 5 min at 4°C. The pellets were dissolved in RIPA buffer (50mM Tris-HCl, 150mM NaCl, 0.5 % Na-Deoxycholate, 1% Triton X 100). Both the cytosolic and the nuclear lysates were adjusted to the same concentration before loading for the proteasome activity assay. Alpha-tubulin and PARP-1 were used as loading controls for cytosolic and nuclear protein fractions, separately.
Indirect immunofluorescence and confocal microscopy
Cells were seeded in six-well plates with cover slips. Cells were fixed in freshly prepared 100% methanol for 1 min at −20°C. After 5 min incubation with 0.3% Triton X-100 in PBS, the cover slips were washed three times in PBS, blocked in PBS with 10% goat serum for 30 min, incubated with primary antibodies for 1 h, washed three times in PBS and incubated with Alexa Fluor® dye-labeled secondary antibodies for 1 h at the concentration of 1:1500 (Invitrogen, Carlsbad, CA). FLAG M2 monoclonal antibody was purchased from Sigma Aldrich (St. Louis, MO). Cells were mounted on glass slides with Pro-Long Gold® antifade reagent with DAPI (Invitrogen, Carlsbad, CA) and visualized on a Leica TCS-SP spectral laser scanning confocal microscope.
Murine model study
PtenM3M4 missense knock-in mutant mice were generated in our lab and were backcrossed more than ten times onto a CD1 genetic background. Briefly, the M3M4 double mutations were targeted into Pten exon 7 which contains the NLS-like sequence (14). Male mouse littermates were sacrificed at 10 weeks ages; megencephalic brains were dissected out for proteasome activity assay and Western blot. All protocols involving mice were approved by the Institutional Animal Care and Use Committee (IACUC) at the Cleveland Clinic.
Statistical analysis
The proteasome activity of lymphoblast cells between each group was compared by student’s t-test. A P<0.05 was considered statistically significant.
Results
Reduced PTEN protein levels and increased proteasome activity is observed in lymphoblasts derived from PHTS patients
To assess the level of PTEN expression in PHTS patients, we investigated PTEN protein expression through Western blot. We identified significantly reduced PTEN protein levels in PHTS lymphoblasts when compared to PTENWT controls (at ~40% of control protein, P<0.01, see Fig. 1A and B). To determine the mechanism of protein loss, we measured PTEN mRNA levels by real-time qRT-PCR in the same samples. Only PTEN-R335X resulted in significantly diminished PTEN mRNA transcript compared to the PTEN-WT controls (at ~65% of WT1, and ~40% of WT2, respectively). The other 2 mutants, R233X and C136R, showed PTEN mRNA levels similar to those of the controls (Fig. 1C). Our data suggest that post-translational degradation is an important pathway for quality control of mutant PTEN protein in PHTS patients.
Figure 1. Diminished PTEN protein levels are accompanied by up-regulated 20S proteasome activity in CS patients with PTEN nonsense or missense mutations.
A, Representative Western blots of whole cell lysates derived from lymphoblast cells isolated from two normal WT controls as well as three CS patients who are heterozygous for PTEN mutations with indicated genotypes (PTEN-R233X, PTEN-R335X and PTEN-C136R). B, Expression levels of PTEN protein from two independent experiments were normalized to GAPDH levels. Asterisks indicate statistically significant difference (**P < 0.01). C, Total RNA from lymphoblast cells isolated from CS patients with mutant PTEN or normal controls with PTEN-WT was extracted and qRT-PCR was performed to measure PTEN mRNA levels. *P <0.05, versus WT controls. D, lymphoblasts isolated from CS patients with PTEN nonsense or missense mutations (n=4 per group) were characterized to measure proteasome activity. The proteasome activity was normalized according to the average proteasome activity levels of the normal PTEN-WT controls (n=10). *P <0.05, **P <0.01.
We then assessed proteasome activity for 12 PHTS patients with germline PTEN nonsense or missense mutations (R233X, R335X and C136R, n=4 in each group) and 10 PTEN-WT controls. We found a ~60% and a ~30% increase in the proteasome activity of lymphoblasts isolated from PHTS patients with R233X or R335X nonsense mutations, respectively (Fig. 1D). We also found a ~30% increase in proteasome activity in the C136R mutants. We therefore conclude that at least a subset of PHTS-related PTEN nonsense or missense germline mutations have diminished PTEN protein levels and proteasome hyperactivity.
PHTS patients with robust proteasome activity are associated with neurological symptoms
Because proteasome hyperactivity is associated with neurodegenerative diseases, and proteasome inhibition can alleviate such diseases, we hypothesize that PHTS patients with increased proteasome activities may present with more neurological phenotypes (15–17). To this end, we analyzed phenotypes in these 12 patients whose proteasome activities were elevated. Surprisingly, 4 out of 12 (33.3%) proteasome hyperactive patients presented with such neurological phenotypes as autism and mental retardation. In contrast, in another series of 39 patients harboring other PTEN germline mutations (G129Q, R130X, R130G, −903G>A) that did not significantly alter proteasome activity, only 3 patients (7.7%) had severe neurological phenotypes (P=0.04, Fisher 2-tailed exact test. Data not shown). Therefore, it is possible that patients with PTEN mutations resulting in unstable PTEN proteins and proteasome hyperactivity are more susceptible to develop neurological phenotypes than patients with normal proteasome activities (Table 1).
Table 1.
Patient genotypes, age at onset of symptoms and stability in lymphoblasts harboring PTEN germline mutations
| Patient | Gender | Age at onset |
*Predicted size (KDa) |
DNA mutation |
Amino acid change |
Exon | Stability | Main manifestation |
|---|---|---|---|---|---|---|---|---|
| ‡900WM | Male | 5yr | 55 | 406T→C | C136R | 5 | Unstable | Macrocephaly, Autism, Global developmental delay, Lipoma |
| 622UC | Female | 53yr | 55 | 406T→C | C136R | 5 | Unstable | Ductal breast Ca., Thyroid adenoma, Thyroid Hurthle cell Ca., Uterine Ca., Lipoma |
| 2447DY | Male | 38yr | 55 | 406T→C | C136R | 5 | Unstable | Macrocephaly, Thyroid adenoma, Hemangioma of skin |
| 2447AY | Male | 9yr | 55 | 406T→C | C136R | 5 | Unstable | Macrocephaly, Hemangioma of skin |
| 12961SW | NA | NA | 27 | 697C→T | R233X | 7 | Unstable | NA |
| ‡972KS | Male | 7yr | 27 | 697C→T | R233X | 7 | Unstable | Macrocephaly, Mental retardation |
| 4026CA | NA | NA | 27 | 697C→T | R233X | 7 | Unstable | NA |
| 420JT | Male | 5 yr | 27 | 697C→T | R233X | 7 | Unstable | Macrocephaly,Cafe-au-lait spots, Tan macules, Congenital genitourinary tract abnormalities |
| ‡531LM | Male | 8yr | 39 | 1003C→T | R335X | 8 | Unstable | Macrocephaly, Global developmental delay |
| 1113KT | Female | 33yr | 39 | 1003C→T | R335X | 8 | Unstable | Macrocephaly, Breast cancer, Lipoma, Hodgkin’s lymphoma |
| ‡3159EK | Female | 10yr | 39 | 1003C→T | R335X | 8 | Unstable | Macrocephaly, Autism, Cafe-au-lait spots, Thyroid adenoma |
| 3147PD | Female | 44yr | 39 | 1003C→T | R335X | 8 | Unstable | Macrocephaly, Thyroid adenoma, Renal cell carcinoma |
Patients with severer neurological phenotypes.
Predicted size (KDa) was calculated online: http://web.expasy.org/compute_pi/
NA = not available,
Missense mutations in PTEN affect protein stability
Since our data clearly show increased proteasome activity in cells derived from PHTS patients, it was necessary to investigate the effect of mutant PTEN protein on proteasome activity. We therefore transfected MCF-7 breast cancer cells with either WT or missense mutant PTEN (K62R, K125E, M3M4 or C136R) and investigated PTEN protein stability through a cycloheximide (CHX) chase study. The WT and K125E mutant PTEN protein was stable after synthesis and was observed at 100% even after 8 hours of CHX treatment. In contrast, PTEN-C136R turned out to be highly unstable, as 50% of the mutant protein was degraded after 2 hours of CHX treatment. Moreover, MCF-7 cells expressing PTEN-K62R or PTEN-M3M4 had PTEN levels decreased by 42% and 55%, respectively, after CHX treatment for 8 hours. The data in Fig. 2A and B clearly show the varying stabilities of different mutant PTEN proteins.
Figure 2. Missense mutations reduce PTEN protein stability via proteasome-mediated PTEN degradation.
A, Degradation of PTEN-C136R, M3M4 and K62R in transfected MCF-7 cells assessed by cycloheximide-chase. B, Plot of PTEN protein decay. Data from the Western blots from Fig. 2A were plotted as the relative ratio of normalized PTEN at each time point to normalized PTEN at time zero. C, HEK-293 cells were transiently transfected with FLAG-tagged WT or missense PTEN plasmids for 1 day and treated with 10µM MG132 or DMSO vehicle control for O.N. Cells were then harvested and probed for transfectant PTEN using anti-FLAG antibody and anti-GAPDH as a loading control. Arrow denotes PTEN that is cleaved by activated caspases. D, Densitometric analyses of PTEN protein expression. PTEN protein levels were normalized against GAPDH, and were presented as fold changes relative to control DMSO; bars, SD. *P<0.05, **P<0.01 when compared to the DMSO control. E and F, Expression of missense mutant PTEN increases proteasome activity in vitro. MCF-7 and HEK-293 cells, respectively, expressing PTEN-WT or missense mutant PTEN were grown to subconfluence and whole cell lysates were harvested by using proteasome lysis buffer. The fluorogenic substrate Suc-Leu-Leu-Val-Tyr-AMC was used to measure proteasome activity in whole-cell lysates. The proteasome activity was quantified and expressed relative to that of the MCF-7 or HEK-293 cells expressing the PTEN-WT control. Columns, mean from three experiments; bars, SD. *P <0.05, **P<0.01 versus PTEN-WT control. G, Graphic representation of PTEN somatic and germline mutations in the proteasome study. The PTEN gene contains 9 exons and encodes a 403 amino-acid protein. The domains within PTEN include an N-terminal phoshpatase domain and a C-terminal C2 domain. A PDZ- binding motif is also labeled in the C-terminus.
Since some of the missense mutations dramatically impair PTEN’s protein stability, we next investigated if degradation of the mutant PTEN was through the ubiquitin-proteasomal pathway. We first utilized HEK-293 cells, which were transfected with PTEN variants (WT, K62R, K125E, M3M4, C136R) and treated for 24 hours with the proteasomal inhibitor, MG132 (10 µM). Proteasomal inhibition results in an increase of the 3 unstable mutants, K62R, M3M4 and C136R, but not of the stable PTEN mutant K125E and WT PTEN (Fig. 2C and D). We then repeated the same experiment in a breast cancer cell line, MCF-7. Similar to HEK-293 cells, inhibition of proteasome by MG-132 results in elevated PTEN protein levels only in cells expressing unstable mutants (K62R, M3M4 and C136R) (Supplementary Fig. S1). Our results suggest that certain missense PTEN mutants are degraded by the proteasomal pathway.
Cells expressing missense PTEN mutants have elevated proteasome activity in vitro
Since we found different mutations leading to different PTEN protein stability, we next sought to determine whether cells expressing mutant PTEN with impaired protein stability would have elevated proteasome activity, as we already detected in PHTS-patient-derived lymphoblasts. Proteasome activity was measured in MCF-7 and HEK-293 cells expressing various PTEN mutations (Fig. 2E–G). Increased proteasome activity was detected in both MCF-7 and HEK-293 cell lines expressing PTEN-M3M4 and PTEN-C136R, which are unstable PTEN mutants. In contrast, cells expressing PTEN-K62R and PTEN-K125E showed similar proteasome activity with the WT control, although PTEN-K62R is also an unstable PTEN mutant (Fig. 2E and F).
Subcellular distribution of proteasome activity in cells expressing PTEN variants
Several mutations in PTEN have been shown to cause mislocalization (18–20). The relatively normal proteasome activity in cells expressing the unstable PTEN-K62R mutants prompted us to investigate proteasome activity at the subcellular levels. Using confocal immunofluorescence microscopy, we were able to define subcellular localization of the PTEN mutants in MCF-7 cells. Both PTEN-K62R and PTEN-K125E are predominantly nuclear localized, whereas PTEN-M3M4, PTEN-C136R, and WT are predominantly cytosolic (Fig. 3A).
Figure 3. Subcellular distribution of proteasome activity in cells expressing PTEN variants.
A, Confocal microscope analyses of MCF-7 cells transfected with FLAG-tagged WT or missense mutant PTEN (M3M4, K62R, C136R and K125E). The subcellular localization and stability of the analyzed PTEN mutants was labeled. B, MCF-7 and C, HEK-293 cells were fractionated. The cytosolic (left panels) and nuclear (right panels) fractions were analyzed for proteasome activity, respectively. Columns, mean from three experiments; bars, SD. *P <0.05, versus PTEN-WT control. The degree of fractionation is demonstrated in Supplementary Figure S2.
Since nuclear proteasome activity accounts for a minimal proportion of total activity (21), it is possible that activity elicited by PTEN-K62R was too insignificant to create an observable change in the whole cell readout. To directly analyze proteasome activity in cytosolic and nuclear compartments, we performed subcellular fractionation and measured proteasome activity in both fractions. MCF-7 and HEK-293 cells overexpressing PTEN-M3M4 and PTEN-C136R showed significantly increased proteasome activity only in the cytosol but not in the nucleus. In contrast, overexpression of PTEN-K62R led to significantly increased proteasome activity only in the nucleus but not in the cytosol (Fig. 3 B and C. Supplementary Fig. S2).
Elevated proteasome activity in mice expressing mutant Pten
To further demonstrate that PTEN-M3M4 is associated with increased proteasome activity in vivo, we measured the activity of the 20S proteasome in a PtenM3M4 knock-in murine model. Heterozygous (PtenWT/M3M4) mice express the Pten-M3M4 protein monoallelically. In comparison to WT littermate controls, the 20S proteasome activity was increased by ~40% in the megencephalic brain tissues of the heterozygous mice (Fig 4A). These observations prompted us to further examine whether the ubiquitin levels were also upregulated in PtenWT/M3M4 heterozygous mice. Ubiquitin protein levels were compared in brain tissues pooled from two PtenWT/M3M4 heterozygous mice or from two PtenWT/WT littermate controls. Strikingly, the megencephalic brains from the PtenWT/M3M4 heterozygous mice showed significantly increased ubiquitin abundance when compared to normocephalic brains from WT controls (Fig. 4B, upper panel). Decreased Pten levels, together with elevated ubiquitinated PTEN levels, were also confirmed in the brain tissues of the PtenWT/M3M4 heterozygous mice compared to those of the WT control littermates (Fig. 4B, middle panel, and Fig. 4C). Thus, these data confirm our hypothesis that proteasome activity can be elevated in MCF-7 cells, HEK-293 cells, and in a mouse model that express PTEN carrying specific mutations.
Figure 4. Heterozygous PtenM3M4 knock-in mice have hyperactive proteasomes and increased protein ubiquitination.
A, protein was extracted from brain tissues of PtenWT/WT and PtenM3M4/WT male littermates (n=2). Proteasome activity was quantified and expressed relative to that of the PtenWT/WT. *P <0.05. B, elevated levels of ubiquitinated proteins and decreased Pten protein levels in PtenM3M4/WT mouse brain tissues. C, elevated ubiquitination of the Pten M3M4 mutant in the brain. The same brain tissues used in Fig. 4B were extracted to get whole cell lysates and were then immunoprecipitated with an anti-ubiquitin antibody, and then immunoblotted with an antibody to PTEN.
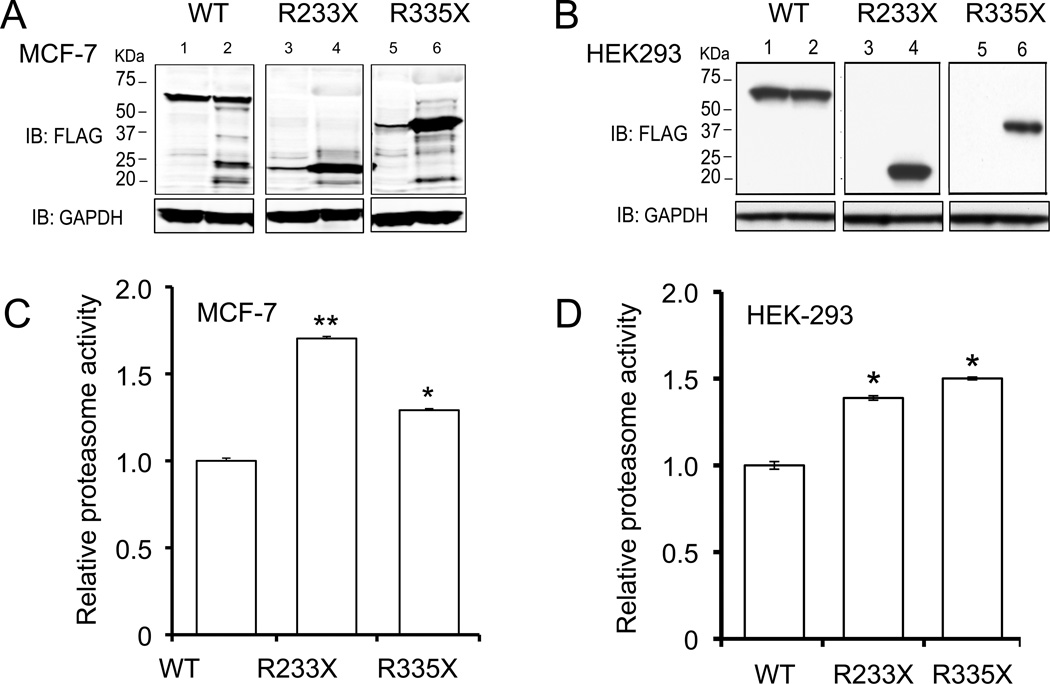
Nonsense mutations in PTEN affect protein stability and proteasome activity
To study the effect of nonsense mutations on PTEN protein stability and proteasome activity, we introduced the 2 most common PHTS-related PTEN nonsense mutations (R233X and R335X) into MCF-7 and HEK-293 cells. Western blot data from both cell lines showed low to no observable PTEN-R233X and R335X proteins in contrast to PTEN-WT (Fig. 5A and B, lanes 3&5). We next asked if proteasomal degradation also plays a role in the degradation of nonsense-mutant PTEN. We compared protein levels by Western Blot after transient transfection of FLAG-tagged PTEN-WT or PTEN-R233X or -R335X) into MCF-7 and HEK293 cells, in the presence of proteasome inhibitor. Surprisingly, there was a significantly increased amount of truncated protein following proteasome inhibitor treatment in comparison to untreated cells (Fig. 5A and B, lanes 4&6). Thus, these two nonsense PTEN mutants may undergo some proteasome degradation, at least in vitro.
Figure 5. Overexpressing PTEN nonsense mutations increases 20S proteasome activity in vitro.
A and B, Nonsense mutations result in diminished PTEN protein that can be rescued by the proteasome inhibitor, MG-132. MCF-7 (A) and HEK-293 (B) cells were transfected with FLAG-tagged WT PTEN or truncated PTEN (R233X, R335X). After 24 h, cells were treated with 10μM MG-132 (lanes 2, 4 and 6) or DMSO control (lanes 1, 3 and 5). Cells were harvested for Western blots after 48 h of transfection. C and D, Relative proteasome activity of MCF-7 (C) and HEK-293 (D) cells expressing WT or nonsesnse mutant PTEN. Columns, mean from three experiments; bars, SD. *P <0.05, **P< 0.01, versus WT control.
To confirm our observations in PHTS, we analyzed proteasome activity in both cell lines to determine if the instability was associated with proteasome hypersensitivity. Similar to our in vivo data, proteasome activity was inversely correlated with protein levels and was significantly elevated in comparison to the WT control (Fig. 5C and D). Such data further validates our hypothesis that proteasome hyperactivity is, in part, due to PTEN protein instability.
Both the intact protein phosphatase activity and the steady-state of PTEN determine PTEN’s inhibitory effect on proteasome activity
The activated proteasome in PHTS-derived cells and animal model prompted us to investigate the mechanism(s) by which PTEN mutations lead to proteasome hyperactivity. PTEN has both lipid and protein phosphatase activity (22, 23). The lipid phosphatase activity of PTEN has been shown to downregulate the phosphatidylinositol-3-kinase (PI3K)/AKT pathway, whereas the protein phosphatase activity of PTEN has been shown to regulate various cell-proliferation pathways, such as the Mitogen Activated Protein Kinase-ERK (MAPK/ERK) pathway (23). We hypothesize that PTEN mutations may lead to upregulated proteasome activity through two possible mechanisms. Firstly, mutations lead to loss of PTEN phosphatase activity and subsequent activation of the PI3K/AKT and the MAPK/ERK pathways. This may increase proteasome activity in cells harboring such mutations. Secondly, mutations leading to misfolded PTEN protein further induce proteotoxic stress. This will also activate proteasomes. To answer this question unambiguously, FLAG epitope-tagged PTEN or empty vector were expressed in MCF-7 cells. Proteasome activity assays show that cells expressing WT PTEN had an 18% decrease of proteasome activity when compared to cells expressing the empty vector (P<0.01) (Fig. 6A, left). Western blot reveals PTEN suppressing both PI3K/AKT and MAPK/ERK pathways (Fig. 6A, right).
Figure 6. Both intact protein phosphatase activity and the steady-state of PTEN determine PTEN’s inhibitory effect on proteasome activity.
A. Expression of PTEN suppresses proteasome activity and inhibits the PI3K/AKT and MAPK pathways. MCF-7 cells were transfected with PTEN or the empty vector. Cells were harvested after 24 h for proteasome activity (left) and Western blot (right). B. Inhibition of the AKT pathway does not significantly alter proteasome activity. MCF-7 cells were treated with the AKT inhibitor, perifosine (0, 5 and 10µM) for 24 h. Cells were then harvested for proteasome activity assay (left) and Western blot analysis (right). C. Expression of constitutively active AKT1 (MyrAKT) does not alter proteasome activity. Proteasome activity of MCF-7 cells expressing MyrAKT or a control vector (left) for 24 h. Western blot for basal levels of AKT and P-Akt in MCF-7 cells expression of MyrAKT or a control vector (Right). D. Inhibition of the MAPK pathway significantly decreases proteasome activity. MCF-7 cells were treated with a mitogen-activated protein kinase (MAPK) inhibitor, PD98059 (0, 5 and 10µM) for 24 h. Cells were then harvested for proteasome activity assay (left) and Western blot analysis (right). E. PTEN mutations lead to changes in PTEN stability and the PI3K/AKT, MAPK/ERK signaling pathways. (Left) MCF-7 cells were transiently transfected with FLAG-tagged WT or mutant PTEN. Cells were then harvested after 24 h for FLAG-PTEN, P-AKT, and P-ERK by Western blotting. (Right) Normalization of FLAG-PTEN, P-AKT and P-ERK of cells expressing mutant PTEN to those of cells expressing PTEN-WT. The expression of each protein was normalized to the GAPDH control, and the relative protein levels were normalized to those of the WT control. F. Model of the mechanisms through which mutant PTEN enhances proteasome activity. PTEN mutations that impair the protein phosphatase activity of PTEN will have enhanced proteasome activity through uncontrolled upregulation of the MAPK/ERK pathway, whereas PTEN mutations that impair the stability will activate the proteotoxic stress pathway and induce proteasome activity. Those two effects can be additive if mutations have both defects.
Next, we investigated the effects of the PI3K/AKT and MAPK/ERK signaling pathways on proteasome activity. We first treated MCF-7 cells with the AKT inhibitor, perifosine (24). Although perifosine can significantly decrease P-AKT, it clearly had no obvious effect on proteasome activity (Fig. 6B). Similarly, transfection of the active AKT1 (MyrAKT, i.e., AKT1 fused with an N-terminal myristoylation signal sequence) failed to change proteasome activity (Fig. 6C). Surprisingly, when MCF-7 cells were treated with the MAPK/ERK inhibitor-PD98059, proteasome activity was suppressed in a dose-dependent manner (13% decrease at 5µM, *P<0.05; 27% decrease at 10µM, **P<0.01. Fig. 6D). This also mimicked the effect of PTEN-WT transfection in the same cell line. Thus, it is the protein, but not the lipid, phosphatase activity of PTEN that contributes, at least in part, to the inhibitory effect of PTEN on proteasome activity.
We further investigated the impairment of the lipid and protein phosphatase activity of PTEN by different mutations. By using P-AKT as a surrogate of the PI3K pathway, we demonstrated impaired lipid phosphatase activity in PTEN-K125E, C136R, M3M4, R233X and R335X. Similarly, by using P-ERK as a surrogate of the MAPK/ERK pathway, we found impaired protein phosphatase activity in PTEN-C136R, M3M4, R233X and R335X (Fig. 6E). Interestingly, all the four PTEN mutants that have impaired protein phosphatase activity (C136R, M3M4, R233X and R335X) were associated with increased proteasome activity (see Fig. 1D, Fig. 2E and F).
Discussion
In the present study, we found increased proteasome activity in lymphoblast cells from PHTS patients and in cell lines expressing germline or somatic PTEN nonsense and missense mutations. The instability of both the nonsense and missense PTEN mutants can be rescued by blocking proteasome activity through a proteasome inhibitor, MG-132, whose clinical analog, bortezomib has already been used to treat patients with multiple myeloma (25) or patients with mantle cell lymphoma (26). In addition, our study revealed that certain PTEN missense or nonsense mutations that affect proper PTEN folding and/or protein phosphatase activity are associated with elevated proteasome activity in PHTS patient-derived cells and in breast cancer cells. Taken together, we propose that there are two mechanisms for gain-of-function proteasomic activity by PTEN mutations. PTEN mutations that impair the protein phosphatase activity will effect enhanced proteasome activity through uncontrolled MAPK/ERK pathway, whereas PTEN mutations that impair PTEN protein stability will activate proteotoxic stress and induce proteasome activity. Those two effects can be additive in mutations that have both defects. The detailed mechanisms have been illustrated in Fig. 6F.
PTEN is a 403-amino acid protein with 2 major functional domains: an N-terminal domain encompassing exons 1–6 and a C-terminal domain encompassing exons 6–9 (27). According to The Human Gene Mutation Database (www.hgmd.cf.ac.uk/), ~50% of PTEN mutations are missense or nonsense mutations. Thus, understanding the pathogenic effects of PTEN missense/nonsense mutations will benefit patients who harbor such mutations. Here, we demonstrate that 3 missense mutations (K62R, M3M4, C136R) and 2 common nonsense mutations (R233X, R335X) exhibit enhanced proteasomal degradation of PTEN providing a mechanism for their impaired protein stability. Such quantitative loss of PTEN results in the functional insufficiency of protein function. Compared to missense PTEN mutations, the 2 common nonsense mutations are highly unstable, as PTEN protein cannot be detected if proteasomal activity is not inhibited. This may suggest the proteasomal degradation of missense mutant PTEN is incomplete whereas the degradation of nonsense mutant PTEN is complete. An alternative explanation might be that the missense mutants studied here are subject to proteasomal degradation secondary to misfolding, whereas these 2 common nonsense mutations result in protein which undergo both nonsense-mediated decay (Eng, unpublished observations) and proteasomal degradation.
We additionally demonstrate that PHTS patients with certain PTEN nonsense or missense mutations have highly activated proteasomes in their derived lymphoblast cells. Moreover, MCF-7 breast cancer cells and HEK-293 cells expressing these missense mutant PTEN (PTEN-M3M4, C136R) and nonsense mutant PTEN (PTEN-R233X and PTEN-R335X) proteins, all showed increased proteasome activity compared to the same cell lines expressing the PTEN-WT control. We believe that our results here identify a potential intrinsic link between presumably misfolded mutant PTEN and the elevated proteasome activity. Interestingly, PHTS patients with destabilizing PTEN mutations and proteasome hyperactivity are more susceptible to develop neurological symptoms such as mental retardation and autism than patients with normal proteasome activities. In addition to PHTS, PTEN is mutated in patients with autism spectrum disorders (ASDs). Rodríguez-Escudero et al identified distinctive functional patterns among PTEN mutations found in tumors and in the germline of PHTS and ASD patients. They revealed that ASD-associated hereditary PTEN mutations did not substantially abrogate PTEN activity in vivo, whereas most of PHTS-associated mutations did (28). By studying neurological symptoms in PHTS patients, our results do suggest that PTEN stability and subsequent proteasome activity may be another factor that influences neurological phenotypes in PHTS.
Missense mutations may affect not only protein instability but intracellular localization. Our studies have revealed that localization of mutant PTEN contributes to total and subcellular proteasome activity. PTEN is predominantly localized in the cytosol. Cells overexpressing the cytosolic-localized mutant PTEN-M3M4 and PTEN-C136R have very robust proteasome activity in whole cell lysates and the cytosolic compartment when compared to cells overexpressing other PTEN mutants or WT PTEN. In contrast, K62R and K125E, which are nuclear mislocalized PTEN mutants, have very different patterns of proteasome activity. The PTEN-K125E protein, although nuclear mislocalized, is relatively stable consistent with the accompanying normal proteasome activity, suggesting that it may not be a major substrate of the ubiquitin-proteasome system. To our surprise, the K62R mutant protein, which is both unstable and nuclear mislocalized, shows relatively normal proteasome activity in whole cell lysates but elevated nuclear proteasome activity when compared to the WT control cells and cells expressing other PTEN mutants. Therefore, mislocalized mutant PTEN appears to only induce proteasome activity in the compartment where it is degraded. Nuclear predominant mutant PTEN (eg K62R) induces nuclear proteasome activity whereas cytosolic predominant mutant PTEN (eg PTEN-M3M4) induces cytosolic proteasome activity. Indeed, the nuclear ubiquitin-proteasome system is responsible for the proteasomal degradation of many short-lived nuclear proteins (29). For instance, MyoD, which is a nuclear transcription factor that is pivotal in skeletal muscle differentiation, is degraded by the nuclear proteasome system (30). Increased nuclear proteasome activity is associated with stress and drug resistance in solid tumors (31). Further studies are needed to elucidate the specific mechanism and pathogenesis of the proteasome hyperactivity induced by nuclear mislocalized mutant PTEN-K62R.
One major goal of this study was to elucidate the mechanisms of proteasome activation in PTEN mutant cells. A detailed molecular and functional analysis shows that the inhibition of MAPK activation by PD98059 led to suppressed proteasome activity, whereas no effect was observed with inhibition of the AKT activation by perifosine. (Fig. 6). We revealed that both PTEN-WT overexpression and PD98059 treatment only suppressed proteasome activity by ~20% in MCF-7 (Fig. 6A), whereas in the same cell line expressing unstable PTEN mutants (such as PTEN-M3M4 and PTEN-C136R, see Fig. 2E), the latter induced more than 50% increase of proteasome activity when compared to cells expressing PTEN-WT. Therefore, it is plausible to conclude that PTEN mutants most likely cause proteasome hyperactivity via two different mechanisms, namely, induction of proteotoxic stress and loss of protein phosphatase activity.
The proper turnover of the mutant PTEN protein by proteasomes may have essential meaning. In both human and mouse model, PTEN dose predisposes to cancer susceptibility (7, 32). Patients with PTEN mutations that encode unstable proteins will result in PTEN dosage effects, whereas patients with PTEN mutations that encode stable proteins may result in a dominant-negative state. In addition, some mutations that abolish PTEN’s phosphatase activity may still have PTEN’S tumor suppressor effects (33). Truncated PTEN can also change the binding ability to microRNAs that affect PTEN protein levels (34).
Proteasome hypersensitivity may be a hallmark of human cancers. Chen et al. reported that the activity of proteasomes was increased in more than 90% of primary breast cancer tissue specimens. In contrast, no activation was observed in benign solid tumors (11). Another study revealed increased proteasome subunit protein expression and proteasome activity in colon cancer (35). In cancer cells, intrinsic stress response pathways are frequently activated. Accumulation of misfolded PTEN protein may lead to proteotoxic stress (36). This can lead to progressive cellular dysfunction such as activation of pro-survival pathways and proliferation pathways (such as heat shock factors). In recent years, proteasome inhibitors have been increasingly developed and tested in human cancers. Thus, our study has some impact on clinical practice by underscoring proteasome inhibitors in the treatment of breast and other cancers with proteasome-related PTEN instability. Indeed, two proteasome inhibitors (bortezomib and BU-32) have been tested to be effective in cultured breast cancer cells and in breast cancer xenografts (37, 38).
In conclusion, we identified that proteasome hyperactivity and proteotoxic stress may be a common phenomenon in PHTS patients with different PTEN nonsense or missense mutations, at least in certain subsets. Our observations here may also explain why there only exists loose genotype-phenotype correlation in PHTS: seemingly disparate types and locations of mutations result in proteins with common fates, in this situation, protein instability and proteasome hyperactivity. We also found that relative proteasome hyperactivity can be affected by PTEN protein stability, protein phosphatase activity, and subcellular localization. These data contribute to a better understanding that PTEN nonsense and missense mutations have multiple deleterious effects, and the combination of PI3K pathway inhibitors and agents targeting proteasomes may show promise for prevention or treatment of breast tumors in a subset of such mutation carriers or in sporadic malignancies showing similar PTEN protein dysfunctional endpoints.
Supplementary Material
Acknowledgements
This work was supported by R01CA118980 and P01CA124570 from the National Cancer Institute, Bethesda, MD (to CE). CE was a recipient of the Doris Duke Distinguished Clinical Scientist Award, is an American Cancer Society Clinical Research Professor, generously funded, in part, by the F.M. Kirby Foundation, and is the Sondra J. and Stephen R. Hardis Endowed Chair of Cancer Genomic Medicine at the Clinical Clinic.
Footnotes
Conflicts of interest: The authors have declared no relevant conflicts of interest.
References
- 1.Eng C. PTEN: one gene, many syndromes. Hum Mutat. 2003;22:183–198. doi: 10.1002/humu.10257. [DOI] [PubMed] [Google Scholar]
- 2.Li DM, Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res. 1997;57:2124–2129. [PubMed] [Google Scholar]
- 3.Myers MP, Pass I, Batty IH, Van der Kaay J, Stolarov JP, Hemmings BA, et al. The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Natl Acad Sci U S A. 1998;95:13513–13518. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zbuk KM, Eng C. Cancer phenomics: RET and PTEN as illustrative models. Nat Rev Cancer. 2007;7:35–45. doi: 10.1038/nrc2037. [DOI] [PubMed] [Google Scholar]
- 5.Mester JL, Tilot AK, Rybicki LA, Frazier TW, Eng C. Analysis of prevalence and degree of macrocephaly in patients with germline PTEN mutations and of brain weight in Pten knock-in murine model. Eur J Hum Genet. 2011;19:763–768. doi: 10.1038/ejhg.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res. 2012;18:400–407. doi: 10.1158/1078-0432.CCR-11-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan MH, Mester J, Peterson C, Yang Y, Chen JL, Rybicki LA, et al. A clinical scoring system for selection of patients for PTEN mutation testing is proposed on the basis of a prospective study of 3042 probands. Am J Hum Genet. 2011;88:42–56. doi: 10.1016/j.ajhg.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang SI, Puc J, Li J, Bruce JN, Cairns P, Sidransky D, et al. Somatic mutations of PTEN in glioblastoma multiforme. Cancer Res. 1997;57:4183–4186. [PubMed] [Google Scholar]
- 9.Tashiro H, Blazes MS, Wu R, Cho KR, Bose S, Wang SI, et al. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 1997;57:3935–3940. [PubMed] [Google Scholar]
- 10.Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6:79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Madura K. Increased proteasome activity, ubiquitin-conjugating enzymes, and eEF1A translation factor detected in breast cancer tissue. Cancer Res. 2005;65:5599–5606. doi: 10.1158/0008-5472.CAN-05-0201. [DOI] [PubMed] [Google Scholar]
- 12.Strauss SJ, Higginbottom K, Juliger S, Maharaj L, Allen P, Schenkein D, et al. The proteasome inhibitor bortezomib acts independently of p53 and induces cell death via apoptosis and mitotic catastrophe in B-cell lymphoma cell lines. Cancer Res. 2007;67:2783–2790. doi: 10.1158/0008-5472.CAN-06-3254. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Romigh T, He X, Tan MH, Orloff MS, Silverman RH, et al. Differential regulation of PTEN expression by androgen receptor in prostate and breast cancers. Oncogene. 2011;30:4327–4338. doi: 10.1038/onc.2011.144. [DOI] [PubMed] [Google Scholar]
- 14.Mester JL, Tilot AK, Rybicki LA, Frazier TW, 2nd, Eng C. Analysis of prevalence and degree of macrocephaly in patients with germline PTEN mutations and of brain weight in Pten knock-in murine model. Eur J Hum Genet. 19:763–768. doi: 10.1038/ejhg.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma M, Burre J, Sudhof TC. Proteasome inhibition alleviates SNARE-dependent neurodegeneration. Science translational medicine. 2012;4:147ra13. doi: 10.1126/scitranslmed.3004028. [DOI] [PubMed] [Google Scholar]
- 16.Aquilano K, Rotilio G, Ciriolo MR. Proteasome activation and nNOS down-regulation in neuroblastoma cells expressing a Cu,Zn superoxide dismutase mutant involved in familial ALS. Journal of neurochemistry. 2003;85:1324–1335. doi: 10.1046/j.1471-4159.2003.01783.x. [DOI] [PubMed] [Google Scholar]
- 17.Sawada H, Kohno R, Kihara T, Izumi Y, Sakka N, Ibi M, et al. Proteasome mediates dopaminergic neuronal degeneration, and its inhibition causes alpha-synuclein inclusions. J Biol Chem. 2004;279:10710–10719. doi: 10.1074/jbc.M308434200. [DOI] [PubMed] [Google Scholar]
- 18.He X, Ni Y, Wang Y, Romigh T, Eng C. Naturally occurring germline and tumor-associated mutations within the ATP-binding motifs of PTEN lead to oxidative damage of DNA associated with decreased nuclear p53. Hum Mol Genet. 2011;20:80–89. doi: 10.1093/hmg/ddq434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lobo GP, Waite KA, Planchon SM, Romigh T, Nassif NT, Eng C. Germline and somatic cancer-associated mutations in the ATP-binding motifs of PTEN influence its subcellular localization and tumor suppressive function. Hum Mol Genet. 2009;18:2851–2862. doi: 10.1093/hmg/ddp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trotman LC, Wang X, Alimonti A, Chen Z, Teruya-Feldstein J, Yang H, et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128:141–156. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klimaschewski L. Ubiquitin-dependent proteolysis in neurons. News Physiol Sci. 2003;18:29–33. doi: 10.1152/nips.01408.2002. [DOI] [PubMed] [Google Scholar]
- 22.Myers MP, Stolarov JP, Eng C, Li J, Wang SI, Wigler MH, et al. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci U S A. 1997;94:9052–9057. doi: 10.1073/pnas.94.17.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waite KA, Eng C. Protean PTEN: form and function. Am J Hum Genet. 2002;70:829–844. doi: 10.1086/340026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondapaka S, Singh S, Dasmahapatra G, Sausville E, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Molecular Cancer Therapeutics. 2003;2:1093–1103. [PubMed] [Google Scholar]
- 25.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 26.Kane RC, Dagher R, Farrell A, Ko CW, Sridhara R, Justice R, et al. Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res. 2007;13:5291–5294. doi: 10.1158/1078-0432.CCR-07-0871. [DOI] [PubMed] [Google Scholar]
- 27.Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, et al. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Escudero I, Oliver MD, Andres-Pons A, Molina M, Cid VJ, Pulido R. A comprehensive functional analysis of PTEN mutations: implications in tumor- and autism-related syndromes. Hum Mol Genet. 2011;20:4132–4142. doi: 10.1093/hmg/ddr337. [DOI] [PubMed] [Google Scholar]
- 29.von Mikecz A. The nuclear ubiquitin-proteasome system. J Cell Sci. 2006;119:1977–1984. doi: 10.1242/jcs.03008. [DOI] [PubMed] [Google Scholar]
- 30.Floyd ZE, Trausch-Azar JS, Reinstein E, Ciechanover A, Schwartz AL. The nuclear ubiquitin-proteasome system degrades MyoD. J Biol Chem. 2001;276:22468–22475. doi: 10.1074/jbc.M009388200. [DOI] [PubMed] [Google Scholar]
- 31.Ogiso Y, Tomida A, Lei S, Omura S, Tsuruo T. Proteasome inhibition circumvents solid tumor resistance to topoisomerase II-directed drugs. Cancer Res. 2000;60:2429–2434. [PubMed] [Google Scholar]
- 32.Alimonti A, Carracedo A, Clohessy JG, Trotman LC, Nardella C, Egia A, et al. Subtle variations in Pten dose determine cancer susceptibility. Nat Genet. 2010;42:454–458. doi: 10.1038/ng.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song MS, Carracedo A, Salmena L, Song SJ, Egia A, Malumbres M, et al. Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell. 2011;144:187–199. doi: 10.1016/j.cell.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pezzolesi MG, Platzer P, Waite KA, Eng C. Differential expression of PTEN-targeting microRNAs miR-19a and miR-21 in Cowden syndrome. Am J Hum Genet. 2008;82:1141–1149. doi: 10.1016/j.ajhg.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arlt A, Bauer I, Schafmayer C, Tepel J, Muerkoster SS, Brosch M, et al. Increased proteasome subunit protein expression and proteasome activity in colon cancer relate to an enhanced activation of nuclear factor E2-related factor 2 (Nrf2) oncogene. 2009;28:3983–3996. doi: 10.1038/onc.2009.264. [DOI] [PubMed] [Google Scholar]
- 36.Dai C, Dai S, Cao J. Proteotoxic stress of cancer: implication of the heat-shock response in oncogenesis. J Cell Physiol. 2012;227:2982–2987. doi: 10.1002/jcp.24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones MD, Liu JC, Barthel TK, Hussain S, Lovria E, Cheng D, et al. A proteasome inhibitor, bortezomib, inhibits breast cancer growth and reduces osteolysis by downregulating metastatic genes. Clin Cancer Res. 2010;16:4978–4989. doi: 10.1158/1078-0432.CCR-09-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agyin JK, Santhamma B, Nair HB, Roy SS, Tekmal RR. BU-32: a novel proteasome inhibitor for breast cancer. Breast Cancer Res. 2009;11:R74. doi: 10.1186/bcr2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.