Abstract
Experimental and clinical evidence suggests that long chain n-3 fatty acids may protect against sudden cardiac death, the leading cause of mortality in hemodialysis patients. Here we investigated whether long chain n-3 fatty acids have a protective relationship with sudden cardiac death in 100 patients who died of sudden cardiac death during the first year of starting hemodialysis and 300 patients who survived. Individuals were selected from a nationally representative cohort of over 1000 U.S. hemodialysis units in 2004–2005. The odds of sudden cardiac death were calculated by quartile of long chain n-3 fatty acids levels over the first year. There was a significant inverse relationship between long chain n-3 fatty acids and the risk of sudden cardiac death even after adjusting for relevant co-morbid conditions, biochemical values, and dietary fats. The odds of sudden cardiac death at 1 year for the second, third, and fourth quartile groups of long chain n-3 fatty acids were 0.37, 0.22, and 0.20, respectively, compared to the lowest quartile. This significant inverse relationship was maintained even during the highest-risk first few months on hemodialysis. Thus, long chain n-3 fatty acids are strongly and independently associated with a lower risk of sudden cardiac death in hemodialysis patients throughout the first year of hemodialysis.
Keywords: sudden cardiac death, hemodialysis, omega-3, n-3, fatty acids
INTRODUCTION
Of the growing number of individuals initiated on dialysis annually in the United States, 94% choose hemodialysis as their initial mode of therapy. The five-year survival rate on hemodialysis is a dismaying 35%1, with the risk of death being highest in the first few months after starting treatment2. The single most common cause of mortality in hemodialysis patients is sudden cardiac death, which accounts for approximately one out of every four deaths.1, 3–5. With an annual incidence of 6–7%, the risk of sudden cardiac death in hemodialysis patients even exceeds that for high-risk diseases like heart failure6. Unfortunately, no interventions have been identified that clearly prevent hemodialysis-associated sudden cardiac death, including mainstay therapies like beta blockers and implantable defibrillators.
Long chain n-3 fatty acids have been demonstrated in experimental and clinical models to protect against malignant ventricular arrhythmias. They have also been shown to reduce the risk of sudden cardiac death in some but not all primary and secondary prevention trials in non-dialysis populations7, 8. We and others have reported that U.S. hemodialysis patients consume insufficient dietary long chain n-3 fatty acids and consequently have low blood levels9–11, and that such levels are inversely related to total mortality12–14. Yet no studies have examined the relationship between long chain n-3 fatty acid blood levels and sudden cardiac death in hemodialysis patients during or after the early high-risk period.
We therefore used a frequency matched case-control design to test the hypothesis that serum phospholipid long chain n-3 fatty acids levels were inversely related to the risk of sudden cardiac death during the first year after initiating hemodialysis. We performed the study in a large contemporary cohort that was representative of the general U.S. hemodialysis population.
RESULTS
Table 1 describes the characteristics at time of initiating hemodialysis of the 100 patients who died of sudden cardiac death during the first year of treatment and their 300 frequency matched controls. The patients who died of sudden cardiac death were more likely to have a history of coronary artery disease (19.0 vs 9.0%, P=0.007), congestive heart failure (19.0 vs 10.3%, P=0.023), and stroke (7.0 vs 2.7%, P=0.048), but less likely to have hypertension (25.0 vs 41.3%, P=0.003). They also had comparatively lower serum albumin (3.38 vs 3.54g/dL, P=0.007), creatinine (5.47 vs 6.24mg/dL, P=0.005), and phosphorus levels (4.19 vs 4.68mg/dL, P=0.007), and were far more likely to have started hemodialysis using a catheter than an arteriovenous fistula or graft (73.4 vs 48.6%, P<0.001).
Table 1.
Baseline Characteristics*
| Cases (n=100) | Controls (n=300) | P-value | |
|---|---|---|---|
|
| |||
| Age (years) | 66.6 (14.3) | 66.3 (14.1) | 0.84 |
|
| |||
| Male, n (%) | 58 (58) | 174 (58) | > 0.99 |
|
| |||
| Race, n (%) | > 0.99 | ||
| Black | 31(31.0) | 93 (31.0) | |
| White | 68 (68.0) | 204 (68.0) | |
| Other | 1 (1.0) | 3 (1.0) | |
|
| |||
| Ethnicity, n (%) | 0.08 | ||
| Non-Hispanic | 93 (93.0) | 259 (86.0) | |
| Hispanic | 7 (7.0) | 41 (14.0) | |
|
| |||
| Body Mass Index (kg/m2) | 25.5 (6.7) | 26.4 (6.6) | 0.21 |
|
| |||
| Cause of End-Stage Renal Disease, n (%) | 0.20 | ||
| Diabetes | 52 (52.0) | 134 (45.0) | |
| Other | 48 (48.0) | 166 (55.0) | |
|
| |||
| Initial Type of Vascular Access, n (%) | < 0.001 | ||
| First access fistula | 19 (20.2) | 101 (34.6) | |
| First access graft | 6 (6.4) | 49 (16.8) | |
| First access catheter | 69 (73.4) | 142 (48.6) | |
|
| |||
| Comorbidities, n (%) | |||
| Hypertension | 25 (25.0) | 124 (41.3) | 0.003 |
| Coronary Artery Disease/Myocardial | 19 (19.0) | 27 (9.0) | 0.007 |
| Infarction | |||
| Peripheral Vascular Disease | 6 (6.0) | 14 (4.7) | 0.60 |
| Congestive Heart Failure | 19 (19.0) | 31 (10.3) | 0.023 |
| Atrial Fibrillation | 4 (4.0) | 9 (3.0) | 0.63 |
| Anemia | 34 (34.0) | 85 (28.3) | 0.28 |
| Stroke | 7 (7.0) | 8 (2.7) | 0.048 |
|
| |||
| Systolic BP (mmHg) | 139 (27) | 144 (22) | 0.07 |
| Diastolic BP (mmHg) | 72 (14) | 73 (12) | 0.62 |
|
| |||
| Medications, n (%) | |||
| ACE inhibitor or ARB | 36 (36.0) | 133 (44.3) | 0.14 |
| Beta Blocker | 59 (59.0) | 184 (61.3) | 0.68 |
| Aspirin | 35 (35.0) | 94 (31.3) | 0.50 |
| Statin | 30 (30.0) | 129 (43.0) | 0.021 |
|
| |||
| Pre-Dialysis Laboratory Values† | |||
| Serum Albumin (g/dL) | 3.38 (0.5) | 3.54 (0.5) | 0.007 |
| Serum Cholesterol (mg/dL) | 147.20 (52.3) | 151.80 (43.6) | 0.56 |
| Serum Creatinine (mg/dL) | 5.47 (2.1) | 6.24 (2.7) | 0.005 |
| Serum Ferritin (ng/ml) | 426.90 (825.0) | 294.50 (469.9) | 0.14 |
| Serum Phosphorus (mg/dL) | 4.19 (1.5) | 4.68 (1.6) | 0.007 |
| Serum Potassium (mEq/L) | 4.27 (0.5) | 4.36 (0.6) | 0.18 |
| Serum Calcium (mg/dL) | 8.40 (0.9) | 8.46 (0.8) | 0.56 |
| Serum PTH (pg/mL) | 270.30 (241.5) | 260.90 (316.1) | 0.78 |
| Serum Alkaline Phosphatase (units/L) | 97.6(45.8) | 101.2(77.8) | 0.68 |
| Serum Glucose (mg/dL) | 154.60 (87.1) | 159.10 (75.1) | 0.68 |
| Hemoglobin (g/dL) | 10.27 (1.5) | 10.26 (1.3) | 0.95 |
| White Blood Cells (x 103/μL) | 8.72 (3.8) | 8.33 (3.1) | 0.37 |
|
| |||
| Standardized Kt/V | 1.27 (1.2) | 1.19 (0.4) | 0.30 |
mean ± standard deviation unless otherwise indicated
To convert the values for albumin to grams per liter, multiply by 10. To convert the values for cholesterol to mmol per liter, multiply by 0.02586. To convert the values for creatinine to μmol per liter, multiply by 88.4. To convert the values for ferritin to μg per liter, multiply by 1. To convert the values for phosphorus to mmol per liter, multiply by 0.3229. To convert the values for potassium to mmol per liter, multiply by 1. To convert the values for calcium to mmol per liter, multiply by 0.2495. To convert the values for PTH to ng per liter, multiply by 1. To convert the values for glucose to mmol per liter, multiply by 0.05551. To convert the values for hemoglobin to g per liter, multiply by 10. To convert the values for white blood cell count to x109 per liter, multiply by 1.
Baseline serum phospholipid fatty acid levels in the case and control groups are shown in Table 2. The patients dying of sudden cardiac death had greater median amounts of the most abundant saturated and monounsaturated serum fatty acids (i.e. palmitic [16:0], oleic [18:1n-9]) but less polyunsaturated fats such as arachidonic acid [20:4n-6] and docosahexaenoic acid [DHA, 22:6n-3]. Long chain n-3 fatty acids were significantly lower in cases than controls (median (1st,3rd quartile) 3.2 (2.6,4.0) versus 4.0 (3.3,4.7), P<0.001).
Table 2.
Baseline Serum Phospholipid Fatty Acid Levels*
| Fatty Acid | Cases (n=100) | Controls (n=300) | P-value† |
|---|---|---|---|
| Saturated | |||
| 16:00 | 23.0 (21.6,24.5) | 22.5 (21.0,23.7) | 0.008 |
| 18:00 | 17.3 (15.9,19.0) | 17.7 (15.8,19.3) | 0.47 |
| Monounsaturated | |||
| 18:1n9 | 16.5 (14.5,19.6) | 15.1 (13.2,17.4) | <0.001 |
| Polyunsaturated | |||
| OMEGA-6 | |||
| 18:2n6 | 18.1 (16.9,20.9) | 18.8 (16.6,20.5) | 0.90 |
| 20:4n6 | 9.3 (8.1,11.1) | 10.7 (9.1,12.5) | <0.001 |
| 22:4n6 | 0.5 (0.4,0.6) | 0.5 (0.4,0.6) | 0.98 |
| 22:5n6 | 0.4 (0.3,0.5) | 0.4 (0.3,0.5) | 0.65 |
| OMEGA-3 | |||
| 18:3n3 | 0.3 (0.3,0.5) | 0.4 (0.3,0.5) | 0.44 |
| 20:5n3 | 0.3 (0.3,0.4) | 0.3 (0.3,0.4) | 0.22 |
| 22:5n3 | 0.7 (0.6,0.8) | 0.8 (0.7,0.9) | <0.001 |
| 22:6n3 | 2.3 (1.7,2.9) | 2.9 (2.3,3.5) | <0.001 |
| Long Chain n-3 | 3.2 (2.6,4.0) | 4.0 (3.3,4.7) | <0.001 |
| Long Chain n-6 | 10.2 (8.9,12.1) | 11.7 (10.0,13.5) | <0.001 |
Reported as median (1st quartile, 3rd quartile) of weight %
Wilcoxon rank sum
The odds of sudden cardiac death by phospholipid n-3 fatty acids levels over the first year of hemodialysis is reported in Table 3 and were significantly and inversely related to the amount of serum long chain n-3 fatty acids. After adjusting for relevant factors and potential confounders, the relationship remained relatively unchanged and significant (P<0.001), with the highest quartile having an 80% lower odds of sudden cardiac death compared to the lowest quartile. Further adjustment for long chain n-6 fatty acids did not weaken the relationship. Results were not qualitatively different when long chain n-3 fatty acids were treated as a continuous variable (data not shown). When long chain n-3 fatty acids were examined separately, 22:5n-3 and 22:6n-3, but not 20:5n-3, were found to have significant inverse relationships with the risk of sudden cardiac death (data not shown). Of note, the rate of hyperkalemia (defined as greater than 6.0 mEq/L) during the one year study period was similar between groups (cases- 5.0%, controls 5.3%, P=0.90).
Table 3.
Odds Ratio for Sudden Cardiac Death According to Baseline Long Chain Serum Phospholipid n-3 Fatty Acids
| Variable | Quartiles of Polar Long Chain n-3 Fatty Acids | P-value for Quartile* | |||
|---|---|---|---|---|---|
|
| |||||
| 1 | 2 | 3 | 4 | ||
|
| |||||
| Fatty Acid level (%) | |||||
| N | 100 | 100 | 100 | 100 | |
| Mean | 2.53 | 3.45 | 4.15 | 5.83 | |
| Range | (1.27, 3.07) | (3.08, 3.82) | (3.82, 4.50) | (4.51, 15.11) | |
|
| |||||
| Model 1; OR (95% CI) |
1.0 -- |
0.43 (0.24, 0.78) | 0.22 (0.11, 0.42) | 0.20 (0.10, 0.40) | < 0.001 |
|
| |||||
| Model 2; OR (95% CI) |
1.0 -- |
0.39 (0.20, 0.76) | 0.24 (0.12, 0.48) | 0.22 (0.10, 0.47) | < 0.001 |
|
| |||||
| Model 3; OR (95% CI) |
1.0 -- |
0.37 (0.19, 0.75) | 0.21 (0.10, 0.45) | 0.20 (0.09, 0.46) | < 0.001 |
|
| |||||
| Model 4; OR (95% CI) |
1.0 -- |
0.37 (0.17, 0.79) | 0.22 (0.09, 0.51) | 0.20 (0.08, 0.51) | 0.001 |
Model 1: Unadjusted
Model 2: Adjusted for cause of end-stage renal disease (diabetes mellitus or other cause), history of coronary artery disease/myocardial infarctions, congestive heart failure, atrial fibrillation, baseline systolic blood pressure, use of beta blockers, and initial type of vascular access
Model 3: Adjusted for Model 2 and pre-dialysis serum creatinine, serum albumin, and serum potassium levels
Model 4: Adjusted for Model 3 and serum long chain n-6 fatty acids
Testing the overall difference between the four groups
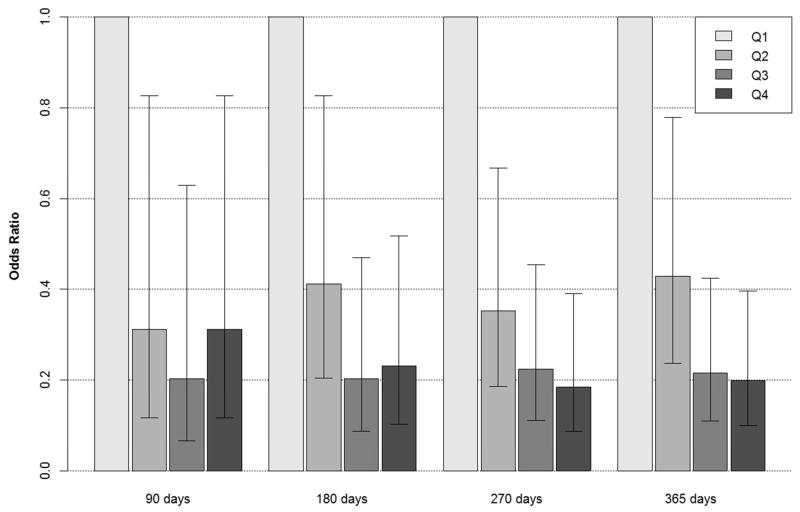
Compared to the lowest quartile of long chain n-3 fatty acids, the odds of sudden cardiac death were significantly lower for individuals in all other quartiles at days 90, 180, 270, and 365 (Figure 1).
Figure 1. Odds of Sudden Cardiac Death for Each Quarter of the First Year on Hemodialysis by Quartile of Long Chain Phospholipid n-3 Fatty Acids.
At 90 days the odds for the second, third, and fourth quartiles (compared to the first and lowest quartile) are 0.31 (95% confidence interval: 0.12–0.83), 0.20 (0.07–0.63), and 0.31 (0.12–0.83), respectively. At 180 days the odds are 0.41 (0.21–0.83), 0.20 (0.09–0.47), and 0.23 (0.10–0.51), respectively. At 270 days the odds are 0.35 (0.19–0.67), 0.22 (0.11–0.45), and 0.19 (0.09–0.39), respectively. At 365 days the odds are 0.43 (0.24–0.78), 0.22 (0.11–0.42), and 0.20 (0.10–0.40), while the odds for the fourth compared to second quartile are 0.46 (0.23–0.95).
DISCUSSION
This case-control study performed in a nationally representative cohort of patients initiating hemodialysis found that serum phospholipid long-chain n-3 fatty acids were inversely associated with sudden cardiac death events during the first year of dialysis, including during the highest-risk first few months. This relationship held true even after controlling for factors that may or do influence the risk for sudden cardiac death in hemodialysis patients. Adjusting for dietary fatty acids did not appreciably influence our findings, suggesting that the effects of long chain n-3 fatty acids are independent of consumption of other dietary fats.
The exceptional vulnerability of hemodialysis patients to sudden cardiac death is well described but not fully understood3–5, 15. In contrast to the non-dialysis population, where sudden cardiac death is most commonly ascribed to acute myocardial ischemic events, in hemodialysis patients diffuse cardiac fibrosis and diastolic dysfunction, autonomic instability and sympathetic overactivity, deranged mineral metabolism and vascular calcification, systemic inflammation, and treatment-associated myocardial stunning and rapid fluid and serum electrolyte shifts predominate as the most likely alternative culprits16. Long chain n-3 fatty acids have been reported to ameliorate many of these unique dialysis-related conditions through a variety of mechanisms8, 17–20.
Roughly half of the major randomized trials testing the effects of long chain n-3 fatty acids supplementation on cardiovascular and mortality endpoints have reported positive results21–24, while a major limitation of many of the negative clinical trials was inadequate statistical power due to low event rates, presumably from the effective use of risk-reducing medications like statins and beta blockers8. In contrast, no therapy in the hemodialysis population has been demonstrated to reduce the risk of sudden cardiac death5, 25. Even the utility of implantable defibrillators in hemodialysis patients is controversial, and given the predominance of diastolic dysfunction and the risk for infections and hemodialysis access compromise, they are unlikely to be recommended routinely26, 27. The possibility that long chain n-3 fatty acids can be used to lower the risk of sudden death is therefore especially appealing. Long chain n-3 fatty acid blood levels are highly modifiable by dietary consumption of fish or fish oil supplements28, which are relatively inexpensive and generally well-tolerated. In addition, U.S. hemodialysis patients have low blood long chain n-3 fatty acid levels compared to Americans without kidney disease and even foreign dialysis patients29, 30. Because the putative cardioprotective effects of long chain n-3 fatty acids are maximal when dietary intake is lowest31, hemodialysis patients in the United States are an optimal population for supplementation studies.
There has been no published research into the relationship between long chain n-3 fatty acids and sudden cardiac death. However, the general consensus of three previous observational studies in incident and prevalent hemodialysis and peritoneal dialysis patients was that long chain n-3 fatty acids, assessed by either dietary surveys or blood levels, are inversely related to total mortality12–14. A randomized, controlled trial studying the effects of long chain n-3 fatty acids on cardiovascular events and death in 206 Danish hemodialysis patients found no improvement in the primary composite endpoint but did observe a reduction in myocardial infarctions30. Effects on sudden cardiac death were not evaluated. An important study limitation was the very high blood levels of long chain n-3 fatty acids—nearly double that in our study population--making it much harder to demonstrate cardiovascular risk reduction31. In contrast, a more recent randomized North American study of 201 hemodialysis patients with much lower baseline long chain n-3 fatty acids reported improvements in the secondary outcome of cardiovascular event-free survival32.
Our study has limitations that warrant attention. We used ICD-9 coding by patient providers to identify individuals who died of sudden cardiac death, and further excluded in-hospital events to minimize event misclassification. While ICD-9 codes for sudden cardiac death have a good sensitivity in hemodialysis patients33, the lack of eyewitness or autopsy confirmation of sudden cardiac death could have led to random misclassification. However, such a bias would have supported the null hypothesis. We also did not have information available on cardiac structure and function, dialysate bath composition, residual renal function, or intradialytic weight gain, variables postulated but not yet proven to play a role in the sudden cardiac death of hemodialysis patients. Though we measured long chain n-3 fatty acid levels at time of hemodialysis initiation, levels could have changed over time as dietary intake changed. Despite this, baseline levels still remained highly predictive of sudden cardiac death. Finally, due to the biologically grounded and hypothesis driven nature of our case-control study design, which focused specifically on patients who died of sudden cardiac death, we could not determine the association between long chain n-3 fatty acid levels and other important outcomes such as all-cause mortality or cardiovascular mortality. Such analyses can be performed in future studies.
The use of serum phospholipids as a surrogate for the phospholipid content of heart cells could possibly be considered a limitation. However, there is no evidence at present to suggest that phospholipids in the heart or peripheral blood cells are better predictive markers than those in serum. While our findings were limited to the first year on hemodialysis, other investigations suggest that long chain n-3 fatty acids have protective effects even after this period12, 14. Finally, our results demonstrate association and not causation. We cannot exclude the possibility that residual confounding from dietary or other factors existed despite having controlled for many of the most recognized and powerful prognostic factors.
The study has a number of strengths. It directly measured blood fatty acid content rather than relying on dietary surveys. The study cohort was contemporaneous and representative of the overall U.S. hemodialysis population. The analysis included the entire first year of hemodialysis, an important attribute in light of the relative lack of information available on outcomes and interventions during the critical first few months after starting hemodialysis. This early period is also associated with the highest risk of death2, which is why mortality rate reduction over the first year of dialysis has become the highest priority for professional nephrology organizations and dialysis companies34.
In summary, long chain n-3 fatty acids are strongly associated with a lower risk of sudden cardiac death in incident hemodialysis patients during the entire first year of dialysis. Because sudden cardiac death is the most common cause of mortality in such patients and lacks an effective treatment, and because hemodialysis patients are especially amenable to the putative cardioprotective benefits of long chain n-3 fatty acid supplementation due to their low dietary intake, there is a compelling need to confirm our findings in a randomized, controlled fashion.
METHODS
Study Population and Collection of Serum Samples
All study subjects participated in the Accelerated Mortality on Renal Replacement (ArMORR) project, a nationally representative prospective cohort study of patients who initiated chronic hemodialysis at any one of over 1000 US dialysis centers operated by Fresenius Medical Care, North America35, Between July 1, 2004, and June 30, 2005, 10,044 incident hemodialysis patients representing 1,056 US dialysis units were prospectively enrolled into ArMORR. ArMORR contains detailed demographic and clinical data, including comorbid conditions, laboratory results, and blood samples at time of dialysis initiation. All clinical data were collected prospectively and entered uniformly into a central database by the patients’ practitioners at the point-of-care based on medical records and physical examination. All clinical data points arriving at Fresenius underwent rigorous quality assurance/quality control (QA/QC) auditing because these data are directly linked to Medicare billing services, and routine QA/QC measures are mandated by the Clinical Quality Group and Data Entry Error Reduction Task Force at Fresenius. Blood samples collected for clinical care were drawn at individual dialysis units and shipped the same day on ice to Spectra East (Rockland, NJ, USA), a good clinical practice (GCP)-accredited central laboratory. Study subjects’ blood samples were individually labeled with a yellow sticker, thus notifying technicians of the need to handle these samples especially expeditiously. Samples were not sent from Friday or Saturday dialysis sessions to ensure that all samples were processed immediately. If samples were not received on ice or contained insufficient quantities of blood, they were not included in the ArMORR study. After blood was extracted for clinical testing, the remaining serum was immediately placed in new tubes and frozen at −80°C. They were then sent in batches on dry ice to the ArMORR investigators where the samples were aliquotted and immediately stored at −80°C (and subsequently liquid nitrogen). Because many aliquots were available for each study subject, the need to expose samples to multiple freeze/thaw cycles was subsequently avoided. This study was approved by the Institutional Review Board of the Massachusetts General Hospital, Boston, MA, which waived the need for informed consent.
Identification of Patients Dying of Sudden Cardiac Death and Controls
Sudden cardiac death events in ArMORR were defined as deaths occurring (1) out of hospital that were (2) identified by International Classification of Diseases (ICD)-9 diagnosis codes for “cardiac arrest” (ICD-9: 427.5) and “sudden death, cause unknown” (ICD-9: 798.1, 798.2, 798.9)36, The ICD-9 codes, frequently used to study sudden cardiac death in dialysis patients4, 37, 38, were documented by the subject’s health care providers at time of death on a dialysis unit mandatory discharge form. One hundred patients who died from sudden cardiac death within one year of initiating hemodialysis and who had sufficient amounts of stored sera were identified as cases on an a priori basis. Three hundred hemodialysis patients who survived the first year of dialysis (controls) were frequency matched by age, gender, and race in random fashion using SAS 9.3 software package (Cary, NC).
Laboratory Analysis
Biochemial assays were performed at Spectra East laboratory as previous described using standard assays. Serum was used to determine fatty acids in the phospholipid fraction in blinded fashion. Serum lipids were extracted with chloroform/methanol (2:1, vol/vol). Serum phospholipids were the primary measurement because they reflect cardiac membrane phospholipid content, an important mediator of cardiac electrophysiology, better than other serum fatty acid fractions39, Solid phase extraction was used to isolate the phospholipid fraction, with methanol being eluted via a silica cartridge (300 mg filling, Grace, Deerfield, IL)40, Phospholipids were then methylated directly with 10% BF3 in methanol and converted to fatty acid methyl esters (FAME). The resulting FAME were concentrated in isooctane (HPLC grade, Fisher Scientific, Pittsburg, PA) and analyzed by gas chromatography (GC) (HP 7890A series, autosampler 7693, GC ChemStation Rev. B.04.03, Agilent Technologies, Palo Alto, CA) with a DB-225 column (30 m, 0.25 mm i.d., 0.15 mm film thickness, Agilent Technologies, Palo Alto, CA) equipped with a flame ionization detector41, Sample peaks were identified by comparison to authentic FAME standards (Nu-Chek-Prep Inc., Elysian, MN). Results of FAME analysis were obtained by area percentage reports and reported in weight percent. Long chain n-3 fatty acids were defined as the area percent of eicosapentaenoic acid (20:5n-3) + docosapanetaenoic acid (22:5n-3) + docosahexaenoic acid (22:6n-3), and long chain n-6 fatty acids as the area percent of 20:4n-6+22:4n-6+22:5n-6.
Statistical Analysis
Based on preliminary data 100 cases and 300 controls were estimated to have 80% power with a 5% type I error to detect an odds ratio of 1.96 between serum phospholipid long chain n-3 fatty acids above versus below the median. Baseline characteristics were summarized by mean +/− standard deviation for continuous variables and frequency for categorical variables. Two-sample t or Chi-square tests were used for group comparisons as appropriate. Summary statistics for each fatty acid (expressed as percentage of total fatty acids) were compared between groups using nonparametric Wilcoxon rank sum test due to their skewed distribution.
To evaluate the association between baseline long chain n-3 fatty acids and sudden cardiac death, we first categorized the subjects into four groups by quartiles of long chain n-3 fatty acids. We analyzed the outcome of death status due to sudden cardiac death at 90, 180, 270, and 365 days after starting dialysis. Since this is a frequency matched case control study, we used the common odds ratio and logistic regression model for analysis (unlike matched case control design, where a conditional logistic regression model is more appropriate). We evaluated the odds ratio of SCD for each of the second, third, and fourth quartile group compared to the first quartile group and calculated the corresponding 95% confidence interval. We also perform logistic regression model with death status at 365 days as the response variables to evaluate the effect of long chain n-3 fatty acids on the risk of sudden cardiac death while adjusting for potential confounders and other important factors, including cause of end-stage renal disease (diabetes mellitus/other), history of coronary artery disease/myocardial infarction, congestive heart failure, or atrial fibrillation, baseline systolic blood pressure, use of beta blockers, initial type of vascular access, and pre-dialysis serum creatinine, serum albumin, and serum potassium levels. Age, gender and race were not included in the model since they were not significantly different between groups and had minimal effect on the coefficient estimates of long chain n-3 fatty acids. To test the sensitivity of the odds ratio estimate to the misspecification of confounder variables, we also ran models with long chain n-3 fatty acids alone and with other covariates. The odds ratio estimate and its confidence interval estimates were similar. We performed a secondary analysis to test confounding by other fatty acid classes. Each fatty acid group (saturated, monounsaturated, trans, long chain n-6) was entered separately into the model. Fatty acids that changed the coefficient estimate of long chain n-3 by more than 15% were included in model 4. C statistics for Models 1, 2, 3, and 4 were 0.67, 0.75, 0.78, and 0.78, respectively, indicating their reasonable predictive ability. All statistical tests were performed at a two-sided 5% significance level using SAS 9.3 software.
Acknowledgments
ANF was supported by funding from the National Institutes of Health (DK084403) and the National Kidney Foundation. RT is supported by funding from the National Institutes of Health (DK094872, HL112746).
Footnotes
DISCLOSURES
RT is a consultant to Fresenius Medical Care North America.
References
- 1.U.S. Renal Data System. USRDS 2012 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2012. [Google Scholar]
- 2.Chan KE, Maddux FW, Tolkoff-Rubin N, et al. Early outcomes among those initiating chronic dialysis in the United States. Clin J Am Soc Nephrol. 2011;6:2642–2649. doi: 10.2215/CJN.03680411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shastri S, Tangri N, Tighiouart H, et al. Predictors of sudden cardiac death: a competing risk approach in the hemodialysis study. Clin J Am Soc Nephrol. 2012;7:123–130. doi: 10.2215/CJN.06320611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parekh RS, Plantinga LC, Kao WH, et al. The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int. 2008;74:1335–42. doi: 10.1038/ki.2008.449. [DOI] [PubMed] [Google Scholar]
- 5.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 6.Passman R, Herzog CA. End-stage renal disease: sudden cardiac death: stratifying risk in dialysis patients. Nat Rev Nephrol. 2011;7:133–135. doi: 10.1038/nrneph.2010.166. [DOI] [PubMed] [Google Scholar]
- 7.London B, Albert C, Anderson ME, et al. Omega-3 fatty acids and cardiac arrhythmias: prior studies and recommendations for future research: a report from the National Heart, Lung, and Blood Institute and Office Of Dietary Supplements Omega-3 Fatty Acids and their Role in Cardiac Arrhythmogenesis Workshop. Circulation. 2007;116:e320–335. doi: 10.1161/CIRCULATIONAHA.107.712984. [DOI] [PubMed] [Google Scholar]
- 8.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 9.Friedman A, Moe S, Perkins S, et al. Fish consumption and omega-3 fatty acid status and determinants in long-term hemodialysis. Am J Kidney Dis. 2006;47:1064–1071. doi: 10.1053/j.ajkd.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 10.Saifullah A, Watkins BA, Saha C, et al. Oral fish oil supplementation raises blood omega-3 levels and lowers C-reactive protein in haemodialysis patients--a pilot study. Nephrol Dial Transplant. 2007;22:3561–3567. doi: 10.1093/ndt/gfm422. [DOI] [PubMed] [Google Scholar]
- 11.Madsen T, Christensen JH, Svensson M, et al. Marine n-3 polyunsaturated fatty acids in patients with end-stage renal failure and in subjects without kidney disease: a comparative study. J Ren Nutr. 2011;21:169–175. doi: 10.1053/j.jrn.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Friedman AN, Saha C, Watkins BA. Feasibility study of erythrocyte long-chain omega-3 polyunsaturated fatty acid content and mortality risk in hemodialysis patients. J Ren Nutr. 2008;18:509–512. doi: 10.1053/j.jrn.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kutner NG, Clow PW, Zhang R, et al. Association of fish intake and survival in a cohort of incident dialysis patients. Am J Kidney Dis. 2002;39:1018–1024. doi: 10.1053/ajkd.2002.32775. [DOI] [PubMed] [Google Scholar]
- 14.Noori N, Dukkipati R, Kovesdy CP, et al. Dietary omega-3 fatty acid, ratio of omega-6 to omega-3 intake, inflammation, and survival in long-term hemodialysis patients. Am J Kidney Dis. 2011;58:248–256. doi: 10.1053/j.ajkd.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. Renal Data System. USRDS 2011 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2010. [Google Scholar]
- 16.Pun PH, Middleton JP. Sudden Cardiac Death in Hemodialysis Patients: A Comprehensive Care Approach to Reduce Risk. Blood Purif. 2012;33:183–189. doi: 10.1159/000334154. [DOI] [PubMed] [Google Scholar]
- 17.Abedin M, Lim J, Tang TB, et al. N-3 fatty acids inhibit vascular calcification via the p38-mitogen-activated protein kinase and peroxisome proliferator-activated receptor-gamma pathways. Circ Res. 2006;98:727–729. doi: 10.1161/01.RES.0000216009.68958.e6. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Shearer GC, Chen Q, et al. Omega-3 fatty acids prevent pressure overload-induced cardiac fibrosis through activation of cyclic GMP/protein kinase G signaling in cardiac fibroblasts. Circulation. 2011;123:584–593. doi: 10.1161/CIRCULATIONAHA.110.971853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh DY, Talukdar S, Bae EJ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer R, Dechend R, Qadri F, et al. Dietary n-3 polyunsaturated fatty acids and direct renin inhibition improve electrical remodeling in a model of high human renin hypertension. Hypertension. 2008;51:540–546. doi: 10.1161/HYPERTENSIONAHA.107.103143. [DOI] [PubMed] [Google Scholar]
- 21.Burr ML, Fehily AM, Gilbert JF, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 22.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 23.Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 24.Investigators G-H. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 25.Tangri N, Shastri S, Tighiouart H, et al. Beta-Blockers for prevention of sudden cardiac death in patients on hemodialysis: a propensity score analysis of the HEMO Study. Am J Kidney Dis. 2011;58:939–945. doi: 10.1053/j.ajkd.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Charytan DM, Patrick AR, Liu J, et al. Trends in the use and outcomes of implantable cardioverter-defibrillators in patients undergoing dialysis in the United States. Am J Kidney Dis. 2011;58:409–417. doi: 10.1053/j.ajkd.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 27.Dasgupta A, Montalvo J, Medendorp S, et al. Increased complication rates of cardiac rhythm management devices in ESRD patients. Am J Kidney Dis. 2007;49:656–663. doi: 10.1053/j.ajkd.2007.02.272. [DOI] [PubMed] [Google Scholar]
- 28.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47:348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Wu JH, Lemaitre RN, King IB, et al. Association of plasma phospholipid long-chain omega-3 Fatty acids with incident atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2012;125:1084–1093. doi: 10.1161/CIRCULATIONAHA.111.062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svensson M, Schmidt E, Jorgensen K, et al. N-3 fatty acids as secondary prevention against cardiovascular events in patients who undergo chronic hemodialysis: A randomized, placebo-controlled intervention trial. Clin J Am Soc Nephrol. 2006;1:780–786. doi: 10.2215/CJN.00630206. [DOI] [PubMed] [Google Scholar]
- 31.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 32.Lok C, Moist L, Hemmelgarn B, et al. Effect of fish oil supplementation on graft patency and cardiovascular events among patients with new synthetic arteriovenous hemodialysis grafts. JAMA. 2012;307:1809–1816. doi: 10.1001/jama.2012.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pun PH, Herzog CA, Middleton JP. Improving ascertainment of sudden cardiac death in patients with end stage renal disease. Clin J Am Soc Nephrol. 2012;7:116–122. doi: 10.2215/CJN.02820311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. [Accessed October 19, 2012];Performance Excellence and Accountability in Kidney Care. http://www.kidneycarequality.com/AboutPeak.htm.
- 35.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 36.Services DoHaH. 9th. rev., clinical modification: IDC-9-CM. Washington, D.C: 1980. The international classification of diseases. [Google Scholar]
- 37.Zheng ZJ, Croft JB, Giles WH, et al. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 38.Herzog CA, Li S, Weinhandl ED, et al. Survival of dialysis patients after cardiac arrest and the impact of implantable cardioverter defibrillators. Kidney Int. 2005;68:818–825. doi: 10.1111/j.1523-1755.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- 39.Harris W, Schacky Cv. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Seifert MF, Lim SY, et al. Bone mineral content is positively correlated to n-3 fatty acids in the femur of growing rats. Br J Nutr. 2010;104:674–685. doi: 10.1017/S0007114510001133. [DOI] [PubMed] [Google Scholar]
- 41.Watkins BA, Li Y, Allen KG, et al. Dietary ratio of (n-6)/(n-3) polyunsaturated fatty acids alters the fatty acid composition of bone compartments and biomarkers of bone formation in rats. J Nutr. 2000;130:2274–2284. doi: 10.1093/jn/130.9.2274. [DOI] [PubMed] [Google Scholar]