Abstract
Malignant and atypical meningiomas are resistant to standard therapies and associated with poor prognosis. Despite progress in the treatment of other tumors with therapeutic vaccines, this approach has not been tested preclinically or clinically in these tumors. Spontaneous canine meningioma is a clinically meaningful but underutilized model for preclinical testing of novel strategies for aggressive human meningioma. We treated 11 meningioma-bearing dogs with surgery and vaccine immunotherapy consisting of autologous tumor cell lysate combined with toll-like receptor ligands. Therapy was well tolerated, and only one dog had tumor growth that required intervention, with a mean follow up of 585 days. Interferon gamma elaborating T cells were detected in the peripheral blood of two cases, but vaccine-induced tumor-reactive antibody responses developed in all dogs. Antibody responses were polyclonal, recognizing both intracellular and cell surface antigens, and heat shock protein 60 was identified as one common antigen. Tumor-reactive antibodies bound allogeneic canine and human meningiomas, demonstrating common antigens across breed and species. Histological analysis revealed robust infiltration of antibody-secreting plasma cells into the brain around the tumor in post-treatment compared to pre-treatment samples. Tumor-reactive antibodies were capable of inducing antibody dependent cell-mediated cytotoxicity to autologous and allogeneic tumor cells. These data demonstrate the feasibility and immunologic efficacy of vaccine immunotherapy for a large animal model of human meningioma and warrant further development towards human trials.
Keywords: IM01 Animal/transgenic models for tumor immunobiology, CL07 Cancer Vaccines
Introduction
Meningioma is the most common primary brain neoplasm, with over 100,000 patients diagnosed in the United States between 2004 and 2008 (1). Newly diagnosed tumors are managed by surgical resection alone. Roughly 6,000 patients will need additional treatment in the United States every year due to recurrence (2), which often occurs with invasive or malignant disease (3, 4). Current salvage approaches include re-operation, radiation, radiosurgery and chemotherapy; there is controversy regarding the perceived clinical benefit of these interventions (5–8). The three-year recurrence rate in re-operated World Health Organization (WHO) grade I meningioma is 50% (9), with risk of recurrence even greater in grade II and III tumors (10–12). Radiation, while modestly effective in benign disease, has been associated with cognitive deficits, secondary malignancies, and the transformation of the tumor to a higher-grade neoplasm (2).
One challenge in treating aggressive meningiomas is the paucity of animal models with which to test combinations of surgery and systemic therapy in a meaningful manner. Spontaneous meningiomas in dogs make up 45% of primary canine brain tumors (13) and are an under-utilized resource for preclinical studies. Canine and human meningiomas share many features, including histological resemblance, overexpression of growth factor receptors, deletion of chromosomal segments, and losses of function in tumor suppressor genes (14–16). Over 40% of canine meningiomas were atypical or malignant in one study (17). Dogs develop many other cancers that are prevalent in humans, and tumors progress 5–7-fold faster in dogs relative to humans (18). We hypothesized that preclinical studies in canine meningioma would enable accurate and rapid testing of immunotherapy for aggressive meningioma.
Cancer vaccines have been tested in multiple malignancies, including gliomas, with evidence of clinical activity (19, 20). A meta analysis covering over 100 clinical trials revealed that response rates to vaccination with peptides containing defined T cell epitopes were less than half of that achieved with whole cell-based vaccines (21). The higher response rate achieved with whole cell vaccines (as used in this study) could be due to greater antigenic coverage or the potential to induce tumor-binding antibodies. Meningiomas are not subject to the same principles of immune privilege as cells within the brain parenchyma such as gliomas (22). Relative to gliomas, meningiomas may be better candidates for immunotherapy because they: (1) are not insulated by the endothelial tight junctions that limit large molecule (e.g. antibody) diffusion; (2) are in direct contact with cerebrospinal fluid that drains to the venous circulation and cervical lymph nodes for antigen presentation to T and B lymphocytes; (3) lack the T cell trafficking checkpoints present in the Virchow-Robin space (e.g., glia limitans); and (4) are relatively slow growing tumors that may be more susceptible to adaptive immune responses. However, invasive, atypical, and malignant meningiomas can infiltrate the brain parenchyma, requiring penetration of antibodies and/or lymphocytes for control of post-surgical, microscopic disease.
Tumor cell lysates mixed with synthetic toll-like receptor (TLR) ligands function as effective vaccines to induce anti-tumor immune responses in glioma-bearing animals (23, 24). The mechanisms of lysate/TLR ligand vaccines have been thoroughly characterized and tested in many cancer patients; however, the activity of this type of vaccine against meningioma is unknown. TLR activation on antigen presenting cells facilitates the induction of adaptive immune responses by enhancing antigen presentation, expression of co-stimulatory molecules, cytokine production, and homing to secondary lymphoid organs. The TLR 9 ligand CpG olidgodeoxynucleotide induced clinical responses in select melanoma and renal cell carcinoma patients (25, 26). The FDA-approved TLR 7 ligand imiquimod exhibited adjuvant activity in cancer clinical trials (20, 27), with excellent efficacy as a single topical agent against skin cancers (28).
We conducted a vaccine immunotherapy trial for pet dogs with symptomatic, spontaneous meningiomas. Dogs underwent surgery followed by vaccination with autologous tumor cell lysate that was combined with imiquimod or CpG. Herein we report safety, robust extension of survival, homology among antibody epitopes between dogs and humans, and vaccine-induced, local antibody production in the brain. Vaccination for canine meningioma reveals promising avenues for further development towards human trials.
Materials and Methods
Surgery, Vaccination Production, and Administration
Dogs were enrolled after obtaining owner consent according to an approved protocol from the University of Minnesota IACUC. A presumptive diagnosis of meningioma was based on the MRI characteristics of a solitary extra-axial mass in the brain, with heterogeneous T1W signal, usually isointense, homogenous T2W signal, sharply defined borders, homogenous contrast enhancement, evidence of a dural tail, that may have associated cysts, peritumoral edema and falx-shift. Surgical resection was performed under general anesthesia using the appropriate approach based on tumor location. Dogs were hospitalized with supportive care for 1–2 days after surgery. Corticosteroids used to minimize peritumoral edema were discontinued by 48 hours before vaccination. Part of the tumor specimen was used for histological diagnosis, and the remainder of the tumor was cultured for vaccine production.
Cultures were established by mincing specimens with scalpels and digestion at 37 °C for 15 minutes in suspension with TrypLE™ Express (Invitrogen/Life Technologies). Cell suspensions were filtered through a 100 μm filter, washed with PBS, and placed in culture in 10 cm culture plates pre-coated with 1:10 Matrigel™ (BD Biosciences), in serum-free neural stem cell media consisting of DMEM:F12 (1:1), with L-glutamine, sodium bicarbonate, penicillin/streptomycin (100 U/mL), B27 and N2 supplements (Gibco), and 0.1-mg/mL Normocin™ (InVivoGen). Semi-weekly, cells were given 20 ng/mL rhEGF and rhFGF (R&D systems) and were cultured at 5% O2 and 5% CO2. Harvest for vaccination involved scraping cells from one 10 cm dish. Cells were washed thrice in PBS, and underwent five freeze-thaw alternations by transfer from warm water bath to liquid N2 followed by radiation (200 Gy). Protein was measured by Bradford assay with standard Coomassie reagent (Pierce), and lysates were stored at −80 °C. GMP-grade CpG 685 ODN was kindly provided by Dr. Wei Chen/SBI Biotechnology (Tokyo, Japan), and imiquimod cream was acquired through the University of Minnesota Boynton Health Services Pharmacy. In five dogs, CpG (2.0 mg) was mixed with thawed lysates immediately before intradermal injection at two sites in the back of the neck. Six other dogs received imiquimod cream (5%; 0.5 g) at two intradermal injection sites in the back of the neck 15 minutes prior to lysate injection. The maximally achievable lysate protein concentration was given to each dog, which varied by tumor volume and the ability of the tumor to proliferate in culture. Lysate doses ranged from 200 to 1,500 μg protein (average of 595 μg), and average dose did not vary significantly between CpG and imiquimod-treated dogs.
Tumor Volume Measurements
Twenty consecutive surgical human cases (of MAH) were analyzed for volume by MRI, as calculated by [Length × width × height]/2 for tumor and intracranial volume. The same procedure was carried out for the eleven dogs in this study and the results were compared as described in supplementary methods.
Western Immunoblot
Cultured tumor cells or homogenized flash-frozen tumors were used for blotting. Cells were lysed, protease and phosphatase inhibitors (Calbiochem, San Diego, CA) were added, and for SDS-PAGE, lysates were diluted in laemmli reducing sample buffer, heated, and centrifuged. Protein standards (BioRad, Hercules, CA) were loaded next to each 40 μg lysate and resolved on NuPAGE 4–12% Bis/Tris gels (Invitrogen). Proteins were transferred to nitrocellulose (Amersham) at 5 V constant voltage using semi-dry transfer (BioRad). The membranes were blocked in 5% NFDM/TTBS at room temperature for 1 hour and cut appropriately into identical blots, each with a molecular weight standard (Bio-Rad) run adjacent to lysate. Each membrane was incubated at room temperature for 1 hour in normal, pre- or post-vaccination sera diluted 1:1000 in 5% NFDM/TTBS, washed six times for 10 min each in TTBS, followed by room temperature for 1 hour in rabbit anti-canine IgG HRP-conjugated secondary antibody (Jackson ImmunoResearch) at 1:50,000 in 5% NFDM/TTBS. Bands were detected using ECL Western Blotting Detection System (Amersham) and HyBlot CL autoradiography film (Denville Scientific). Densitometry was performed by the Gel Analysis tool in ImageJ 1.45s software (NIH), and values were normalized by dividing by heavy and light chain densities (areas under the curve) from prevaccination lanes.
Detection of Tumor Cell Surface-Reactive Antibodies
A Becton Dickinson Custom Canto three-laser flow cytometer was used for data acquisition. Tumor cells were removed from Matrigel -coated 10 cm culture plates by scraping or BD™ Dispase (BD Biosciences), washed with PBS thrice, and incubated with heat-inactivated normal dog, prevaccination, or 3-month post-vaccination serum at 1:100 dilutions at 4° C for 30 minutes. Cells were then washed thrice in PBS and incubated with 1μg anti-canine IgG (H+L)—FITC (American Qualex) at 4° C for an additional 30 minutes before washing and analyzing.
Immunohistochemistry Staining and Quantification of Lymphocyte Infiltration
For lymphocyte analysis, 5μm tissue sections were cut, prepared with standard procedures, and the following antibodies were used: CD3 (AbD Serotec) at 1:2000, CD20 (Thermo Scientific) at 1:2000, and IgG (H+L; Jackson ImmunoResearch) at 1:2000. Tissue sections were incubated with the primary antibodies for 1 hour, rinsed, and a biotinylated secondary antibody was applied for 30 minutes. CD20 and IgG antibodies were followed with undiluted Rabbit Link (Covance), and a biotin conjugated donkey anti-rat IgG (H+L) (Jackson ImmunoResearch) at 1:500 was used with CD3. Sections were rinsed, incubated in hydrogen peroxidase, and a tertiary streptavidin horseradish peroxidase link (Covance) for 30 minutes. The immune complex was visualized using 3,3′-diaminobenzidine as the chromogen. Sections were lightly counterstained with hematoxylin, dehydrated, coverslipped, and scanned using the Aperio Scanscope XT.
All surgical resection specimens and all necropsy blocks containing tumor or inflammation as seen by H&E staining were included in counting of CD3+ and CD20+ cells. Samples were blinded, and all 10X fields in slides were captured and saved as image files. Automated cell quantification was conducted with a customized macro and the particle analysis tool in Fiji software (ImageJ 1.46j, NIH). Counts underwent statistical analysis as described in supplemental methods before unblinding.
Antibody-Dependent Cell-Mediated Cytotoxicity
Dog blood from healthy donors was collected in anti-coagulant tubes, lysed osmotically, and peripheral blood leukocytes (PBLs) were washed thrice with PBS. PBLs were resuspended in complete RPMI 1640 (supplemented with 10% heat-inactivated FBS, 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 0.1 mg/mL Normocin™ from InVivoGen) in a 96-well plate at 2.5 × 106 cells/mL, and stimulated 14 hours with 30 μM of the TLR7/8 ligand resiquimod before being washed and co-cultured with antibody-coated tumor cells. Primary meningioma cultures were coated with antibody as described in the cell surface binding procedure. Two washes were performed before addition to PBLs at an effector:target ratio of 25:1. Tumor cell lysis was determined by measurement of lactate dehydrogenase (LDH) activity as indicated by the manufacturer’s protocol after seven hours of co-culture (Roche Applied Science). Percent specific lysis of tumor cells was calculated by: [Sample − (tumor only + PBL only)]/(lysed tumor + PBL only) × 100.
Statistical Analysis
Samples were analyzed by unpaired t-test (tumor/brain volume, flow cytometry, LDH activity). Histological cell counts were analyzed by an unpaired t-test with Welch’s correction for unequal variances with 95% confidence intervals. Survival was analyzed using a logrank test with 95% confidence intervals. All statistics were conducted using GraphPad Prism version 4.0c for OS X (GraphPad Software, San Diego CA, www.graphpad.com).
Results
Canine Meningiomas Model Aggressive Human Disease
Meningiomas from dogs treated in our study share histological features with human tumors of the same subtype (Fig. 1A), in addition to features that signify poor survival in humans. Brain invasion is an independent negative prognostic factor that prompted the reclassification of otherwise WHO grade I meningiomas as grade II (29). Two canine cases exhibited gross tumor invasion into the brain at resection, and three additional cases showed brain invasion upon postmortem analysis (Fig. 1B, left, and Supplementary Table 1). Tumor invasion into the skull was also present in two cases (Fig. 1B, right). In addition, canine meningiomas occupied more than twice the volume of the brain relative to human tumors (Fig. 1C). All dogs were symptomatic at the time of diagnosis, and in ten dogs, the presenting clinical sign was seizures. The rapid recurrence and progression of this disease in canines is consistent with the common appearance of invasion into brain, which, in humans, predicts poor outcomes.
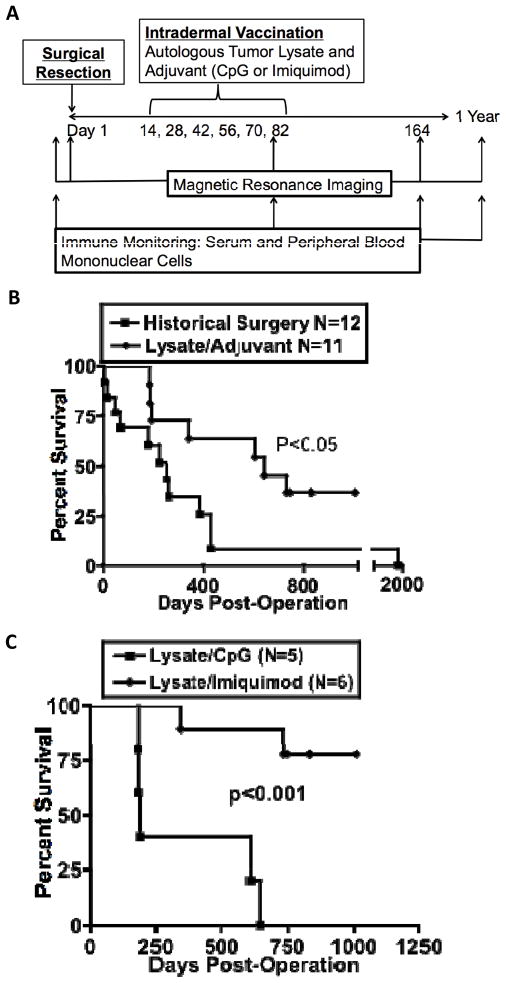
Figure 1.
Canine Meningiomas Model Aggressive Human Disease. (A) Meningioma surgery specimens stained with H&E, left to right, from cases 3,4, and 1. (B) Necropsy specimens from two dogs with tumors that, in humans, are categorized as WHO grade I on biopsy. (C) Tumor volume and brain volume of the eleven dogs treated with surgery plus immunotherapy and twenty consecutive intra-institutional human meningioma patients. *, P<0.05.
Extension of Survival Following Vaccine Immunotherapy
Eleven dogs underwent craniotomy for tumor removal after radiographic diagnosis of an intracranial neoplasm consistent with meningioma. Following histological confirmation, dogs were administered vaccinations biweekly with lysate derived from their tumors in combination with CpG or imiquimod (Fig. 2A). Mean follow-up time was 585 days, with 36% (4/11) of dogs alive (Supplementary Table 1; Fig. 2 B&C). Relative to historical controls, median survival is extended in the immunotherapy cohort (645 versus 222 days, p<0.05), with no censoring of dogs that died from other causes (Fig. 2B and Supplementary Table 2). Neither vaccination cohort contained a case of frank tumor progression, although more deaths occurred in the CpG cohort (Fig. 2C). One CpG-treated dog developed two meningiomas, of which the second was not identified before vaccination due to small size. The second tumor was subsequently removed after its growth caused breakthrough seizures, and four other vaccines were given using tumor lysate of the second tumor.
Figure 2.
Biweekly Tumor Lysate/Adjuvant Vaccination Schedule Results in Prolonged Survival of Spontaneous Canine Meningioma. (A) Study design and immune monitoring scheme. (B) Surgery controls were conducted at four institutions, and all cases were confirmed to be meningioma by histopathology. (C) Comparison of CpG and Imiquimod treatment groups.
Tumor-Reactive Antibodies Bind Cell Surface Antigens and Cross-React with Human Meningiomas
Immune responses before and after vaccination were measured in peripheral blood mononuclear cells (PBMCs) and sera. Interferon gamma elaborating tumor-reactive T cells were significantly increased in post-vaccination PBMCs in two of nine dogs tested, suggesting a low frequency of circulating T cells three months after surgery (Supplementary Table 3). In contrast, polyclonal tumor-reactive antibody responses to whole tumor lysate (Fig. 3A) and cell surface antigens (Fig 3B&C) were detected in all eleven dogs (Supplementary Table 3). Probing of allogeneic tumor cells and lysates indicated recognition of common tumor antigens among dogs, some of which were present on the cell surface (Fig. 4A–C). Post-vaccination antibodies recognized recombinant human HSP60 and HSP60 from autologous and allogeneic tumor (Fig. 4A), demonstrating the common expression and recognition of this antigen. Remarkably, post-vaccination canine serum recognized human meningioma tissue, indicating antigen conservation between species (Fig. 4D).
Figure 3.
Vaccination Induces Antibody Responses to Meningioma Surface Antigens. (A) Western immunoblot of autologous tumor cells from culture (cases 10 and 3) or snap-frozen tumor specimen (case 5). Normal dog serum (“ND”) was used as a control. (B) Live autologous tumor cells were stained with serum from prevaccination or postvaccination (at 82 days). (C) Aggregate data of three dogs from B. *, P<0.05; **, P<0.005.
Figure 4.
Antibodies Recognize Allogeneic Dog and Human Meningiomas. (A) Western immunoblot of tumor cells from three cases and recombinant human heat shock protein (HSP) 60. (B) Representative histograms of cell surface binding of primary tumor cells from case 7. (C) Quantification of serum antibody surface binding to an allogeneic papillary (non-study dog—recognized by case 3) and a meningothelial meningioma (case 7—recognized by cases 4,6,7, and 9). (D) Western immunoblot of a human meningioma probed with postvaccination serum from case 3. *, P<0.05; **, P<0.005.
Vaccination Induces T, B and Plasma Cell Infiltration into Peritumoral Brain
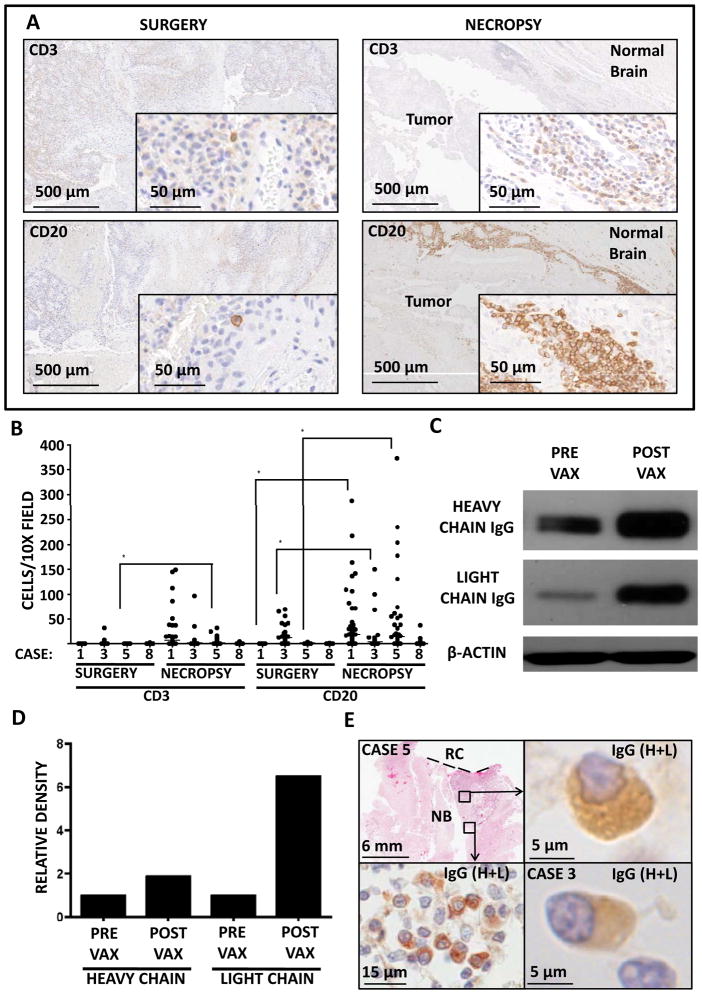
We conducted analyses of B and T cell infiltration in surgical and necropsy tissue in the four cases in which it was available. Three CpG-treated dogs died within three weeks of the final vaccination and exhibited robust, focal increases in B (with relatively less T) cell infiltration into peritumoral brain (Figs. 5A&B; Cases 1, 3, and 5). One imiquimod-treated dog developed acute lymphoblastic lymphoma and was euthanized 25 weeks after the final vaccination. This dog showed mild B cell infiltration (and no T cell infiltration) into the brain (Fig. 5B; case 8). As mentioned above, case 3 presented with a second meningioma in the contralateral hemisphere between the fifth and sixth vaccinations, which was subsequently resected. Western analysis revealed a profound increase in IgG penetration into the second tumor relative to the first, pre-vaccination tumor (Fig. 5C&D). Moreover, staining for canine IgG exposed plasma cells in the brains of three CpG-treated dogs (Fig. 5E). While previous studies have reported plasma cell entry into the brain in antibody-mediated autoimmune (31) and anti-pathogen (32) responses, this is the first account of induced plasma cell homing into brain tumors. In situ antibody production was further suggested by focal areas of extracellular IgG staining seen in plasma cell-containing areas of brain tissue but not others (data not shown).
Figure 5.
Vaccination Induces B and Plasma Cell Infiltrates in Peritumoral Brain. (A) Representative images from CD3 and CD20 IHC of biopsies and necropsies from case 1. (B) Quantification of CD3 and CD20 stains from cases 1,3,5, and 8. (C) IgG blotted on case 3 primary tumor (prevaccination) and contralateral tumor (postvaccination). (D) Densitometry of heavy and light chain bands from C. (E) Immunohistochemical stain of canine IgG (H+L), indicating plasma cell infiltrates in normal brain areas of cases 3 and 5. *, P<0.005.
Recognition of Non-Neoplastic Brain and Meningeal Antigens by Post-vaccination Sera
Two dogs (cases 3 and 5) were euthanized seven and twenty days after the most recent vaccination. Both animals presented with uncontrollable seizures and tumor recurrence was assumed. At necropsy, case 3 had a microscopic focus of residual tumor and case 5 had no evidence of tumor. To evaluate vaccine-induced autoantibody production, sera from these and three other dogs was probed against normal dog brain, arachnoid/pia mater, and dura mater. Secondary antibody revealed heavy and light chain IgG and IgM deposited in meninges, but not brain parenchyma (Fig. 6A, left). These results are consistent with the physiologic permeability of immunoglobulin into these tissues. Serum from cases 3 and 5 reacted to arachnoid/pia and brain parenchyma, respectively. Consistent with the autoreactivity of case 5 sera, analysis of necropsy brain tissue from this dog revealed IgG accumulation on or in neurons distal to the site of resection (Fig. 6B). The binding of the sera to normal brain antigens in these dogs sets them apart from two CpG treated dogs and one imiquimod treated dog that remained healthy and had non-reactive sera (Fig. 6A).
Figure 6.
Reactivity with Normal Brain Correlates with Neurologic Symptoms in CpG-Treated Dogs. (A) Brain, arachnoid/pia mater, and dura mater were probed with secondary anti-canine IgG (H+L) alone, with serum from an allogeneic healthy dog or postvaccination sera from five cases. Arrows indicate reactivity in dogs with neurologic symptoms following vaccination. (B) Necropsy specimen from case 5 stained for canine IgG (H+L).
Postvaccination Sera are Capable of Antibody Dependent Cell-Mediated Cytotoxicity
Anti-tumor effector activities of antibodies encompass multiple mechanisms, including antibody-dependent cell-mediated cytotoxicity (ADCC). Since antibodies reacted with cell surface antigens (Fig. 3B&C; Fig. 4 B&C; Fig 7 A&B), and antibody production in situ could enable opsonization of invasive meningioma cells behind an intact blood brain barrier, we tested whether tumor-reactive antibodies could trigger ADCC. Peripheral blood leukocytes killed few tumor cells when co-cultured with meningioma cells, or when tumor cells were pre-incubated with pre-vaccination serum; however, ADCC occurred when tumor cells were incubated with post-vaccination serum (Fig. 7C). Post-vaccination serum also triggered ADCC against allogeneic meningioma cells (Fig. 7D), demonstrating that allogeneic vaccination may be an effective strategy in canines with meningioma.
Figure 7.
Post Vaccination Serum Enables Antibody-Dependent Cell-Mediated Cytotoxicity. (A) Case 3 postvaccination serum antibodies bound autologous tumor and (B) allogeneic tumor of papillary histology from a non-study dog. Postvaccination serum also enabled killing of (C) autologous and (D) allogeneic tumor when combined with allogeneic peripheral blood leukocytes (PBL). *, P<0.05; **, P<0.005.
Discussion
As many as 57,000 dogs a year develop meningiomas in the US (13, 33), and these dogs are an under-utilized resource for preclinical study. Because the prognosis for canine meningioma is dismal (34), both dogs and humans could benefit from these studies. Our data show the canine model resembles many histological subtypes observed in humans and has features associated with poor prognosis in humans. The large size of the canine brain allows for surgery as a component of therapy, enabling a more realistic clinical interpretation of systemic interventions.
The survival outcomes of this study call for further investigation into the predictive potential of the canine model, especially with regard to tumor and immune biology in the two species. Different responses to vaccination may occur due to differential antigen expression, TLR expression in leukocyte cell types, tumor growth kinetics, the relationship between histologic subtype, biological behavior and clinical outcome.
Addition of lysate/adjuvant vaccination to surgery resulted in favorable survival compared to historical controls, but a prospectively designed, randomized study is required to make firm conclusions on therapeutic efficacy. Nonetheless, only case 3 experienced tumor growth despite treatment. The survival data may underestimate the benefit of vaccination because censoring was not used (Fig. 2B&C). Two dogs included in the analysis died of other malignancies, and two dogs lacked postmortem analysis but were assumed to die of age-related causes (Supplementary Table 1). The three other deaths were due to euthanasia after dogs presented with uncontrollable seizures assumed to be caused by tumor recurrence. Postmortem analysis in these CpG-treated dogs found minimal or no tumor burden, but immunoreactivity to normal brain structures may have been treatment related and contributed to the acute onset of neurologic symptoms (Fig. 6A&B). Similar seizure activity in glioma-bearing dogs in clinical trials at our institution has been controlled using additional anti-convulsants, corticosteroids or induction of general anesthesia (data not shown). It is therefore possible that the seizures in meningioma-bearing dogs could have been controlled. Whether CpG or imiquimod is more efficacious is still unresolved from the current study due to the small number of dogs and many non-tumor-related deaths. Given the high level of tumor control and the superior safety profile of imiquimod, however, a prospective randomized trial to compare surgery alone and in combination with vaccines of tumor lysate and a novel TLR 7/8 ligand was initiated.
Our study is a starting point for investigation of immunotherapy for meningioma. The data indicate that B cell activation and antibody production is the predominant mechanism of immunity induced by vaccination. Tumor-reactive antibodies were detected in the serum of all dogs regardless of lysate dose, demonstrating feasibility of autologous lysate/TLR vaccine production. Antibodies exhibited considerable inter-case and –species cross reactivity (Fig. 4 A&D; Supplementary Table 3). B cell infiltration outnumbered T cell infiltration in postmortem brain tissue adjacent to the resection cavity (Fig. 5B). In contrast, only two dogs had increased tumor-reactive T cell responses as measured by interferon-gamma ELISpot (Supplementary Table 3). However, it is likely that reactive CD4 T cells were primed following vaccination because antibody responses often require CD4 T cell help. Tumor-reactive T cells in the blood may not represent what occurs in the tumor draining lymph nodes or tumor site. A limitation of our study was that ELISpot was carried out only in pre-vaccination and 3-month post-vaccine blood samples. Nevertheless, infiltrating CD3+ cells were observed in dogs that died within two weeks of the final vaccination (Fig. 5 A&B), suggesting some T cell activation. Future studies will examine T cell responses in greater detail.
Tumor antigens in lysate vaccines could include over-expressed normal antigens, mutated (neo-) antigens, oncofetal antigens (expressed during development), or tumor-specific carbohydrates, glycoproteins, or lipoproteins. That two dogs with severe post-vaccine seizure activity had auto-reactive sera to brain and meninges suggests that severe autoreactivity is possible but infrequent (Fig. 6 A&B). Lysates may be depleted of these autoantigens to yield greater tumor specificity and less risk. Since most dogs had no autoreactivity or refractory seizures, risk of autoimmunity must be weighed against the threat to life posed by aggressive meningiomas.
Identification of meningioma antigens recognized by post-vaccination sera will enable the discovery of crucial epitopes for fully synthetic vaccine strategies in more widespread application. We identified HSP60 as one antigen recognized by sera following vaccination. HSP60 can translocate to the cell surface upon stress or malignant transformation (35), but it was not expressed on the tumor cell surface of case 3 (data not shown), arguing against cell surface protein as the functional target of this antibody. Sera that recognized HSP60 also reacted with a human meningioma, and reactivity was observed at 60 kDa; however, more study is needed to determine if HSP60 is a relevant target in human meningioma.
Antibody-mediated autoimmunity is well characterized, with autoimmune conditions in the CNS such as multiple sclerosis being re-evaluated in light of clinical benefit seen from B cell depleting therapies (36). Brain tumor immunotherapy, once focused on T cell-dependent mechanisms, is similarly expanding its breadth of effector mechanisms. Orthotopic mouse models of glioma indicate that survival benefit from immunotherapy is absent in B cell deficient, μMT tumor-bearing mice (37 and unpublished results). The importance of B cells and antibodies in brain tumor immunity are relatively unstudied. Our study documents for the first time the induction of plasma cells homing to brain tumors as a consequence of therapeutic intervention. B cell and plasma cell infiltration into the brain also indicates promise for immunotherapy to act against invasive cells that exist behind an intact blood brain barrier. ADCC due to in situ antibody production in brain is a novel immune effector mechanism relevant to any brain tumor. Our data suggest that vaccination can induce a “Trojan horse”-like infiltration of plasma cells that can in turn trigger ADCC, and thus create excitement for translation of this approach to human meningioma and other CNS cancers.
Supplementary Material
Acknowledgments
Financial Support: (B.M.A.) Torske Klubben Fellowship for Minnesota Residents, Medical Scientist Training Program Grant T32 GM008244 and the Cancer Biology Training Grant T32 CA009138—36; (M.A.H.) the American Brain Tumor Association Discovery Grant supported by the Anonymous Family Foundation; (J.R.O.) 1R21NS070955-01, R01 CA154345, R01 CA160782, the American Cancer Society grant RSG-09-189-01-LIB, Minnesota Medical Foundation, the Hedberg Family Foundation, and the Children’s Cancer Research Fund.
We thank Dr. Robert Schmidt (Washington University, St. Louis MO), for the micrograph of the human papillary meningioma; Dr. Wei Chen (University of Minnesota Department of Pediatrics) for CpG 685; Guillermo Marques and John Oja (UIC, University of Minnesota) for assistance in quantification and micrograph capture; Jose L. Gallardo and Patrick T. Grogan for vaccine production; and Nick J. Erickson (University of Minnesota), for assistance in IHC quantification.
Footnotes
Conflicts of Interest: The authors declare no conflicts.
References
- 1.CBTRUS. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2008. Hinsdale, IL: Central Brain Tumor Registry of the United States; 2012. website: www.cbtrus.org. [Google Scholar]
- 2.Vogelbaum MA, Leland Rogers C, Linskey MA, Mehta MP. Opportunities for clinical research in meningioma. J Neurooncol. 2010;99:417–22. doi: 10.1007/s11060-010-0375-6. [DOI] [PubMed] [Google Scholar]
- 3.Pearson BE, Markert JM, Fisher WS, et al. Hitting a moving target: evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus. 2008;24:E3. doi: 10.3171/FOC/2008/24/5/E3. [DOI] [PubMed] [Google Scholar]
- 4.Willis J, Smith C, Ironside JW, Erridge S, Whittle IR, Everington D. The accuracy of meningioma grading: a 10-year retrospective audit. Neuropathol Appl Neurobiol. 2005;31:141–9. doi: 10.1111/j.1365-2990.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain MC. Hydroxyurea for recurrent surgery and radiation refractory high-grade meningioma. J Neurooncol. 2012;107:315–21. doi: 10.1007/s11060-011-0741-z. [DOI] [PubMed] [Google Scholar]
- 6.Soyuer S, Chang EL, Selek U, Shi W, Maor MH, DeMonte F. Radiotherapy after surgery for benign cerebral meningioma. Radiother Oncol. 2004;71:85–90. doi: 10.1016/j.radonc.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Aghi MK, Carter BS, Cosgrove GR, et al. Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery. 2009;64:56–60. doi: 10.1227/01.NEU.0000330399.55586.63. discussion. [DOI] [PubMed] [Google Scholar]
- 8.Chamberlain MC, Johnston SK. Hydroxyurea for recurrent surgery and radiation refractory meningioma: a retrospective case series. J Neurooncol. 2011;104:765–71. doi: 10.1007/s11060-011-0541-5. [DOI] [PubMed] [Google Scholar]
- 9.Jung HW, Yoo H, Paek SH, Choi KS. Long-term outcome and growth rate of subtotally resected petroclival meningiomas: experience with 38 cases. Neurosurgery. 2000;46:567–74. doi: 10.1097/00006123-200003000-00008. discussion 74–5. [DOI] [PubMed] [Google Scholar]
- 10.Perry A. Meningiomas. In: McLendon RRM, editor. Russell & Rubinstein’s pathology of tumors of the nervous system. 7. London: Hodder Arnold; 2006. pp. 427–74. [Google Scholar]
- 11.Rogers L, Gilbert M, Vogelbaum MA. Intracranial meningiomas of atypical (WHO grade II) histology. J Neurooncol. 2010;99:393–405. doi: 10.1007/s11060-010-0343-1. [DOI] [PubMed] [Google Scholar]
- 12.Perry A. Unmasking the secrets of meningioma: a slow but rewarding journey. Surg Neurol. 2004;61:171–3. doi: 10.1016/s0090-3019(03)00488-9. [DOI] [PubMed] [Google Scholar]
- 13.Snyder JM, Shofer FS, Van Winkle TJ, Massicotte C. Canine intracranial primary neoplasia: 173 cases (1986–2003) J Vet Intern Med. 2006;20:669–75. doi: 10.1892/0891-6640(2006)20[669:cipnc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Adamo PF, Cantile C, Steinberg H. Evaluation of progesterone and estrogen receptor expression in 15 meningiomas of dogs and cats. Am J Vet Res. 2003;64:1310–8. doi: 10.2460/ajvr.2003.64.1310. [DOI] [PubMed] [Google Scholar]
- 15.Platt SR, Scase TJ, Adams V, et al. Vascular endothelial growth factor expression in canine intracranial meningiomas and association with patient survival. J Vet Intern Med. 2006;20:663–8. doi: 10.1892/0891-6640(2006)20[663:vegfei]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Dickinson PJ, Surace EI, Cambell M, et al. Expression of the tumor suppressor genes NF2, 4. 1B, and TSLC1 in canine meningiomas. Vet Pathol. 2009;46:884–92. doi: 10.1354/vp.08-VP-0251-D-FL. [DOI] [PubMed] [Google Scholar]
- 17.Sturges BK, Dickinson PJ, Bollen AW, et al. Magnetic resonance imaging and histological classification of intracranial meningiomas in 112 dogs. J Vet Intern Med. 2008;22:586–95. doi: 10.1111/j.1939-1676.2008.00042.x. [DOI] [PubMed] [Google Scholar]
- 18.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8:147–56. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
- 19.Okada H, Kalinski P, Ueda R, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29:330–6. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prins RM, Soto H, Konkankit V, et al. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17:1603–15. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neller MA, Lopez JA, Schmidt CW. Antigens for cancer immunotherapy. Semin Immunol. 2008;20:286–95. doi: 10.1016/j.smim.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Wu A, Oh S, Gharagozlou S, et al. In vivo vaccination with tumor cell lysate plus CpG oligodeoxynucleotides eradicates murine glioblastoma. J Immunother. 2007;30:789–97. doi: 10.1097/CJI.0b013e318155a0f6. [DOI] [PubMed] [Google Scholar]
- 24.Pluhar GE, Grogan PT, Seiler C, et al. Anti-tumor immune response correlates with neurological symptoms in a dog with spontaneous astrocytoma treated by gene and vaccine therapy. Vaccine. 2010;28:3371–8. doi: 10.1016/j.vaccine.2010.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson JA, Kuzel T, Drucker BJ, Urba WJ, Bukowski RM. Safety and efficacy of PF-3512676 for the treatment of stage IV renal cell carcinoma: an open-label, multicenter phase I/II study. Clin Genitourin Cancer. 2009;7:E58–65. doi: 10.3816/CGC.2009.n.025. [DOI] [PubMed] [Google Scholar]
- 26.Pashenkov M, Goess G, Wagner C, et al. Phase II trial of a toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J Clin Oncol. 2006;24:5716–24. doi: 10.1200/JCO.2006.07.9129. [DOI] [PubMed] [Google Scholar]
- 27.Adams S, O’Neill DW, Nonaka D, et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol. 2008;181:776–84. doi: 10.4049/jimmunol.181.1.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schon MP, Schon M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190–9. doi: 10.1038/sj.onc.1210913. [DOI] [PubMed] [Google Scholar]
- 29.Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM. Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol. 1997;21:1455–65. doi: 10.1097/00000478-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Louis DNOH, Wiestler OD, Cavenee WK. WHO Classification of Tumours of the Central Nervous System. 4. Lyon, France: IARC Press; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henderson AP, Barnett MH, Parratt JD, Prineas JW. Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Ann Neurol. 2009;66:739–53. doi: 10.1002/ana.21800. [DOI] [PubMed] [Google Scholar]
- 32.Burgoon MP, Keays KM, Owens GP, et al. Laser-capture microdissection of plasma cells from subacute sclerosing panencephalitis brain reveals intrathecal disease-relevant antibodies. Proc Natl Acad Sci U S A. 2005;102:7245–50. doi: 10.1073/pnas.0502323102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Pet Ownership & Demographics Sourcebook. American Veterinary Medical Association; 2007. [Google Scholar]
- 34.Axlund TW, McGlasson ML, Smith AN. Surgery alone or in combination with radiation therapy for treatment of intracranial meningiomas in dogs: 31 cases (1989–2002) J Am Vet Med Assoc. 2002;221:1597–600. doi: 10.2460/javma.2002.221.1597. [DOI] [PubMed] [Google Scholar]
- 35.Shin BK, Wang H, Yim AM, et al. Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J Biol Chem. 2003;278:7607–16. doi: 10.1074/jbc.M210455200. [DOI] [PubMed] [Google Scholar]
- 36.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–88. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 37.Daga A, Orengo AM, Gangemi RM, et al. Glioma immunotherapy by IL-21 gene-modified cells or by recombinant IL-21 involves antibody responses. Int J Cancer. 2007;121:1756–63. doi: 10.1002/ijc.22901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.