Abstract
The recent approval of Provenge has brought new hope for anti-cancer vaccine therapies. However, the immunosuppressive tumor microenvironment seems to impair the efficacy of vaccine therapies. The abnormal tumor vasculature creates a hypoxic microenvironment that polarizes inflammatory cells toward immune suppression. Moreover, tumors systemically alter immune cells’ proliferation, differentiation and function via secretion of growth factors and cytokines. For example, vascular endothelial growth factor (VEGF), a major pro-angiogenic cytokine induced by hypoxia, plays a critical role in immunosuppression via these mechanisms. Hence, anti-angiogenic treatment may be an effective modality to potentiate immunotherapy. Here we discuss the local and systemic effects of VEGF on tumor immunity, and propose a potentially translatable strategy to re-engineer the tumor immune microenvironment and improve cancer immunotherapy by using lower “vascular normalizing” doses of anti-angiogenic agents.
Introduction
It is now widely accepted that tumors can be immunogenic. Therefore, active cancer immunotherapies – designed to harness the power of the immune system to selectively eliminate malignant cells whilst sparing normal tissues – have recently re-entered the limelight (1, 2). In 2010, the US Food and Drug Administration approved the first therapeutic cancer vaccine for clinical use (Provenge®)(3), and presently over a dozen of other cancer vaccines are being evaluated in randomized phase II or III clinical trials (1). Despite this renewed hope for cancer immunotherapies, survival benefits from vaccination alone remain modest. Indeed, anti-cancer vaccines face many challenges – a critical one being the immunosuppressive tumor microenvironment (1, 2, 4, 5). As such, the judicious combination of an immunotherapy with an agent that reprograms the tumor microenvironment is an attractive therapeutic strategy. Growing body of evidence indicates that anti-angiogenic agents can normalize the abnormal tumor vasculature and potentially re-engineer the tumor immune microenvironment towards a more immunosupportive profile (5–8). In this mini-review, we discuss the interplay among pro-angiogenic molecules, tumor vessels and the host immune system, and how vessel-targeted therapies may improve the efficacy of anti-cancer immunotherapy.
The abnormal tumor vasculature creates an immune-suppressive microenvironment
Unlike the vessels of normal tissue, tumor blood vessels are highly abnormal. Driven by the relentless production of pro-angiogenic factors such as vascular endothelial growth factor (VEGF), tumor angiogenesis proceeds in a dysregulated fashion. As a result, tumor vessels show structural abnormalities including a heterogeneous distribution, tortuosity, dilation, and inadequate perivascular cell (PVC) investment. Functionally, blood flow is at times insufficient and vessels are hyperpermeable. The resultant microenvironment is characterized by patchy hypoperfusion, hypoxia, acidity and a high interstitial fluid pressure (5, 6, 9). These microenvironmental abnormalities may affect immune cell proliferation, infiltration, survival and function. Indeed, both preclinical and clinical reports found relative absence of anti-tumor lymphocytes, but detected abundant regulatory immune cells such as tumor-associated macrophages (TAMs) and regulatory T cells (Tregs)(4, 8, 10–13).
Even though all types of immune cells could infiltrate into tumor parenchyma through functional tumor vessels, suppressive immune cell populations appear to accumulate preferentially in tumors for a number of reasons. First, tumors often express high levels of growth factors, such as colony stimulating factor (CSF)-1 and chemokine (C-C motif) ligand(CCL)-2, which attract monocytes into tumor parenchyma where they differentiate into macrophages (12). Second, the tissue-resident macrophages are known to migrate into hypoxic and necrotic tumor areas where they are converted to TAM phenotype. Third, Treg cells – an immune suppressive lymphoid cell population – may be preferentially recruited into tumors via upregulation of chemotactic factors, such as CCL-22(10)and CCL-28 induced by tumor hypoxia (14). Finally, hypoxia and immunosuppressive factors in the tumor microenvironment can polarize TAMs to immune suppressive phenotype impeding the recruitment and activation of effector lymphocytes (8, 10, 15, 16).
TAMs have at least two phenotypes: M1 and M2. M1-like TAMs can destroy tumor cells through the induction of Th1 cytokines, such as interferon (IFN)-γ and interleukin (IL)-12, and stimulation of cytotoxic T lymphocyte immunity. Conversely, M2-like TAMs express high level of arginase (Arg)1, IL-10, VEGF and transforming growth factor(TGF)-β which suppress Th1 T cell immunity (10, 17). In cancer, the M2-like TAM phenotype predominates (17), in keeping with the “pro-tumor” immune environment. This is partly due to tumor hypoxia, a direct consequence of the abnormal tumor vasculature. Hypoxia promotes the polarization or differentiation of tumor-infiltrating myeloid cells to M2-like TAMs with high expression levels of IL-10 and Arg1 (16). Indeed, more M2-like TAMs are predominantly located in hypoxic tumor areas, and these cells have pro-angiogenic effects (15). Furthermore, TAMs in perfused regions of tumors express lower levels of M2-like genes, including Arg1, CSF-1, TGF-β and matrix metalloproteinase(MMP)-9, than TAMs distant from functional blood vessels (8). These findings suggest the critical role of abnormal tumor vasculature and resulting hypoxia in polarizing TAMs to M2-like phenotype and forming an immunosuppressive tumor microenvironment.
Besides these, hypoxia has more far-reaching consequences on the tumor immune environment. For example, hypoxia alters various metabolic pathways in cancer cells leading to accumulation of immunosuppressive metabolites such as adenosine (18). Hypoxia could also directly suppress T effector cell function (19).
Thus, the excessive production of pro-angiogenic factors creates locally an abnormal tumor microenvironment that leads to the preferential accumulation of immune suppressor cells in the tumors.
Circulating VEGF alters host immune cell proliferation, differentiation and function
In cancer, VEGF is present at high levels not just in the tumor but also in the systemic circulation. In addition to its ability to promote an immunosuppressive local tumor microenvironment, VEGF also exerts a systemic influence on immune cell development and function. Dendritic cells (DCs)are potent antigen-presenting cells (APCs) with a specialized ability to take up antigens and present them to T cells. Elevated levels of circulating VEGF suppress the maturation of DC precursors, promote the proliferation of Tregs, and thus inhibit T cell immune responses (13, 20, 21). Furthermore, treating mice with recombinant VEGF at concentrations similar to those observed in advanced stage cancer patients induces T cell defects via inhibition of Delta ligand signaling through Notch (22). Finally, the accumulation of myeloid-derived suppressor cells (MDSCs)in peripheral immune organs has been observed in both cancer patients and tumor-bearing animals, and excess VEGF is one causative factor (23). These MDSCs can suppress both T cell and DC function through a variety of mechanisms (23). Importantly, this systemic immunosuppression induced by excess VEGF can be reversed by the blockade of VEGF/VEGFR2 signaling pathway (13, 21, 24). Thus, elevated levels of systemic VEGF in tumor-bearing hosts play a key role in interfering with the recognition and destruction of tumor cells by the host immune system.
Combining anti-VEGF therapy with immunotherapy: rationale and current progress
Given that VEGF can interfere with anti-tumor immunity both systemically and through the generation of an abnormal local microenvironment, recent efforts have been inspired by the idea that anti-VEGF therapy could facilitate anti-tumor immunity, offering synergistic benefits when combined with immunotherapy.
Our laboratory has carried out extensive research into the effects of anti-VEGF therapy upon the tumor microenvironment. In both preclinical models and patients, blockade of VEGF can reverse many of the structural and functional abnormalities seen in tumor vessels – a process we have termed “vascular normalization”(5, 6). In short, judicious anti-VEGF therapy can improve tumor vessel perfusion, reduce tumor hypoxia, and in turn can enhance the delivery of systemically administered cytotoxic therapy and improve tumor radiosensitivity (5, 6). Targeting other pro-angiogenic factors, such as platelet-derived growth factor (PDGF), angiopoietins (Ang) and placenta growth factor (PlGF), as well as their cognate receptors and integrins, with antibodies or small molecule inhibitors may also achieve vascular normalization[reviewed in (6)]. A recent clinical study showed that an increase in tumor perfusion and oxygenation after anti-angiogenic treatment with cediranib in glioblastoma was associated with prolonged overall survival (9, 25). It is conceivable that this improved vessel function could enhance the delivery of immune effector cells into tumors, and that a reduction in hypoxia could reprogram immune suppressors in the local microenvironment. Together with the neutralization of the systemic immunosuppressive activity of VEGF, the prospect of combined VEGF-blockade and immunotherapy holds great promise, and is supported by growing body of evidence as discussed below (8, 24, 26, 27).
I: Genetically induced vascular normalization can improve anti-tumor immunity
Recently published animal studies using elegant genetic models have illustrated how normalization of the tumor vasculature can improve anti-tumor immunity. In a spontaneous pancreatic insulinoma mouse model (RIP1-Tag5/RGS5−/−), RGS5 deficiency normalized tumor vessels - resulting in increased PVC coverage and perfusion– leading to increased delivery of adoptively transferred T cells and improved mouse survival (7). In a second study, overexpression of the histidine-rich glycoprotein (HRG) induced a normalized vessel phenotype in solid tumors (through a PlGF-dependent mechanism), evidenced by increased PVC coverage, greater blood perfusion and reduced hypoxia. In keeping with this phenotype, HRG overexpression augmented DC and CD8+ T cell tumor infiltration and polarized TAMs away from an M2-like phenotype (28). Together, these findings show a mechanistic link between vessel normalization and enhanced immune cell infiltration and function.
II: Pharmacologically-induced vascular normalization can improve anti-tumor immunity
Anti-angiogenic drugs have been incorporated as the clinical standard of care for several malignancies (29). A number of studies have demonstrated the benefits of anti-angiogenic treatment on the efficacy of cancer immunotherapy in several cancer models (Table 1). Such combinations of anti-VEGF with immunotherapies have been most successful against highly immunogenic tumors (26, 27), and little or no benefit has been demonstrated when using immune tolerant neoplasms (27). This may be owing to the high dose of anti-angiogenic agents often used in animal models (26, 27), which can lead to shorter time-windows of normalization due to rapid and excessive pruning of tumor vessels and resulting hypoxia (5, 6). This may further restrict the access of effector T cells into tumor parenchyma, enhance the immunosuppressive tumor microenvironment and thus compromise the efficacy of an anti-cancer immunotherapy.
Table 1.
Benefits of combined anti-angiogenic and active immune therapies
| Anti-angiogenic strategy |
Immunotherapy | Tumor model | Results |
|---|---|---|---|
| Anti-mouse VEGF mAb |
Peptide-pulsed DCs | Sarcoma | ↑The number and function of DCs; ↑Tumor growth delay.(24) |
| Anti-VEGFR2 mAb |
GM-CSF secreting, neu specific whole tumor cell vaccine |
Neu expressing breast carcinoma |
↑Infiltration of CD8+ T cell; ↑Tumor regression (in FVB mice, but not neu-N/FVB mice).(27) |
| Anti-mouse VEGF mAb |
PVI | Melanoma | ↑Immune cell infiltration; ↑Tumor growth delay.(26) |
| Rgs5 knockout | ACT | Insulinoma, fibrosarcoma |
↑Immune cell infiltration; ↑Survival.(7) |
| Low dose anti-VEGFR2 mAb |
Mitomycin-C treated whole breast tumor cell vaccine |
Breast carcinoma |
↑Infiltration of CD8+ and CD4+ T cells; ↓MDSC and Tregs; ↑Survival.(8) |
| Adenoviral delivery of sVEGFR1/R2 |
GM-CSF secreting whole tumor cell vaccine |
Melanoma, colon carcinoma |
↑The number of activated CD4+ and CD8+ T cells; ↓decrease Treg; ↑Survival.(32) |
| SU6668 | Recombinant murine B7.2-IgG fusion protein and irradiated whole tumor cell vaccine |
Breast carcinoma |
↑Infiltration of CD8+ T cells; ↑Tumor growth delay.(33) |
| Sunitinib | Intratumoral IL-12 gene delivery by adenoviral vector plus 4-1BB activation |
Colon carcinoma |
↑Infiltration of CD8+ and CD4+ T cells; ↓MDSC and Tregs; ↑Survival.(34) |
| Sunitinib | Renal cell carcinoma (RCC) patients |
↓MDSC and Tregs; ↑type 1 T-cell activity.(35) |
|
| Low dose TNF-α | Adoptive T cell transfer | Insulinoma | ↑Infiltration of CD8+ T cell; ↑ Survival.(30) |
mAb: monoclonal antibody; DC: dendritic cells; MDSC: myeloid-derived suppressor cells; VEGF: vascular endothelial growth factor; VEGFR2: VEGF receptor 2; GM-CSF: granulocyte-macrophage colony-stimulating factor; PVI: the administration of anti-GP100 transgenic pmel-1 T cells plus gp100 vaccine plus IL-2 injection after 500cGy lymphodepleting whole body irradiation; ACT: adoptive cell transfer. SU6668 is an inhibitor of tyrosine kinase activity of the angiogenic receptors VEGFR2, PDGFRβ, and FGFR1. Sunitinib is an inhibitor of tyrosine kinase activity of VEGFR2, PDGFR, Flt3 and c-kit.
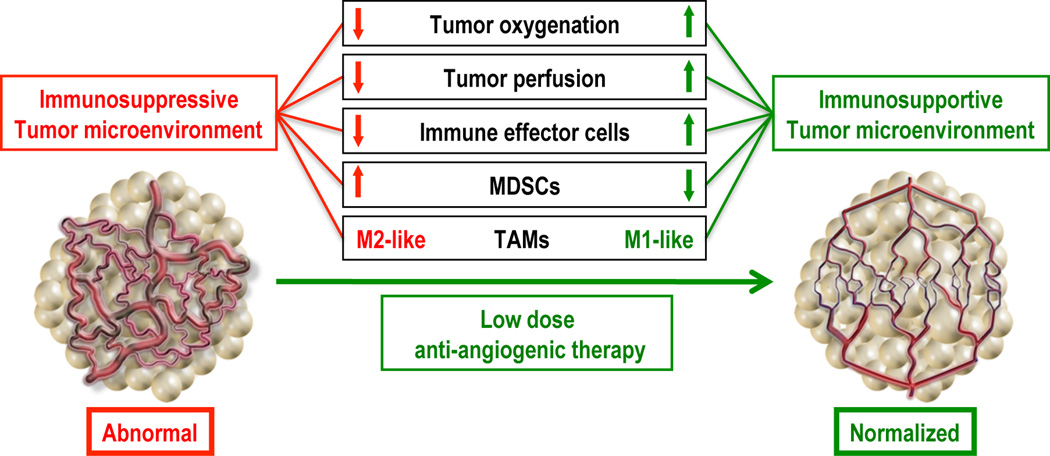
In support of this concept of dose-dependent normalization of vessels with anti-VEGF therapy, we have recently found in two murine breast cancer models that lower doses of anti-VEGFR2 antibody therapy (i.e. DC101, 10 or 20 mg/kg given every three days) induced breast tumor vascular normalization. This included a more homogeneous distribution of perfused tumor vessels, an increase in PVC coverage and a decrease in hypoxia. In contrast, the standard anti-vascular DC101 treatment promoted vessel regression. In keeping with these results, lower “vascular normalizing” dose of DC101 treatment was superior to high dose DC101 in facilitating T cell tumor infiltration, and at polarizing perivascular TAMs from an M2-like to an M1-like phenotype. In addition, low dose DC101 treatment reduced tumor-infiltrating MDSC numbers in MCaP0008 breast tumors, compared to high dose DC101. Based on these mechanisms, synchronizing vascular normalization and T cell activation by a whole breast cancer cell vaccine significantly enhanced tumor growth inhibition in a CD8+ T cell dependent manner in both immune tolerant and immunogenic breast cancer models (8). This is consistent with a recent report that low dose TNFα treatment normalized tumor vasculature and enhanced efficacy of the adoptive T cell transfer therapy (30). Collectively, these data suggest that lower “vascular normalizing” doses of anti-angiogenic therapy redirect the tumor microenvironment from immunosuppressive to immunosupportive and enhance an anti-cancer immunotherapy (Fig. 1).
Fig. 1. Lower “Vascular normalizing” dose of anti-angiogenic treatment reprograms the tumor microenvironment from immunosuppressive to immunosupportive.
The abnormal tumor vasculature creates a hypoxic tumor microenvironment, which impedes T effector cell infiltration into tumors and polarizes TAMs to the immune inhibitory M2-like phenotype to suppress T effector cell function. Lower dose anti-angiogenic treatment normalizes the tumor vasculature and generates a homogeneous distribution of perfused tumor vessels, facilitating the infiltration of T effector cells while reducing MDSC accumulation. In addition, improved vascular perfusion polarizes TAMs to an immune stimulatory M1-like phenotype. Thus, vascular normalization could be an effective strategy to alleviate the immunosuppressive tumor microenvironment and enhance cancer immunotherapy. Adapted from Huang et al, PNAS 2012 (8).
Clinical implications and challenges
Anti-angiogenic drugs have been administered in patients for nearly a decade and are often combined with chemotherapy (6, 29). However, the survival benefits of anti-angiogenic drugs are in the order of a few months and treatment resistance often develops rapidly. In addition, anti-angiogenic agents have failed to improve overall survival in some cancers, such as breast cancer. These issues raise questions about how anti-angiogenic therapy might be more effectively used in cancer treatment. In this respect, an increasing body of evidence points to the benefit of anti-angiogenic treatment when used with tumor immunotherapy.
A successful active tumor immunotherapy requires not only activated tumor antigen specific T cells, but also the access of T cells to malignant cells and an immunosupportive environment to sustain T cell function. These goals can be achieved by normalizing tumor vasculature, which can facilitate the delivery of T effector cells into tumor parenchyma and alleviate the immunosuppressive tumor microenvironment (5–8, 22, 28). Indeed, recent studies suggests that lower “vascular normalizing” dose of anti-angiogenic treatment could be an effective strategy to recondition the tumor immune microenvironment for anti-cancer vaccine therapy (8)and potentially other immunotherapies in a clinical setting, such as the blockade of immune checkpoints (31). The ongoing clinical trial of MPDL3280A [a monoclonal antibody that targets programmed cell death-1 ligand 1 (PD-L1)] in combination with a high dose (15 mg/kg) bevacizumab might shed some light on this interaction (see ClinicalTrials.gov, trial identifier NCT01633970). We propose that vascular normalizing doses of anti-angiogenic treatments could improve the effectiveness of immune checkpoint inhibitors with reduced toxicity.
In light of the above, a number of critical issues remain to be clarified. Tumors are highly heterogeneous. We may need to target different pro-angiogenic factors to induce vascular normalization in different tumors and at different times. Optimal doses of anti-angiogenic agents for vascular normalization may vary by patients or by disease status and hence could be difficult to generalize. A reliable biomarker would be helpful to choose a “vascular normalizing” or “pruning” dose. We observed a strong correlation between an increase in tumor-infiltrating CD8+ T cell and vascular normalization. This could be a potential biomarker. Another critical issue is the schedule. Different types of immunotherapies, for example, whole tumor cell vaccine, DC vaccine and adoptive T cell transfer, require different time to boost anti-cancer immunity, thus the schedules of an anti-angiogenic treatment with a tumor immunotherapy need to be optimized to achieve better anti-cancer efficacy. While these challenges will take some time to overcome, these concepts offer hope of developing new approaches that could enhance treatment efficacy of breast cancer and other malignancies.
Acknowledgments
Grant Support
NIH grants P01-CA080124 (RKJ, DGD, DF); R01-CA115767, R01-CA085140 and R01-CA126642 (RKJ); R21-CA139168 and R01-CA159258 (DGD); R01-CA096915 (DF); and Federal Share/NCI Proton Beam Program Income Grants (RKJ, DGD, DF). Department of Defense Innovator Award W81XWH-10–1-0016 (RKJ) and Postdoctoral Research Fellowship W81XWH-11-1-0619 (YH). American Cancer Society Grant RSG-11–073-01-TBG (DGD). National Foundation for Cancer Research Grant (RKJ). Australian-American Fulbright Commission and an American Society of Clinical Oncology Young Investigator Award (SG).
Footnotes
Conflict of Interest statement:
No potential conflicts for authors Yuhui Huang, Shom Goel, Dan G. Duda and Dai Fukumura. Rakesh K. Jain received research grants from Dyax, MedImmune, and Roche; consultant fees from Dyax, Noxxon, and Zyngenia; owns equity in Enlight, SynDevRx, and XTuit; and serves on the Board of Directors of XTuit and Board of Trustees of H&Q Healthcare Investors and H&Q Life Sciences Investors. No reagents or funding from these companies were used in these studies. Therefore, there is no significant financial or other competing interest in the work.
References
- 1.Schlom J. Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst. 2012;104:599–613. doi: 10.1093/jnci/djs033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quezada SA, Peggs KS, Simpson TR, Allison JP. Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol Rev. 2011;241:104–118. doi: 10.1111/j.1600-065X.2011.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010;15:969–975. doi: 10.1634/theoncologist.2010-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 6.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–414. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emblem KE, Mouridsen K, Bjornerud A, Farrar CT, Jennings D, Borra RJH, et al. Vessel architectural imaging identifies cancer patient responders to anti-angiogenic therapy. Nat Med. 2013 doi: 10.1038/nm.3289. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 11.Schmid MC, Varner JA. Myeloid cells in the tumor microenvironment: modulation of tumor angiogenesis and tumor inflammation. Journal of oncology. 2010;2010:201026. doi: 10.1155/2010/201026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, et al. VEGFA-VEGFR Pathway Blockade Inhibits Tumor-Induced Regulatory T-cell Proliferation in Colorectal Cancer. Cancer Res. 2013;73:539–549. doi: 10.1158/0008-5472.CAN-12-2325. [DOI] [PubMed] [Google Scholar]
- 14.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 15.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 16.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y, Snuderl M, Jain RK. Polarization of tumor-associated macrophages: a novel strategy for vascular normalization and antitumor immunity. Cancer Cell. 2011;19:1–2. doi: 10.1016/j.ccr.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sitkovsky MV, Kjaergaard J, Lukashev D, Ohta A. Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin Cancer Res. 2008;14:5947–5952. doi: 10.1158/1078-0432.CCR-08-0229. [DOI] [PubMed] [Google Scholar]
- 19.Palazon A, Aragones J, Morales-Kastresana A, de Landazuri MO, Melero I. Molecular pathways: hypoxia response in immune cells fighting or promoting cancer. Clin Cancer Res. 2012;18:1207–1213. doi: 10.1158/1078-0432.CCR-11-1591. [DOI] [PubMed] [Google Scholar]
- 20.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y, Chen X, Dikov MM, Novitskiy SV, Mosse CA, Yang L, et al. Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood. 2007;110:624–631. doi: 10.1182/blood-2007-01-065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y, Lin L, Shanker A, Malhotra A, Yang L, Dikov MM, et al. Resuscitating cancer immunosurveillance: selective stimulation of DLL1-Notch signaling in T cells rescues T-cell function and inhibits tumor growth. Cancer Res. 2011;71:6122–6131. doi: 10.1158/0008-5472.CAN-10-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res. 1999;5:2963–2970. [PubMed] [Google Scholar]
- 25.Sorensen AG, Emblem KE, Polaskova P, Jennings D, Kim H, Ancukiewicz M, et al. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2012;72:402–407. doi: 10.1158/0008-5472.CAN-11-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–6180. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manning EA, Ullman JG, Leatherman JM, Asquith JM, Hansen TR, Armstrong TD, et al. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res. 2007;13:3951–3959. doi: 10.1158/1078-0432.CCR-07-0374. [DOI] [PubMed] [Google Scholar]
- 28.Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Jain RK, Carmeliet P. SnapShot: Tumor angiogenesis. Cell. 2012;149 doi: 10.1016/j.cell.2012.05.025. 1408-e1. [DOI] [PubMed] [Google Scholar]
- 30.Johansson A, Hamzah J, Payne CJ, Ganss R. Tumor-targeted TNFalpha stabilizes tumor vessels and enhances active immunotherapy. Proc Natl Acad Sci USA. 2012;109:7841–7846. doi: 10.1073/pnas.1118296109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li B, Lalani AS, Harding TC, Luan B, Koprivnikar K, Huan Tu G, et al. Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy. Clin Cancer Res. 2006;12:6808–6816. doi: 10.1158/1078-0432.CCR-06-1558. [DOI] [PubMed] [Google Scholar]
- 33.Huang X, Wong MK, Yi H, Watkins S, Laird AD, Wolf SF, et al. Combined therapy of local and metastatic 4T1 breast tumor in mice using SU6668, an inhibitor of angiogenic receptor tyrosine kinases, and the immunostimulator B7.2-IgG fusion protein. Cancer Res. 2002;62:5727–5735. [PubMed] [Google Scholar]
- 34.Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer research. 2009;69:2514–2522. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]