Abstract
The aim of this study was to evaluate differences between the small and large intestines (SI and LI) with regard to colonization and immunity during infection with Trichinella spiralis. In orally infected C57BL/6 mice, the gender ratios of worms differed among the SI, cecum, and LI. Mucosal mastocytosis developed in the SI but not in the LI, consistent with reduced IL-9 and IL-13 production by explants from the LI. Despite these differences, worms were cleared at the same rate from both sites. Furthermore, IL-10 production was reduced in the LI, yet it was instrumental in limiting local inflammation. Finally, passive immunization of rat pups with tyvelose-specific antibodies effectively cleared fist-stage larvae from all intestinal regions. We conclude that despite regional differences in immune responsiveness and colonization, immune mechanisms that clear T. spiralis operate effectively throughout the intestinal tract.
Keywords: Trichinella spiralis, Large intestine, Mast cells, IL-10, Colon explant
1. Introduction
Regions of the vertebrate intestine differ with regard to size, physiology, and microbial flora. In addition, the small and large intestine (SI and LI) constitute distinct immune environments with differences in regulatory T cell phenotypes and the production of IL-10 and TGF-β (Autenrieth et al., 1997; Maynard et al., 2007). Endothelial cells displaying MAdCAM-1 recruit α4β7-expressing lymphocytes throughout the intestine, while CCR9-bearing lymphocytes are selectively recruited to the SI by locally produced CCL25/TECK (Gorfu et al., 2009). In this way, immune responses can be directed to the SI. Although many parasites are restricted in their distribution in the gastrointestinal tract, Trichinella spiralis colonizes both the small and large intestines (E. E. Tyzzer, 1916; Roth, 1938) providing an opportunity to investigate variation in regional immune responses and the impact this may have on infection.
2. Materials and methods
2.1 Animals
C57BL/6NHsd and BALB/cNHsd were purchased (Harlan, USA) and IL-10 deficient (B6.129P2-IL10tm1Cgn) mice and Albino Oxford (AO) rats were bred and maintained under specific pathogen-free conditions according to the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care. Experiments were performed with the approval of the Cornell University Institutional Animal Care and Use Committee.
2.2 Parasite and infections
Maintenance of Trichinella spiralis (pig strain), L1 recovery, and intestinal worm burden estimation were carried out as described (Blum et al., 2009; Gagliardo et al., 2002). Adult mice (7 to 10 weeks old) or suckling rat pups (12 to 16 days old) received 400 or 200 L1 by gavage, respectively. Crude L1 parasite extract (cAg) was prepared as described (Appleton and Usack, 1993).
2.3 Tissue collection, preparation, and evaluation
Mice were euthanized with C02. The SI and LI were removed and cut longitudinally, prepared as swiss rolls (Moolenbeek and Ruitenberg, 1981), fixed in Carnoy’s solution, and sectioned for staining with Alcian Blue (pH 0.4) and Nuclear Fast Red. Alternatively, tissues were fixed in formalin prior to sectioning and H & E staining. Mast cells in Alcian Blue-stained sections were estimated per crypt-villus unit (CVU) in a minimum of 50 CVU per section. Scoring of enteropathy in H & E-stained sections was as follows: epithelial hyperplasia (0–3), severity of inflammation (0–4). The sum of these two scores was multiplied by a value assigned to the distribution of inflammatory foci (0–3) for a total score ranging from 0 to 21. Severity of inflammation was defined as follows: no significant inflammation - 0; cellular infiltrate within the lamina propria, mild - 1, moderate - 2, severe and extending into the submucosa - 3; severe with crypt abscess, goblet cell depletion, and ulceration - 4. Neutrophil infiltration was given a score from 0 (no infiltration) to 3 (severe infiltration). Microscopy and image capture were performed with an Olympus BX51 microscope and DP25 camera, using Microsuite Basic Edition software.
2.4 Antibody treatment of rat pups
Monoclonal tyvelose-specific IgG1 (clone 9D4) and polyclonal IgG (nIgG) were prepared as described previously (Appleton and McGregor, 1987; Appleton et al., 1988). Rat pups were treated with 2.5 mg of antibody per 20 g of body weight by gavage, challenged one hour later, and intestinal parasite burdens estimated after 24 and 48 hours (Blum et al., 2009).
2.5 Cytokine measurement
Five-mm pieces of jejunum, ileum, or LI were weighed prior to processing for explant cultures, as described (Egan et al., 2011). Explants were cultured with 50 μg/mL of cAg for 16–18 hours at 37°C. Explant supernatants were centrifuged at 138 × g and assayed for IL-4, IL-5, and IL-10 by ELISA as described previously (Beiting et al., 2007). The same protocol was applied to measure IL-9 (BD Biosciences: 2.5 μg/mL capture clone D8402E8, 0.25 μg/mL detection antibody clone D9302C12), IL-13 (Ebioscience: 2 μg/mL capture clone ebio13A, 0.2 μg/mL detection antibody clone eBio1316H), IL-17A (BD Biosciences: 2 μg/mL capture clone TC11-18H10, 0.17 μg/mL detection antibody clone TC11-8H4.1), and IFN-γ (BD Biosciences: 1 μg/mL capture antibody clone AN-18; Ebioscience: 0.125 μg/mL detection antibody clone XMG1.2). Recombinant cytokine standards were purchased (Ebioscience).
2.6 Statistical analysis
Experiments were performed twice and data were evaluated using Student’s t test or ANOVA with Tukey’s post-hoc test for multiple means. P-values less than 0.05 were considered to be statistically significant.
3. Results
3.1 Distribution of T. spiralis in the intestinal tract
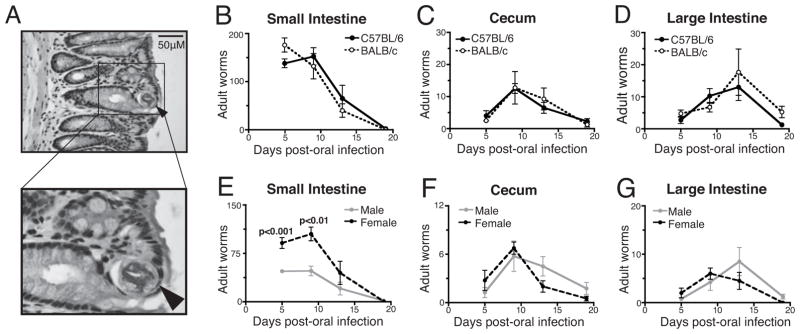
Previous reports have documented the presence of T. spiralis in the large intestine; however, the location of the worms in the tissue has not been described. Ten days post-infection (dpi), adult worms occupied an epithelial habitat similar to that observed in the small intestine (Figure 1A) (Wright, 1979). Peak worm burdens occurred prior to day 5 in the SI, on day 9 in the cecum, and on day 13 in the LI (Figure 1B, C, D). Once established, worms were expelled at comparable rates from each site. Results obtained from C57BL/6 and BALB/c mice were indistinguishable.
Figure 1.
Colonization and expulsion of intestinal T. spiralis. (A) Cross section of adult T. spiralis in an H&E stained section of the LI from a C57BL/6 mouse. Arrow indicates the parasite. (B-D) Parasite colonization and expulsion from the three compartments of the intestine in C57BL/6 and BALB/c mice infected orally with 400 L1. (E-G) Numbers of male and female worms in C57BL/6 mice. P-values represent differences between the numbers of male and female worms (n = 4 mice per group, means compared using Student’s t-test).
The ratio of female to male T. spiralis colonizing the SI has been reported to be approximately 2:1, shifting to 1:1 as worms are expelled (Gursch, 1949). Figure 1 (panels E, F, G) shows the expected transition in gender ratio in the SI of C57BL/6 mice, while equal numbers of male and female parasites were observed at all times in the cecum and LI. Similar results were obtained from BALB/c mice (not shown). These results suggest that females are cleared more rapidly from the SI and are less successful than males in colonizing the distal compartments.
3.2 Cytokine production and mast cell response
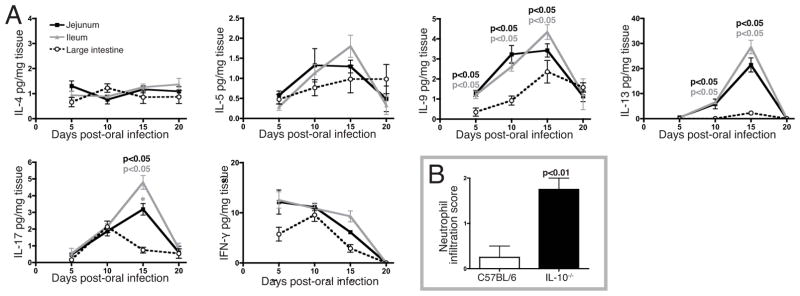
Assay of cytokines in explant cultures revealed no significant differences in IL-4, IL-5, or IFN-γ among the jejunum, ileum, and LI (Figure 2A). Although colonization of the sites was not synchronous, the response kinetics were similar for these cytokines across the sites. In contrast, IL-9, IL-10, and IL-13 were significantly lower in LI explant cultures, and the modest output of these cytokines was delayed compared to the SI; IL-17 was reproducibly lower in the LI only at 15 dpi.
Figure 2.
Cytokine and mast cell responses in the SI and LI. (A) Cytokines in culture supernatants of tissue explants cultured with crude parasite extract. Values are expressed as picograms of cytokine per milligram of tissue. P-values indicate significant differences between LI and jejunum (black) or LI and ileum (gray) (n = 4 mice per group, means compared using ANOVA with Tukey’s post-test). (B) Intestinal mast cells in SI and LI during the course of infection. Values represent the mean number of mast cells per crypt-villus unit (CVU) estimated from a minimum of 50 CVU per mouse. P-values indicate a significant increase in the SI relative to uninfected mice, with no significant changes in the LI (n = 3 mice per group, means compared using ANOVA with Tukey’s post-test).
In the context of T. spiralis infection, intestinal mastocytosis in the SI of mice is driven by IL-9 and IL-10 (Faulkner et al., 1997; Helmby and Grencis, 2003), and mast cells are believed to be essential to the mechanism of worm expulsion (Ha et al., 1983; Knight et al., 2000). The absence of a significant increase in Alcian Blue positive cells in the LI during infection (Figure 2B) correlated with reduced production of IL-9 and IL-10 in that compartment. Despite these deficiencies, worms were expelled at a rate similar to that observed in the SI. Thus, the immune response to T. spiralis in the LI is significantly different from that of the SI, and does not feature mastocytosis, yet expulsion occurs in a timely manner.
3.3 Enteropathy and the influence of IL-10
In C57BL/6 mice, inflammation in the SI increased dramatically during infection, while no reproducible inflammatory response was detected at any time in the LI (Figure 3). In contrast, inflammation increased significantly on day 10 of infection in the LI of IL-10−/− mice, and this inflammation resolved by day 15. We concluded that IL-10 is a key regulator of inflammation in the LI during T. spiralis infection, and that an IL-10-independent regulatory mechanism functions in the LI to quickly resolve inflammation, while SI enteropathy persists.
Figure 3.
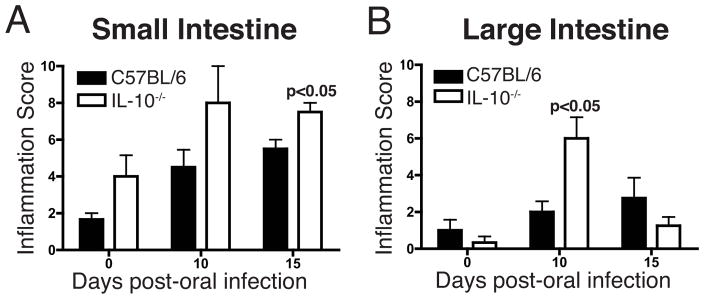
Enteropathy in C57BL/6 and IL-10−/− mice infected with T. spiralis. H&E stained tissues were scored using a 21-point scale (see text). (A) SI (B) LI. P-values represent significant differences relative to uninfected mice (n = 4 mice per group, means compared using Student’s t-test).
To further evaluate the importance of IL-10 in regulating immunity, we measured the cytokines produced by tissue explants from IL-10−/− mice (Figure 4A). These mice were tested in parallel with C57BL/6 mice, allowing direct comparison of results graphed in Figures 2 and 4. Explants from IL-10−/− and C57BL/6 mice yielded nearly identical cytokine quantities, with the exception that IL-17 production by SI explants was significantly greater in IL-10−/− mice. The increase in IL-17 was correlated with a significantly greater influx of neutrophils in IL-10−/−mice at day 15 pi (Figure 4B). Thus, IL-10 regulated IL-17 production in the SI but not the LI, and the transient inflammation observed in the IL-10−/− LI did not correlate with alterations in the production of the cytokines assayed.
Figure 4.
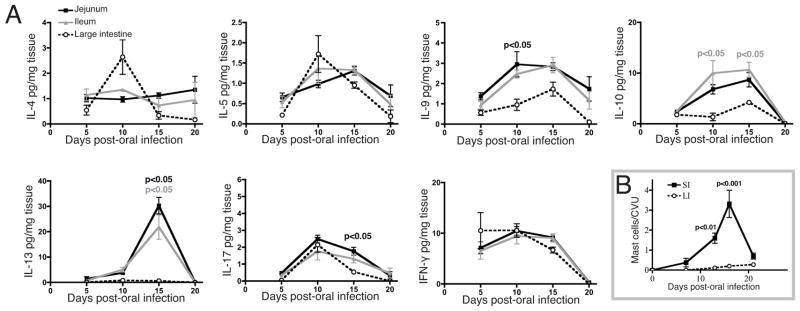
Cytokine response in the SI and LI of IL-10−/− mice. (A) Cytokines in culture supernatants of tissue explants in IL-10−/− mice, expressed as picograms of cytokine per milligram of tissue. P-values indicate significant differences between LI and jejunum (black) or LI and ileum (gray), * represents a difference of p<0.05 between jejunum and ileum (n = 4 mice per group, means compared using ANOVA with Tukey’s post-test). (B) Neutrophil infiltration in the SI at 15 dpi (n = 4 mice per group, means compared using Student’s t-test).
3.4 Protective immunity in the SI and LI
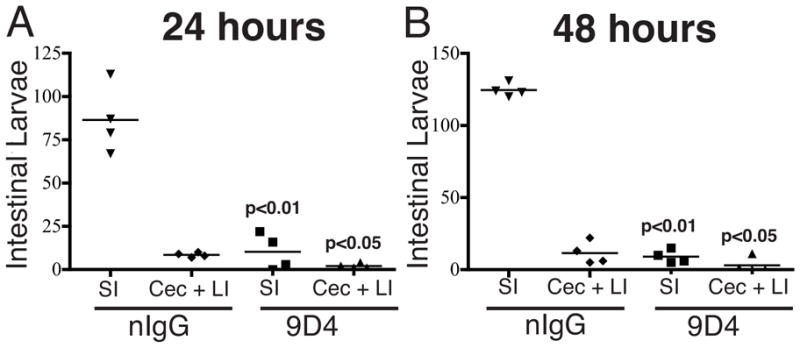
Previously infected rats display a dramatic protective immunity in which 90% of L1 are cleared from the intestine within an hour of challenge. Suckling rats display this rapid expulsion when passively immunized with antibodies specific for tyvelose (Appleton and McGregor, 1984, 1987; Bell et al., 1992). In order to determine whether rapid expulsion occurs in the LI, we estimated worm burdens in passively immunized pups after 24 (Figure 5A) or 48 (Figure 5B) hours. Treatment with specific antibody cleared parasites from all compartments, compatible with the conclusion that antibodies can effectively exclude or eliminate T. spiralis from epithelia in the cecum and LI.
Figure 5.
Protective immunity in the LI of suckling rats. Pups were treated with tyvelose-specific IgG1 (9D4) or naive rat IgG (nIgG) one hour prior to challenge with 200 L1. (A) Parasite burden in the SI, or combined cecum and LI, 24 hours after challenge. (B) Parasite burden in the SI, or combined cecum and LI, 48 hours after challenge. P-values are shown for nIgG versus corresponding compartment in 9D4-treated pups (n = 4 rats per group, means compared using Student’s t-test).
4. Discussion
Immunity to T. spiralis is often compared with immunity to Trichuris muris, a cecal dwelling nematode. Although the two parasites are closely related, the mechanisms of worm clearance are distinct, where mast cells are reported to play a prominent role in expulsion of adult T. spiralis while epithelial cell turnover and function appear to be key to clearance of T. muris (Artis et al., 1999; Ha et al., 1983). The differences in effector mechanisms may be dictated by properties intrinsic to each parasite, or by their distinct intestinal habitats. Although immunity induced by T. spiralis in the SI of mice and rats has been studied in depth, immunity in the LI has been largely overlooked. We set out to compare immune responses across intestinal compartments within the context of T. spiralis infection.
T. spiralis demonstrated a pattern of rolling colonization and expulsion. Females were more numerous in the SI compared to the cecum and LI, perhaps because their size (3–4 mm in length versus 1 mm for males) may increase their susceptibility to physical disruption and nutritional deprivation.
Adult worms move rapidly within the intestinal epithelium, destroying epithelial cells that they occupy (Wright et al., 1987). As the habitable space in one section of intestine is diminished, the parasites may be forced to migrate distally to find healthy cells. Alternatively, the immune response in the SI may make that site inhospitable to the worms, prompting migration to the immunologically naïve cecum and LI. We found that although IL-4, IL-5, and IFN-γ were produced in the LI at early time points, production of the cytokines known to cause expulsion (IL-9, IL-10, and IL-13) was delayed or absent in that compartment. Thus, the distal movement of the parasites may follow a cytokine gradient along the cranial-caudal axis of the intestine. Mucosal mastocytosis was evident in the SI but not in the LI, consistent with weak IL-9 and IL-10 production by LI explants. Mast cells are believed to be essential for the mechanism of worm expulsion from the SI, and their absence in the LI, despite clearance of worms from this site, indicates that the mechanisms of protective immunity may differ between the SI and LI.
Inflammation was more robust in the SI than the LI at 10 and 15 dpi, indicating that the LI may be a more permissive region for the parasite. In the SI, IL-10 controlled IL-17 production and neutrophil infiltration, which have been shown by others to be pathogenic in the intestine (Schaefer et al., 2010; Yen et al., 2006). We did not observe this effect in the LI, and the distinct roles for IL-10 and IL-17 in the SI versus the LI merit further investigation.
We found that mucosal mastocytosis and the cytokines that influence it are not prominent in the LI during expulsion of T. spiralis, and that immunopathology is more tightly regulated in the LI than the SI. Thus, the mechanisms of immunity and enteropathy vary in different regions of the intestine that are colonized by T. spiralis.
Acknowledgments
This work was funded by the NIH-NIAID Grant #AI14490 to JA. We thank Dr. Charlotte Egan for advice regarding the explant culture protocol.
Footnotes
Conflict of interest statement
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appleton JA, McGregor DD. Rapid expulsion of Trichinella spiralis in suckling rats. Science. 1984;226:70–72. doi: 10.1126/science.6474191. [DOI] [PubMed] [Google Scholar]
- Appleton JA, McGregor DD. Characterization of the immune mediator of rapid expulsion of Trichinella spiralis in suckling rats. Immunology. 1987;62:477–484. [PMC free article] [PubMed] [Google Scholar]
- Appleton JA, Schain LR, McGregor DD. Rapid expulsion of Trichinella spiralis in suckling rats: mediation by monoclonal antibodies. Immunology. 1988;65:487–492. [PMC free article] [PubMed] [Google Scholar]
- Appleton JA, Usack L. Identification of potential antigenic targets for rapid expulsion of Trichinella spiralis. Mol Biochem Parasitol. 1993;58:53–62. doi: 10.1016/0166-6851(93)90090-k. [DOI] [PubMed] [Google Scholar]
- Artis D, Potten CS, Else KJ, Finkelman FD, Grencis RK. Trichuris muris: host intestinal epithelial cell hyperproliferation during chronic infection is regulated by interferon-gamma. Exp Parasitol. 1999;92:144–153. doi: 10.1006/expr.1999.4407. [DOI] [PubMed] [Google Scholar]
- Autenrieth IB, Bucheler N, Bohn E, Heinze G, Horak I. Cytokine mRNA expression in intestinal tissue of interleukin-2 deficient mice with bowel inflammation. Gut. 1997;41:793–800. doi: 10.1136/gut.41.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiting DP, Gagliardo LF, Hesse M, Bliss SK, Meskill D, Appleton JA. Coordinated control of immunity to muscle stage Trichinella spiralis by IL-10, regulatory T cells, and TGF-beta. J Immunol. 2007;178:1039–1047. doi: 10.4049/jimmunol.178.2.1039. [DOI] [PubMed] [Google Scholar]
- Bell RG, Appleton JA, Negrao-Correa DA, Adams LS. Rapid expulsion of Trichinella spiralis in adult rats mediated by monoclonal antibodies of distinct IgG isotypes. Immunology. 1992;75:520–527. [PMC free article] [PubMed] [Google Scholar]
- Blum LK, Thrasher SM, Gagliardo LF, Fabre V, Appleton JA. Expulsion of secondary Trichinella spiralis infection in rats occurs independently of mucosal mast cell release of mast cell protease II. J Immunol. 2009;183:5816–5822. doi: 10.4049/jimmunol.0900944. [DOI] [PubMed] [Google Scholar]
- Tyzzer EE, aJAH The effects of radiation on the development of Trichinella spiralis. J Parasitol. 1916;3:43–56. [Google Scholar]
- Egan CE, Maurer KJ, Cohen SB, Mack M, Simpson KW, Denkers EY. Synergy between intraepithelial lymphocytes and lamina propria T cells drives intestinal inflammation during infection. Mucosal Immunol. 2011;4:658–670. doi: 10.1038/mi.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner H, Humphreys N, Renauld JC, Van Snick J, Grencis R. Interleukin-9 is involved in host protective immunity to intestinal nematode infection. Eur J Immunol. 1997;27:2536–2540. doi: 10.1002/eji.1830271011. [DOI] [PubMed] [Google Scholar]
- Gagliardo LF, McVay CS, Appleton JA. Molting, ecdysis, and reproduction of Trichinella spiralis are supported in vitro by intestinal epithelial cells. Infect Immun. 2002;70:1853–1859. doi: 10.1128/IAI.70.4.1853-1859.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorfu G, Rivera-Nieves J, Ley K. Role of beta7 integrins in intestinal lymphocyte homing and retention. Curr Mol Med. 2009;9:836–850. doi: 10.2174/156652409789105525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursch OF. Intestinal phase of Trichinella spiralis, Owen, 1835, Railliet, 1895. J Parasitol. 1949;35:19–26. [PubMed] [Google Scholar]
- Ha TY, Reed ND, Crowle PK. Delayed expulsion of adult Trichinella spiralisby mast cell-deficient W/Wv mice. Infect Immun. 1983;41:445–447. doi: 10.1128/iai.41.1.445-447.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmby H, Grencis RK. Contrasting roles for IL-10 in protective immunity to different life cycle stages of intestinal nematode parasites. Eur J Immunol. 2003;33:2382–2390. doi: 10.1002/eji.200324082. [DOI] [PubMed] [Google Scholar]
- Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000;192:1849–1856. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, Weaver CT. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- Moolenbeek C, Ruitenberg EJ. The “Swiss roll”: a simple technique for histological studies of the rodent intestine. Lab Anim. 1981;15:57–59. doi: 10.1258/002367781780958577. [DOI] [PubMed] [Google Scholar]
- Roth H. On the localization of adult Trichinella spiralis in the intestinal tract. J Parasitol. 1938;24:225–231. [PubMed] [Google Scholar]
- Schaefer JS, Montufar-Solis D, Vigneswaran N, Klein JR. ICOS promotes IL- 17 synthesis in colonic intraepithelial lymphocytes in IL-10−/− mice. J Leukoc Biol. 2010;87:301–308. doi: 10.1189/jlb.0409238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KA. Trichinella spiralis: an intracellular parasite in the intestinal phase. J Parasitol. 1979;65:441–445. [PubMed] [Google Scholar]
- Wright KA, Weidman E, Hong H. The distribution of cells killed by Trichinella spiralis in the mucosal epithelium of two strains of mice. J Parasitol. 1987;73:935–939. [PubMed] [Google Scholar]
- Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]


