Abstract
Hypothesis
We hypothesize that the severity of hearing loss (HL) associated with sporadic vestibular schwannomas (VS) is correlated with tumor secretion of proteins with ototoxic or otoprotective potential.
Background
Since the recognition that HL associated with VS is not solely due to compression of the auditory nerve, elucidating the mechanism by which VS cause HL has been an important task. We previously showed that VS stratified by hearing have differential gene expression. We now focus on identifying differentially expressed proteins in tumor secretions.
Methods
Fresh surgical specimens of VS were incubated in sterile PBS at 37°C to collect secretions. The specimens were divided into a group associated with good hearing (GH, word recognition ≥70% and pure-tone average ≤30 dB, n=11) or poor hearing (PH, n=10). The groups were compared using a customized cytokine array. Statistically significant results were verified with ELISA on a different set of secretions (n=8 for GH and n=10 for PH group).
Results
Of the 37 molecules we studied, 9 were significantly expressed in secretions from VS compared to secretions from control nerves. Secretion of fibroblast growth factor 2 (FGF2) was 3.5-fold higher in VS associated with GH versus PH based on cytokine array analysis (p=0.02), which was validated with ELISA.
Conclusions
This study highlights FGF2, a mitogen known to protect the auditory nerve, as a potential tumor-secreted mediator of hearing protection in VS. If FGF2's significant role in hearing protection in patients with VS is validated, then FGF2 could be utilized as a biomarker for HL in VS and therapeutic targeting of the FGF2 signaling pathway may reduce HL due to VS.
Keywords: Sporadic vestibular schwannoma, acoustic neuroma, hearing loss, cytokine array, FGF2
Introduction
Sporadic vestibular schwannomas (VS), also known as acoustic neuromas, are the most common tumors of the cerebellopontine angle and frequently present with hearing loss (HL) (1). The pathophysiology of this HL remains elusive. Because VS typically arise from the schwann cells of the vestibular part of the eighth cranial nerve, it is commonly assumed that they cause HL by compressing the adjacent auditory portion of the nerve. However, the poor correlation between VS radiological dimensions and degree of HL suggests that nerve compression is not the only cause of HL due to VS (2). Further, a retrospective study of patients with intracanicular VS found no relation between hearing and tumor size or the tumor-induced expansion of the internal auditory canal, reinforcing that nerve compression is not the only modulator of HL (3). Tumor presence does, however, lead to cochlear degeneration and neuronal loss in patients with VS (4). Previous work from our laboratory demonstrated that VS stratified by hearing into good versus poor hearing groups have distinctly different gene expression profiles, suggesting that differential expression of potentially ototoxic or otoprotective molecules may contribute to the degree of HL associated with VS (5). To gain further insight into the mechanisms of HL, the current study focuses on tumor-secreted factors that may contribute to HL or hearing protection in patients with VS.
We have focused on cytokines - small, secreted cell signaling molecules - because they are thought to mediate HL in a variety of diseases, including meningitis, cochlear otosclerosis and labyrinthitis (6,7). Specifically, inhibition of pro-inflammatory cytokines through molecules such as interleukin-1 receptor antagonist (IL1RN) has been proposed as potential treatments against presbycusis and other types of sensorineural HL (8,9). Additionally, corticosteroids, drugs that are known to repress cytokine gene transcription, are known to be effective in many cases of sudden sensorineural HL associated with VS, suggesting a biochemical imbalance or inflammatory response as a potential trigger for loss of hearing (10,11,12).
In addition to cytokines, we studied expression of several non-secreted molecules in incubated medium from VS because tumors are known to shed (13,14). For example, shedding plasma membrane fragments may have proteins attached, including receptors, that can contribute to intercellular signaling (15). We also studied several candidate molecules identified in our recent proteomic analysis of perilymph from patients with VS (16). We investigated a total of 37 proteins using a customized cytokine array. Differential expression was validated with enzyme-linked immunosorbent assays (ELISA). Given FGF2's otoprotective role in other contexts, our results point to FGF2 as a secreted molecule (17) that may contribute to hearing protection in some patients with VS (18, 19).
Materials and Methods
Study Population and Specimen Collection
Surgical VS specimens were collected as previously described (5) from a total of 16 adults with good hearing (GH) and 19 adults with poor hearing (PH). Subject demographics are listed in Supplemental Table 1. Specimens of healthy great auricular nerves (GAN) were collected from 7 adults undergoing unrelated neck dissections. Specimens were placed in sterile saline on ice for 15 minutes while being transported to the laboratory. Specimens were handled according to the institutional review board's study protocol approved by the Human Studies Committee of Massachusetts General Hospital (Protocol No. 2004- P2297/2, PI: K.M.S) and Massachusetts Eye and Ear Infirmary (Protocol No. 05-02-009X, PI: K.M.S), and in accordance with the Helsinki Declaration. Age was defined at the time of diagnosis. Tumor size (largest diameter parallel to the petrous face), word discrimination (WD) and pure tone average (PTA) were the last measurements before tumor surgery. Tumor growth rate was derived from transverse or greatest dimension measurement changes in serial MRI scans. Informed consent was obtained from all subjects.
Cytokine Array
Fresh human specimens were thoroughly washed in sterile phosphate-buffered saline (PBS) three times before being incubated in 500 μL sterile PBS at 37°C for 1h. The specimens were then removed from PBS and the tumor-conditioned PBS was stored at - 80°C until further use. The same procedure was followed for the VS and GAN specimens. Sterile PBS without specimen was incubated in parallel as a negative control.
Human cytokine array membranes coated with 37 specific antibodies (RayBioTM Human Cytokine Antibody Array, RayBiotech, Inc.) were probed with 21 VS secretion samples, 7 GAN secretion samples and 1 blank sterile PBS. Antibodies to 6 targets of particular interest due to our past work were shipped to the company for inclusion on the array: Mu-crystallin homolog (CRYM, Abnova, Walnut, CA, Catalog No. H00001428-M03), αB-crystallin (CRYAB, Santa Cruz Biotechnology,Inc., Santa Cruz, CA, Catalog No. sc-22744), Merlin (NF2, Santa Cruz Biotechnology,Inc., Santa Cruz, CA, Catalog No. sc-331), Fibronectin-1 (FN1, BD Pharmingen, San Diego, CA, Catalog No. 555867), F-actin (ACTA1, Abcam, Cambridge, MA, Catalog No. ab205) and Versican (VCAN, Abcam, Cambridge, MA, Catalog No. ab19345). Proteins analyzed in the cytokine array are listed in Supplemental Table 2. The manufacturer's protocol was followed for experimental procedures and data analysis. Samples were dialyzed twice with PBS, pH 8.
Protein concentrations of the dialyzed samples were measured spectrophotometrically and normalized through dilution in sterile PBS before incubation on the array membranes. Cytokine arrays were processed using standard manufacturer's protocol. Briefly, the membranes were exposed to the blocking buffer at room temperature (RT) for 1 h, incubated with sample at 4°C overnight, washed with Wash Buffer I and II at room temperature (RT), incubated with biotin-conjugated antibodies at 4°C overnight, and washed and incubated with HRP-conjugated streptavidin at RT for 1 h. The membranes were incubated in detection buffer for 1 min, and exposed in Chemidoc (BioRad Laboratories, Hercules, CA) for 175s- 350s to obtain a strong, clear signal. The relative expression levels were compared after a densitometric analysis using Quantity One (BioRad Laboratories, Hercules, CA). Probing with blank PBS treated in the same manner as the samples did not produce positive staining except at the positive control spots coated with the biotinylated IgGs. Proteins were determined to be significantly expressed if the corresponding spots had optical densities more than 2 standard deviations of background values above the mean background level for each array. Statistical significance was determined through an analysis of variance (ANOVA) with alpha set to 0.05.
Enzyme-linked immunosorbent assays (ELISA)
ELISA was conducted to validate the result obtained in cytokine array analysis. Tumor samples were collected and incubated in PBS as described above. A total of 8 VS secretions associated with GH and 10 VS secretions associated with PH were used. Majority of the samples were from different patients than the samples studied in the cytokine array; 4 samples (3 GH and 1 BH samples) overlapped with the cytokine array sample group and are marked with double asterisks in Supplemental Table 1.
ELISA for FGF2 and IL8 (Catalog No. DFB50 and D8000C, Quantikine ELISA, R&D Systems, Minneapolis, MN) were used as per manufacturer's protocol. Tumor secretions were diluted in sterile PBS to have a total protein concentration of 30μg/mL. In brief, duplicates were incubated in the 96-well immunoassay for 2 h along with the standard and control sterile PBS, which served as background. The plate was washed four times with Wash Buffer, incubated with the specific enzyme-linked monoclonal antibody specific for FGF2 for 2 h at RT, washed again, and incubated with the substrate solution. The stop solution was added and the plate was read using BioRad Model 680 (BioRad Laboratories, Hercules, CA) set to 450 nm, with a correction by 655 nm. ANOVA was used for statistical analysis with an alpha of 0.05.
Results
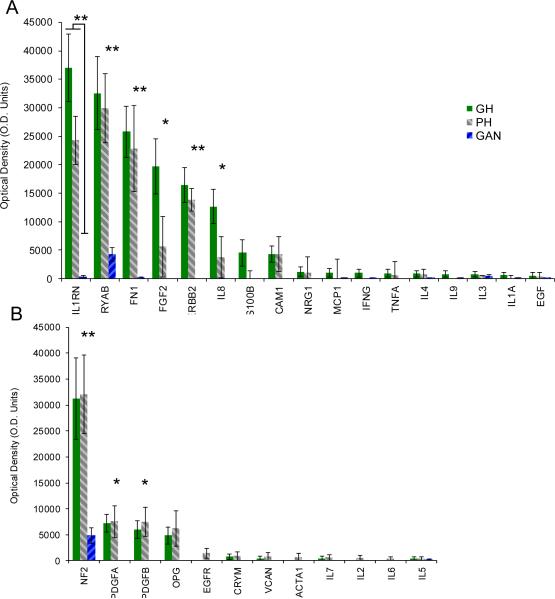
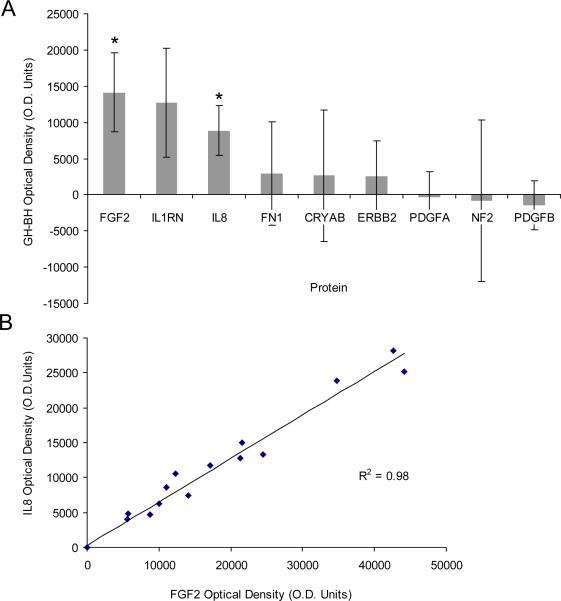
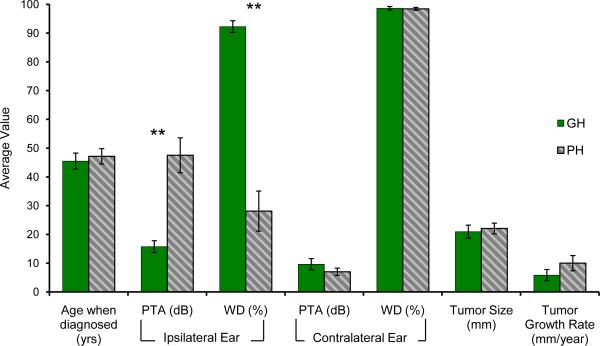
Tumors associated with GH significantly differed from those associated with PH in pure tone average (PTA) (p<0.001) and word discrimination (WD) scores (p<0.001) of the ipsilateral ear, but not with respect to age (p=0.70), sex (p=0.92), tumor size (p=0.71), PTA (p=0.25) or WD (p=0.80) of the contralateral ear (Figure 1 and Supplemental Table 1 summarizing subject demographics). Of the 37 proteins studied, 9 were significantly expressed in VS secretions compared with GAN controls (Figure 2). Of these, 6 demonstrated a trend for higher (Figure 2A) and 3 demonstrated a trend for lower expression (Figure 2B) in the tumors associated with GH versus PH. Two molecules met our criteria for statistically different level of expression between the two groups: FGF2 was 3.5-fold higher (p=0.02) and IL8 was 3.4-fold higher (p=0.02) in the VS associated with GH versus VS associated with PH (Figure 3A). Expression of FGF2 was closely correlated with IL8 expression for any given tumor (R2=0.978) (Figure 3B).
Figure 1.
Patient Demographics. Age, pure tone average (PTA) and word discrimination (WD) for ipsilateral and contralateral ear, tumor size measured in transverse dimension in the cerebellopontine angle (based on most recent MRI scan prior to surgical excision), and tumor growth rate (based on change in transverse or greatest dimension between the first and last preoperative MRI scans) are shown. There is no statistically significant difference in gender between the good (GH) and poor hearing (PH) group. n=16 in GH and n=19 in PH group. Tumor growth rate was available for minority of tumors (14 out of 35) that were followed by serial MRI scans prior to excision. Detailed information on individual samples is provided in the Supplemental Table 1. Error bars represent SEM. **p<0.01.
Figure 2.
Cytokine srray results for 37 proteins studied. Means and SEM are plotted for secretions from VS associated with GH (solid gray columns, n=11) and PH (backward shaded columns, n=10) and control GAN (forward shaded columns, n=7). (A) Protein secreted at higher levels in VS associated with GH versus PH; (B) Proteins secreted at higher levels in VS associated with PH versus GH. IL1B, IL10, IL12B, 1L13, IL15, IL17, TNFB and CEA are not shown as they were not significantly secreted in VS or GAN. Statistical significance of *p≤0.05 or **p<0.01 refers to VS secretions (combining GH and PH) versus GAN secretions.
Figure 3.
Analysis of significantly aberrant pathways in VS. Of the 9 proteins that were secreted by VS at significantly different levels than GAN, FGF2 and IL8 met statistical criteria for differential secretion levels between tumors associated with GH versus PH. (A) The difference in optical density between GH and PH group is plotted as mean +/ −SEM.*p≤0.05 (B) FGF2 and IL8 secretion levels were closely correlated among samples. Each point represents one sample; 2 GH samples and 4 BH samples are not shown as their FGF2 and IL8 levels were within background level.
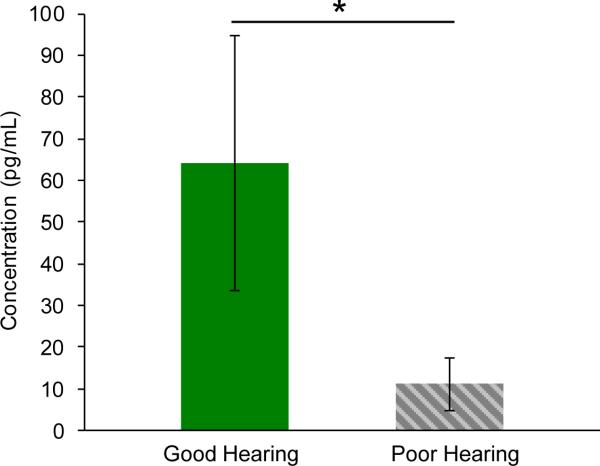
Results of cytokine array analysis were validated on a different set of tumor secretions using FGF2 and IL8 ELISA. The ELISA kits that we used have been extensively studied in the literature (20–23). We again found FGF2 at substantially higher levels in secretions associated with good versus poor hearing (p=0.05). The magnitude of the difference in average expression detected was higher with ELISA (5.8-fold change) (Figure 4) in comparison to the cytokine array, possibly due to the greater sensitivity of the antibody used in ELISA versus the proprietary antibody in the cytokine array, or due to biological differences in tumor samples. The IL8 ELISA showed no IL8 expression in VS secretions. The discrepancy between the IL8 ELISA and the cytokine array results is likely due to cross-reactivity between IL8 and FGF2 on the cytokine array. Their highly correlated expression on the cytokine array (Figure 3B) suggests a technical artifact as the cytokine array was manufactured with an IL8 antibody that was not tested for cross-reactivity with FGF2. In contrast, the IL8 antibody in the ELISA kit was found to have no significant cross-reactivity with FGF2 by the manufacturer.
Figure 4.
ELISA results. Secreted FGF2 levels are on average 5.8-fold higher in VS associated with GH re PH (p=0.05). The calculated protein concentrations are plotted as mean +/− SEM for VS associated with GH (n=8) and VS associated with PH (n=10).
Of the 9 molecules significantly expressed in VS (Figure 2), most are known to be secreted. However, three molecules are typically not secreted (CRYAB, ERBB2 and NF2), and their presence in tumor secretions is consistent with shedding (13–15).
Discussion
A comparative screening of 37 molecules in VS associated with good versus poor hearing identified FGF2 as a potential mediator of hearing protection in VS. The result was robust between the two different techniques applied to different samples despite the large inter-sample variability typically observed in human samples. Nine targets were found to be significantly present in the VS secretions relative to GAN. Some of these targets (CRYAB, NF2, ERBB2, PDGFA, PDGFB, and FGF2) were previously studied in pathophysiology of VS (16, 24–28). Fibroblast growth factor 2 is a known mitogen for proliferation of VS (29) but it has not been previously studied in the context of HL due to VS. Increased levels of FGF2 in tumors associated with GH suggest that FGF2 secreted by the tumors may mediate hearing protection. This conjecture is intriguing because of FGF2's known role in protection of the auditory neurons and hair cells from acoustic trauma, glutamate toxicity and aminoglycoside ototoxicity (17,18,30,31). It is possible that FGF2 secreted by VS, or by the cranial nerves surrounding VS, protect the auditory nerve and cochlea from damage and death caused by various mechanisms, including nerve compression or structural degradation. VS are known to be associated with substantial degeneration of cochlear structures, including loss of hair cells and cochlear neurons (4). FGF2 may be involved in limiting this degeneration in patients with VS that continue to have GH. It is difficult to assess the mechanisms through which FGF2 is asserting its protective role on the cochlear and neuronal cells since no causative studies for HL due to VS have been conducted thus far. It may be that FGF2 is blocking a putative neurotoxic or ototoxic substance, or interfering with the pathway(s) this substance controls, or easing death from nerve compression. Future studies are needed to delineate these possibilities.
Our finding of high levels of FGF2 secretions by VS associated with GH, combined with the known role of FGF2 as mitogen of VS in vitro (29) and its correlated expression with VS growth in vivo (28), is consistent with the published reports of large VS that do not cause HL (2,3,32). Our data suggest that FGF2 may mediate tumor growth and hearing level by different mechanisms. If the pathways that lead to tumor growth are divergent from the pathways that modulate hearing, then tumor size or growth rate do not have to correlate with HL. This notion is supported by our cytokine array analysis where levels of well-established growth modulators, such as ERBB, did not correlate with the hearing level, and FGF2 levels did not correlate with tumor size. We could calculate tumor growth rate for only 14 out of the 35 tumors because only 40% of the studied patients were followed by serial MRI scans prior to surgical excision. Within this small subset of tumors, we did not find a significant correlation between tumor growth rate and hearing outcome. This is in contrast to the prior work that found a correlation between high tumor growth rate or large tumor size and poor hearing (33,34). These divergent findings are likely not only due to the differences in the sample size, but also due to the methodological or definitional differences, such as the definition of tumor size and hearing loss.
If the putative otoprotective and neuroprotective effects of FGF2 secreted by VS are to be explored in future therapies to preserve hearing in patients with VS, exogenous FGF2 would have to be modified to minimize its growth promoting potential. Additionally, if FGF2 levels could be measured in the tumor microenvironment, such as by sampling blood serum or cerebrospinal fluid (35–37), then FGF2 may be explored as a biomarker to estimate the possibility of the tumor leading to HL, and to counsel patients accordingly.
It is reassuring that many proteins previously implicated in pathology of VS and/or HL were found to be significantly elevated in VS compared with control GAN in the present study. Consistent with our study, in which Merlin, the protein encoded by the NF2 gene, was absent in 3 out of 21 VS secretions, another study found approximately 23% of tumors to have alterations or loss of both NF2 alleles (38). About 81% of sporadic schwannomas have mutated NF2 genes with small deletions, and 93% of these mutations result in truncated proteins with defects in all or part of the C-terminus (39). Because the anti-NF2 antibody used in the cytokine array targets the n-terminus of the protein, we could detect NF2, even if mutated. ERBB2, known to shed in other types of cancers (40), was also found to be present in 17 out of the 21 tumor secretions. Our results are consistent with past work demonstrating ERBB2 in VS, where it plays a role in tumor proliferation and survival (41). Similarly, Platelet derived growth factor AA (PDGFA) and Platelet-derived growth factor BB (PDGFB) have been implicated in pathophysiology of VS (27, 42), and we found both to be present in 13 out of 21 VS secretions. We detected a trend, albeit not significant (p=0.93), for higher PDGFA secretion by VS associated with PH in comparison with GH (Fig. 2). This trend is consistent with a cDNA microarray analysis published previously (43). The decrease in significance between the current and prior study may be because we analyzed protein secretions using a larger sample size.
Interestingly, interleukin-1 receptor antagonist (IL1RN) was found to be substantially elevated in tumor secretions associated with GH versus PH, although the result did not meet our criterion for statistical significance (p=0.10). IL1RN serves to block the receptor for interleukin-1 (IL1) and therefore prevent IL1 signaling. Therapeutic blockade of IL1A and IL1B has been suggested to treat sensorineural HL (9). Fibronectin-1 (FN1), seen in our previous work as a biomarker for vestibular schwannomas in perilymph (16), was also found to be significantly upregulated in the VS secretions when compared with GAN secretions (p<0.001).
A larger sample size will be needed in future studies to validate the results of the current study. Moreover, a more elaborate stratification of hearing should be explored in the future (34), as opposed to a potentially simplistic stratification into two categories used in the current study. It is likely that several different mechanisms lead to the spectrum of HL seen with VS, and that these mechanisms vary in significance through a tumor's progression for different patients.
Conclusion
Sporadic vestibular schwannomas secrete cytokines at substantially higher levels than control great auricular nerves. Fibroblast growth factor 2 is secreted at significantly higher levels by VS with GH versus PH. From past literature, FGF2 is known to protect primary auditory neurons and hair cells from ototoxicity. If future studies establish a causative, rather than a correlative, relationship between FGF2 expression and hearing level in VS, then physicians could utilize FGF2 as a biomarker for prognosis of the likelihood of HL in patients with VS, which would influence counseling and surgical decision making. In addition, for tumors that are not growing and hence are followed radiographically, modulation of the FGF2 pathways may help maintain patients' hearing.
Supplementary Material
Acknowledgements
We would like to thank Dr. Kevin Emerick for assisting with sample collection.This project was supported by NIDCD Grants T32 DC00038 and K08DC010419.
Footnotes
Conflicts of Interest: None for all authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mahaley MSJ, Mettlin C, Natarajan n, Laws ERJ, Peace BB. Analysis of patterns of care of brain tumor patients in the united states: A study of the brain tumor section of the AANS and the CNS and the commission on cancer of the ACS. Clin Neurosurg. 1990;36:347–5. [PubMed] [Google Scholar]
- 2.Nadol JB, Diamond PF, Thornton AR. Correlation of hearing loss and radiologic dimensions of vestibular schwannomas (acoustic neuromas) Otol Neurotol. 1996;17(2):312–6. [PubMed] [Google Scholar]
- 3.Caye-Thomasen P, Dethloff T, Hansen S, Stangerup S, Thomsen J. Hearing in patients with intracanalicular vestibular schwannomas. Audiol Neurotol. 2007;12(1):1–12. doi: 10.1159/000096152. [DOI] [PubMed] [Google Scholar]
- 4.Roosli C, Linthicum F, Cureoglu S, Merchant S. Dysfunction of the cochlea contributing to hearing loss in acoustic neuromas: An underappreciated entity. Otol Neurotol. 2012;33(3):473–80. doi: 10.1097/MAO.0b013e318248ee02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stankovic K, Mrugala M, Martuza R, et al. Genetic determinants of hearing loss associated with vestibular schwannomas. Otol Neurotol. 2009;30(5):661–7. doi: 10.1097/MAO.0b013e3181a66ece. [DOI] [PubMed] [Google Scholar]
- 6.Adams J. Clinical implications of inflammatory cytokines in the cochlea: A technical note. Otol Neurotol. 2002;23(3):316–22. doi: 10.1097/00129492-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Satoh H, Firestein GS, Billings PB, Harris JP, Keithley EM. Proinflammatory cytokine expression in the endolymphatic sac during inner ear inflammation. J Assoc Res Otolaryngol. 2003;4(2):139–47. doi: 10.1007/s10162-002-3025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rynne M, Maclean C, Bybee A, McDermott MF, Emery P. Hearing improvement in a patient with variant muckle-wells syndrome in response to interleukin 1 receptor antagonism. Ann Rheum Dis. 2006;65(4):533–4. doi: 10.1136/ard.2005.038091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldbach-Mansky R, Dailey NJ, Canna SW, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1B inhibition. n Engl J Med. 2006;355(6):581–92. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes PJ, Adcock I. Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharmacol Sci. 1993;14(12):436–41. doi: 10.1016/0165-6147(93)90184-l. [DOI] [PubMed] [Google Scholar]
- 11.Lebel MH, Freij BJ, Syrogiannopoulos GA, et al. Dexamethasone therapy for bacterial meningitis. n Engl J Med. 1988;319(15):964–71. doi: 10.1056/NEJM198810133191502. [DOI] [PubMed] [Google Scholar]
- 12.Chen C, Halpin C, Rauch SD. Oral steroid treatment of sudden sensorineural hearing loss: A ten year retrospective analysis. Otol Neurotol. 2003;24(5):728–33. doi: 10.1097/00129492-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Steffensen KD, Waldstrøm M, Jeppesen U, Knudsen HJ, Brandslund I, Jakobsen A. Preoperative serum levels of epidermal growth factor receptor, HER2, and vascular endothelial growth factor in malignant and benign ovarian tumors. Clin Ovarian Cancer. 2008;1(2):127–34. [Google Scholar]
- 14.Black PH. Shedding from normal and cancer-cell surfaces. n Engl J Med. 1980;303(24):1415–6. doi: 10.1056/NEJM198012113032411. [DOI] [PubMed] [Google Scholar]
- 15.Taylor D, Black P. Shedding of plasma membrane fragments. neoplastic and developmental importance. Dev Biol (NY) 1986;3:33–57. doi: 10.1007/978-1-4684-5050-7_3. [DOI] [PubMed] [Google Scholar]
- 16.Lysaght AC, Kao S, Paulo JA, Merchant SN, Steen H, Stankovic KM. Proteome of human perilymph. J Proteome Res. 2011;10(9):3845–51. doi: 10.1021/pr200346q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhai S, Wang D, Wang J, Han D, Yang W. Basic fibroblast growth factor protects auditory neurons and hair cells from glutamate neurotoxicity and noise exposure. Acta Otolaryngol. 2004;124(2):124–9. doi: 10.1080/00016480310015939. [DOI] [PubMed] [Google Scholar]
- 18.Low W, Dazert S, Baird A, Ryan AF. Basic fibroblast growth factor (FGF-2) protects rat cochlear hair cells in organotypical culture from aminoglycoside injury. J Cell Physiol. 1996;167(3):443–450. doi: 10.1002/(SICI)1097-4652(199606)167:3<443::AID-JCP8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 19.Sørensen V, Nilsen T, Wiedłocha A. Functional diversity of FGF-2 isoforms by intracellular sorting. Bioessays. 2006;28(5):504–14. doi: 10.1002/bies.20405. [DOI] [PubMed] [Google Scholar]
- 20.Clyne AM, Zhu H, Edelman ER. Elevated fibroblast growth factor-2 increases tumor necrosis factor-α induced endothelial cell death in high glucose. J Cell Physiol. 2008;217(1):86–92. doi: 10.1002/jcp.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu J, Jiang W, Yang J, et al. Electronegative LDL impairs vascular endothelial cell integrity in diabetes by disrupting fibroblast growth factor 2 (FGF2) autoregulation. Diabetes. 2008;57(1):158–66. doi: 10.2337/db07-1287. [DOI] [PubMed] [Google Scholar]
- 22.Schadendorf D, Moller A, Algermissen B, Worm M, Sticherling M, Czarnetzki BM. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol. 1993;151(5):2667–75. [PubMed] [Google Scholar]
- 23.Ankersmit HJ, Hoetzenecker K, Dietl W, et al. Irradiated cultured apoptotic peripheral blood mononuclear cells regenerate infarcted myocardium. Eur J Clin Invest. 2009;39(6):445–56. doi: 10.1111/j.1365-2362.2009.02111.x. [DOI] [PubMed] [Google Scholar]
- 24.Ousman SS, Tomooka BH, van Noort JM, et al. Protective and therapeutic role for[agr]B-crystallin in autoimmune demyelination. Nature. 2007;448(7152):474–9. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- 25.Hansen MR, Roehm PC, Chatterjee P, Green SH. Constitutive neuregulin-1/ErbB signaling contributes to human vestibular schwannoma proliferation. Glia. 2006;53(6):593–600. doi: 10.1002/glia.20316. [DOI] [PubMed] [Google Scholar]
- 26.Welling DB, Guida M, Goll F, et al. Mutational spectrum in the neurofibromatosis type 2 gene in sporadic and familial schwannomas. Hum Genet. 1996;98(2):189–93. doi: 10.1007/s004390050188. [DOI] [PubMed] [Google Scholar]
- 27.Hanemann CO, Bartelt-Kirbach B, Diebold R, Kampchen K, Langmesser S, Utermark T. Differential gene expression between human schwannoma and control schwann cells. Neuropathol Appl Neurobiol. 2006;32(6):605–14. doi: 10.1111/j.1365-2990.2006.00769.x. [DOI] [PubMed] [Google Scholar]
- 28.Koutsimpelas D, Stripf T, Heinrich UR, Mann WJ, Brieger J. Expression of vascular endothelial growth factor and basic fibroblast growth factor in sporadic vestibular schwannomas correlates to growth characteristics. Otol Neurotol. 2007;28(8):1094–9. doi: 10.1097/MAO.0b013e31814b2787. [DOI] [PubMed] [Google Scholar]
- 29.Weerda HG. Effects of transforming growth factor-ß1 and basic fibroblast growth factor on proliferation of cell cultures derived from human vestibular nerve schwannoma. Acta Otolaryngol. 1998;118(3):337–43. doi: 10.1080/00016489850183412. [DOI] [PubMed] [Google Scholar]
- 30.Zhai S, Cheng J, Wang J, Yang W, Gu R, Jiang S. Protective effect of basic fibroblast growth factor on auditory hair cells after noise exposure. Acta Otolaryngol. 2002;122(4):370–3. doi: 10.1080/00016480260000030. [DOI] [PubMed] [Google Scholar]
- 31.Yin JF, Zhai SF, Guo WF, Hu YF, Shi L. Protective and rescue effects of transgenic bFGF/GFP expression mediated by cationic liposome on gentamicin-induced guinea pig cochlear toxicity. Zhonghua yi xue za zhi. 2002;82(17):1192–4. [PubMed] [Google Scholar]
- 32.Arriaga MA, Long S, Nelson R. Clinical correlates of acoustic neuroma volume. Otol Neurotol. 1993;14(5):465–8. doi: 10.1097/00129492-199309000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Sughrue ME, Yang I, Aranda D, et al. The natural history of untreated sporadic vestibular schwannomas: A comprehensive review of hearing outcomes. J Neurosurg. 2010;112(1):163–167. doi: 10.3171/2009.4.JNS08895. [DOI] [PubMed] [Google Scholar]
- 34.Meyer TA, Canty PA, Wilkinson EP, Hansen MR, Rubinstein JT, Gantz BJ. Small acoustic neuromas: Surgical outcomes versus observation or radiation. Otology & Neurotology. 2006;27(3) doi: 10.1097/00129492-200604000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Larsson A, Sköldenberg E, Ericson H. Serum and plasma levels of FGF-2 and VEGF in healthy blood donors. Angiogenesis. 2002;5(1):107–110. doi: 10.1023/a:1021588227705. [DOI] [PubMed] [Google Scholar]
- 36.Salven P, Orpana A, Teerenhovi L, Joensuu H. Simultaneous elevation in the serum concentrations of the angiogenic growth factors VEGF and bFGF is an independent predictor of poor prognosis in non-hodgkin lymphoma: A single-institution study of 200 patients. Blood. 2000;96(12):3712–3718. [PubMed] [Google Scholar]
- 37.Blasko I, Lederer W, Oberbauer H, et al. Measurement of thirteen biological markers in CSF of patients with Alzheimer's disease and other dementias. Dement Geriatr Cogn Disord. 2006;21(1):9–15. doi: 10.1159/000089137. [DOI] [PubMed] [Google Scholar]
- 38.Sainz J, Huynh DP, Figueroa K, Ragge NK, Baser ME, Pulst S. Mutations of the neurofibromatosis type 2 gene and lack of the gene product in vestibular schwannomas. Hum Mol Genet. 1994;3(6):885–91. doi: 10.1093/hmg/3.6.885. [DOI] [PubMed] [Google Scholar]
- 39.Sughrue ME, Yeung AH, Rutkowski MJ, Cheung SW, Parsa AT. Molecular biology of familial and sporadic vestibular schwannomas: Implications for novel therapeutics. J Neurosurg. 2011;114(2):359–66. doi: 10.3171/2009.10.JNS091135. [DOI] [PubMed] [Google Scholar]
- 40.Colomer R, Montero S, Lluch A, et al. Circulating HER2 extracellular domain and resistance to chemotherapy in advanced breast cancer. Clin Cancer Res. 2000;6(6):2356–62. [PubMed] [Google Scholar]
- 41.Ahmad ZK, Brown CM, Cueva RA, Ryan AF, Doherty JK. ErbB expression, activation, and inhibition with lapatinib and tyrphostin (AG825) in human vestibular schwannomas. Otol Neurotol. 2011;32(5):841–7. doi: 10.1097/MAO.0b013e31821f7d88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Coltrera MD, Gown AM. Cell proliferation in human soft tissue tumors correlates with platelet-derived growth factor B chain expression: An immunohistochemical and in situ hybridization study. Cancer Res. 1994;54(2):560–4. [PubMed] [Google Scholar]
- 43.Lassaletta L, Martanez-Glez V, Torres-Martan M, Rey JA, Gavilajn J. cDNA microarray expression profile in vestibular schwannoma: Correlation with clinical and radiological features. Cancer Genet Cytogenet. 2009;194(2):125–7. doi: 10.1016/j.cancergencyto.2009.06.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.