Abstract
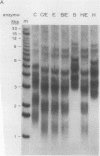
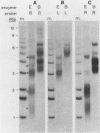
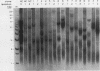
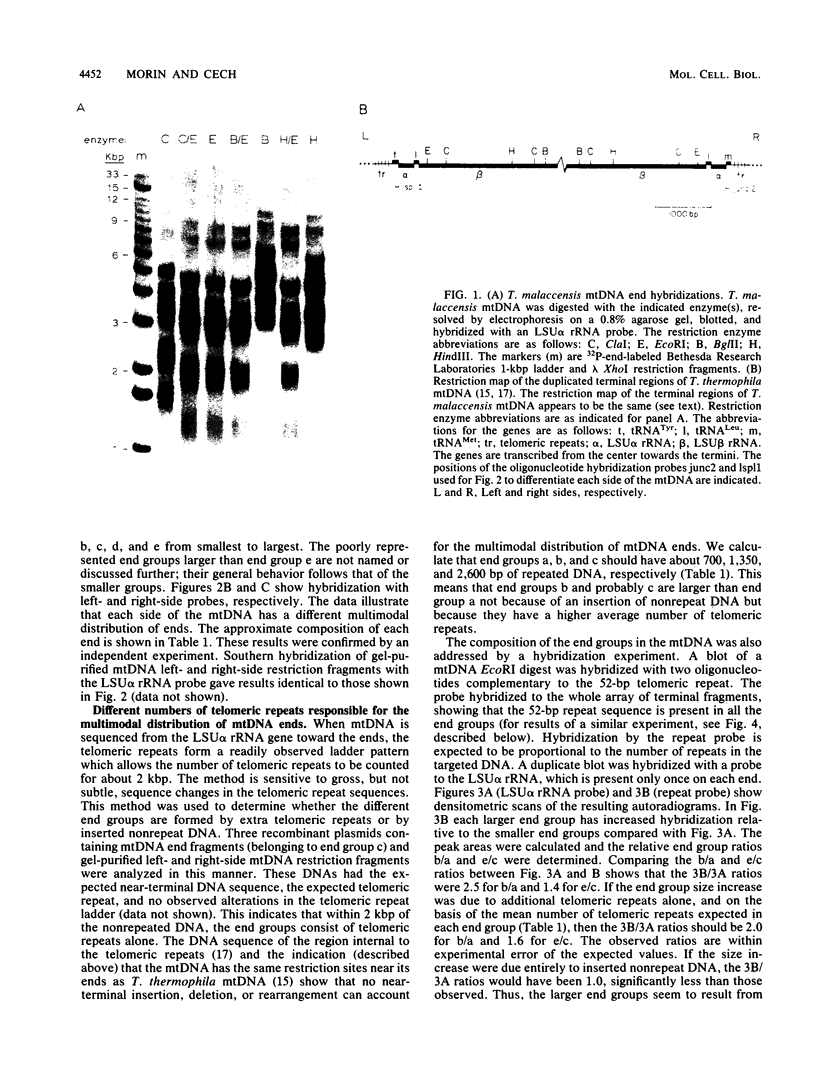
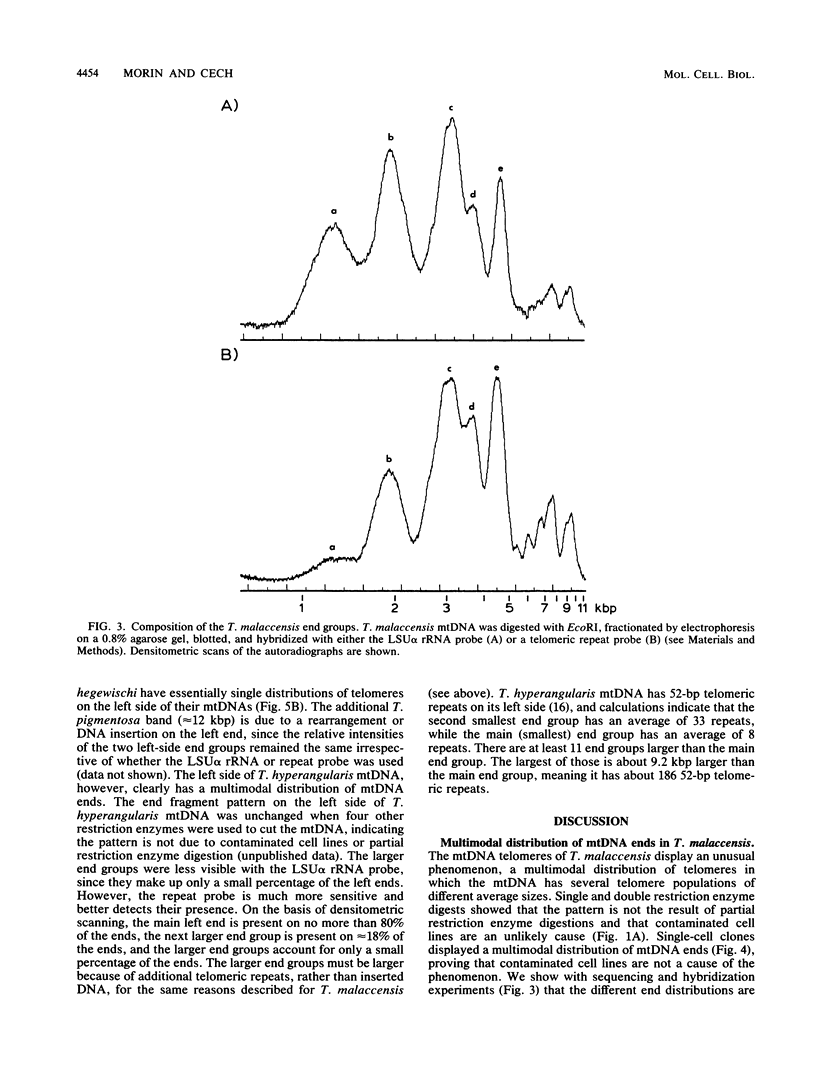
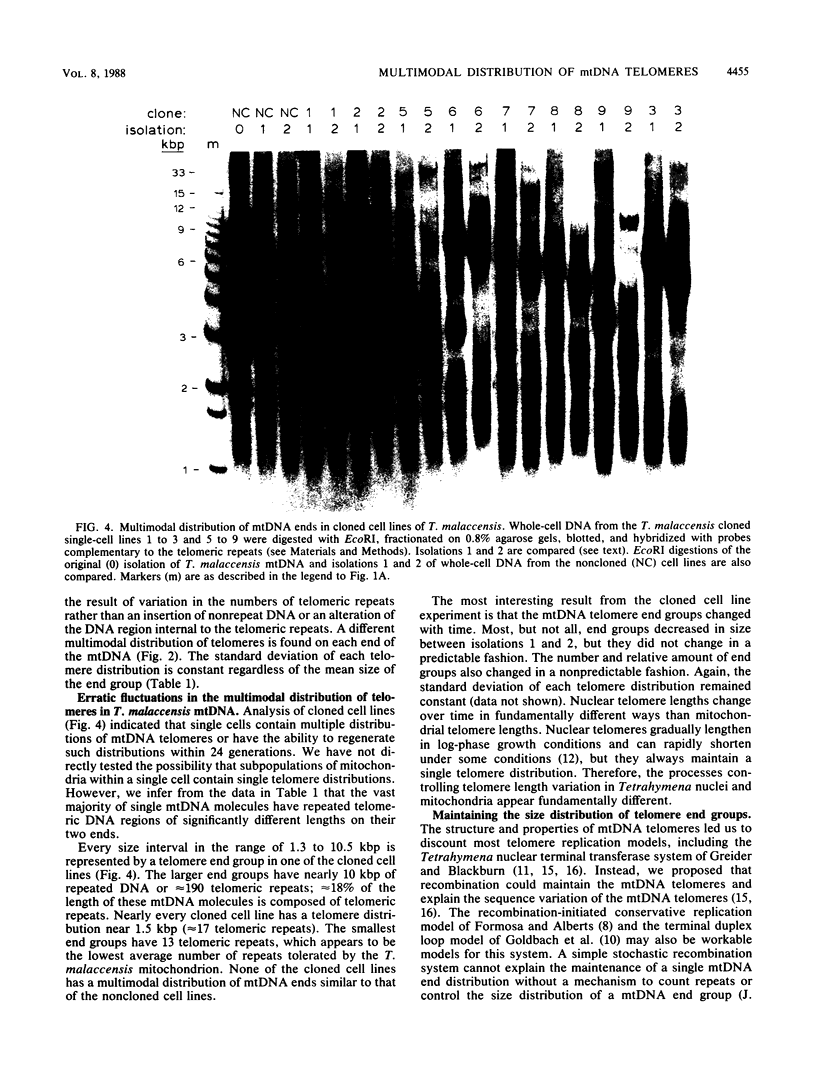
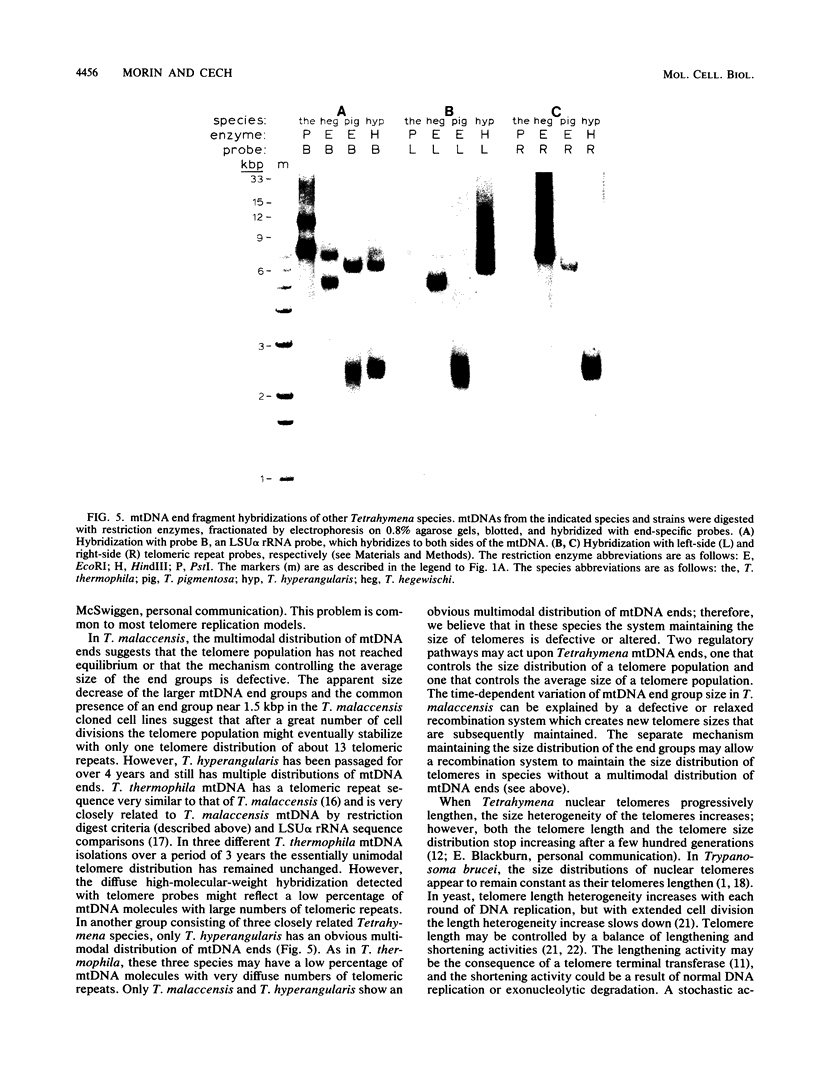
The linear mitochondrial DNA (mtDNA) of Tetrahymena malaccensis has tandem 52-base-pair repeats at its telomeres. The mtDNA has a multimodal distribution of telomeres. Different groups in the distribution have different numbers of telomeric repeats. The standard deviation of the size of each end group is independent of the mean size of the end group. The two sides of the mtDNA have different multimodal distributions of repeats. Cloned cell lines have multimodal distributions of mtDNA telomeres distinct from that of the original cell line. The number of telomere end groups and the average size of the end groups change in an erratic fashion as the cells are passaged and do not reach a stable equilibrium distribution in 185 generations. We propose that the mean size of a telomere end group and the size distribution of an end group are independently regulated. The system controlling the average size of end groups may be defective in T. malaccensis, since a closely related species (T. thermophila) does not have a multimodal distribution of mtDNA telomeres. T. hyperangularis, which has different telomeric repeats on each side of its mtDNA, has a multimodal distribution of mtDNA telomeres on only one side, suggesting that the mechanism controlling the average number of repeats in an end group can be sequence specific. These mitochondrial telomeres provide a new example of the more general phenomenon of expansion and contraction of arrays of repeated sequences seen, for example, with simple-sequence "satellite" DNAs; however, the mitochondrial telomeres change on a very short time scale.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernards A., Michels P. A., Lincke C. R., Borst P. Growth of chromosome ends in multiplying trypanosomes. Nature. 1983 Jun 16;303(5918):592–597. doi: 10.1038/303592a0. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Telomeres: do the ends justify the means? Cell. 1984 May;37(1):7–8. doi: 10.1016/0092-8674(84)90295-2. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Carson M. J., Hartwell L. CDC17: an essential gene that prevents telomere elongation in yeast. Cell. 1985 Aug;42(1):249–257. doi: 10.1016/s0092-8674(85)80120-3. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Rio D. C. Localization of transcribed regions on extrachromosomal ribosomal RNA genes of Tetrahymena thermophila by R-loop mapping. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5051–5055. doi: 10.1073/pnas.76.10.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formosa T., Alberts B. M. DNA synthesis dependent on genetic recombination: characterization of a reaction catalyzed by purified bacteriophage T4 proteins. Cell. 1986 Dec 5;47(5):793–806. doi: 10.1016/0092-8674(86)90522-2. [DOI] [PubMed] [Google Scholar]
- Gall J. G., Atherton D. D. Satellite DNA sequences in Drosophila virilis. J Mol Biol. 1974 Jan 5;85(4):633–664. doi: 10.1016/0022-2836(74)90321-0. [DOI] [PubMed] [Google Scholar]
- Goldbach R. W., Bollen-de Boer J. E., van Bruggen E. F., Borst P. Replication of the linear mitochondrial DNA of Tetrahymena pyriformis. Biochim Biophys Acta. 1979 May 24;562(3):400–417. doi: 10.1016/0005-2787(79)90104-7. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985 Dec;43(2 Pt 1):405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Larson D. D., Spangler E. A., Blackburn E. H. Dynamics of telomere length variation in Tetrahymena thermophila. Cell. 1987 Jul 31;50(3):477–483. doi: 10.1016/0092-8674(87)90501-0. [DOI] [PubMed] [Google Scholar]
- Lustig A. J., Petes T. D. Identification of yeast mutants with altered telomere structure. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1398–1402. doi: 10.1073/pnas.83.5.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazrimas J. A., Hatch F. T. A possible relationship between satellite DNA and the evolution of kangaroo rat species (genus Dipodomys). Nat New Biol. 1972 Nov 22;240(99):102–105. doi: 10.1038/newbio240102a0. [DOI] [PubMed] [Google Scholar]
- Morin G. B., Cech T. R. Mitochondrial telomeres: surprising diversity of repeated telomeric DNA sequences among six species of Tetrahymena. Cell. 1988 Feb 12;52(3):367–374. doi: 10.1016/s0092-8674(88)80029-1. [DOI] [PubMed] [Google Scholar]
- Morin G. B., Cech T. R. Phylogenetic relationships and altered genome structures among Tetrahymena mitochondrial DNAs. Nucleic Acids Res. 1988 Jan 11;16(1):327–346. doi: 10.1093/nar/16.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin G. B., Cech T. R. The telomeres of the linear mitochondrial DNA of Tetrahymena thermophila consist of 53 bp tandem repeats. Cell. 1986 Sep 12;46(6):873–883. doi: 10.1016/0092-8674(86)90069-3. [DOI] [PubMed] [Google Scholar]
- Pays E., Laurent M., Delinte K., Van Meirvenne N., Steinert M. Differential size variations between transcriptionally active and inactive telomeres of Trypanosoma brucei. Nucleic Acids Res. 1983 Dec 10;11(23):8137–8147. doi: 10.1093/nar/11.23.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta A. F., Dani G. M., Spear B. B., Zakian V. A. Elaboration of telomeres in yeast: recognition and modification of termini from Oxytricha macronuclear DNA. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1475–1479. doi: 10.1073/pnas.81.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritossa F., Scalenghe F., Di Turi N., Contini A. M. On the cell stage of X-Y recombination during rDNA magnification in Drosophila. Cold Spring Harb Symp Quant Biol. 1974;38:483–490. doi: 10.1101/sqb.1974.038.01.052. [DOI] [PubMed] [Google Scholar]
- Shampay J., Blackburn E. H. Generation of telomere-length heterogeneity in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1988 Jan;85(2):534–538. doi: 10.1073/pnas.85.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shampay J., Szostak J. W., Blackburn E. H. DNA sequences of telomeres maintained in yeast. Nature. 1984 Jul 12;310(5973):154–157. doi: 10.1038/310154a0. [DOI] [PubMed] [Google Scholar]
- Smith G. P. Unequal crossover and the evolution of multigene families. Cold Spring Harb Symp Quant Biol. 1974;38:507–513. doi: 10.1101/sqb.1974.038.01.055. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Base sequence and evolution of guinea-pig alpha-satellite DNA. Nature. 1970 Aug 22;227(5260):794–798. doi: 10.1038/227794a0. [DOI] [PubMed] [Google Scholar]
- Suyama Y., Miura K. Size and structural variations of mitochondrial DNA. Proc Natl Acad Sci U S A. 1968 May;60(1):235–242. doi: 10.1073/pnas.60.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Blackburn E. H. Cloning yeast telomeres on linear plasmid vectors. Cell. 1982 May;29(1):245–255. doi: 10.1016/0092-8674(82)90109-x. [DOI] [PubMed] [Google Scholar]
- Walmsley R. M., Petes T. D. Genetic control of chromosome length in yeast. Proc Natl Acad Sci U S A. 1985 Jan;82(2):506–510. doi: 10.1073/pnas.82.2.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. D. Origin of concatemeric T7 DNA. Nat New Biol. 1972 Oct 18;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- Zahler A. M., Prescott D. M. Telomere terminal transferase activity in the hypotrichous ciliate Oxytricha nova and a model for replication of the ends of linear DNA molecules. Nucleic Acids Res. 1988 Jul 25;16(14B):6953–6972. doi: 10.1093/nar/16.14.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]