Abstract
Many and complex plant-bacteria inter-relationships are found in the rhizosphere, since plants release a variety of photosynthetic exudates from their roots and rhizobacteria produce multifaceted specialized compounds including rich mixtures of volatiles, e.g., the bouquet of Serratia odorifera 4Rx13 is composed of up to 100 volatile organic and inorganic compounds. Here we show that when growing on peptone-rich nutrient medium S. odorifera 4Rx13 and six other rhizobacteria emit high levels of ammonia, which during co-cultivation in compartmented Petri dishes caused alkalization of the neighboring plant medium and subsequently reduced the growth of A. thaliana. It is argued that in nature high-protein resource degradations (carcasses, whey, manure and compost) are also accompanied by bacterial ammonia emission which alters the pH of the rhizosphere and thereby influences organismal diversity and plant-microbe interactions. Consequently, bacterial ammonia emission may be more relevant for plant colonization and growth development than previously thought.
Introduction
The rhizosphere defines the area of soil directly bordering the plant roots. This is a preferred habitat for many microorganisms, which has been known to contain up to 1011 microbial cells per gram root [1]. A complex exchange of organic and inorganic molecules is a prerequisite for such strong microbial growth. Plants, for example, secrete 20–50% of photosynthetically assimilated carbon as root exudates in the form of sugars, flavonoids, aliphatic acids, amino acids, organic acids and proteins [2], [3], [4], [5]. Microorganisms metabolize these rhizosphere deposits and release products themselves that influence organisms of this habitat in many different ways [6], [7], [8]. In the last decade, several reports established that rhizobacteria also emit complex mixtures of volatiles; which in turn influence the development of plants, fungi and other organisms positively or negatively [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]. Subsequent analysis of the headspace volatiles of the bacteria revealed spectra of different composition and complexity [8]. Although in many cases the bioactive components have yet to be determined, four bacterial volatiles - 2,3-butanediol, acetoin, 2-pentylfuran and CO2 - were demonstrated to act as plant-growth-promoting compounds [9], [20], [21]. In contrast, dimethyl disulfide, 2-phenylethanol and hydrogen cyanide were reliably shown to act as phytotoxic volatiles [17], [19], [22], [23]. Hydrogen cyanide is released from many Pseudomonas spp. and Chromobacterium spp. [19], [24], and dimethyl disulfide and 2-phenylethanol were found in the volatile blends of many bacterial species [8].
A survey showed that particularly rich volatile mixtures were released from species of the genera Chromobacter, Streptomyces and Serratia [8]. The emission spectrum of Serratia odorifera 4Rx13, which was isolated from the rhizosphere of Brassica napus [25], comprises approximately one hundred volatiles [22]. Although many compounds could be detected, only a few were unequivocally identified, such as sodorifen, 2-phenylethanol, dimethyl disulfide, dimethyl trisulfide, methanethiol, methanol, ethanol and CO2. Among these, dimethyl disulfide and 2-phenylethanol were shown to reduce plant growth [17], [22]. However, respective growth reductions were only visible in Petri dish experiments when high doses of both compounds (e.g. IC50: 20 µg) were applied. Such DMDS and 2-phenylethanol levels, however, were not reached by bacteria growing on NB medium in the Petri dish. Therefore it was hypothesized that additional inhibiting volatiles were released by S. odorifera 4Rx13 and preliminary investigations suggested ammonia to play a key role [22]. Since the bacterial emission of ammonia was not intensively studied, yet, we surveyed nine bacterial species and delved into a possible contribution of ammonia influencing the growth of A. thaliana.
Materials and Methods
Organisms and the Co-cultivation of Bacteria and Plants
Bacteria which originated from the rhizosphere of potato or oilseed rape were selected: Serratia odorifera 4Rx13, S. plymuthica HRO-C48, S. plymuthica 3Re4-18, Pseudomonas fluorescens L13-6-12, P. fluorescens 3Re2-7, Bacillus subtilis B2g, Stenotrophomonas maltophilia R3089, S. rhizophila P69, Staphylococcus epidermidis 2P3-18a [11].
Bacterial strains were cultivated either on nutrient broth (NBII) [11] or on synthetic medium (DMG) [26]. Arabidopsis thaliana Col-0 was sterilized and cultivated on Murashige-Skoog (MS) medium as described [12], [17], [22], [27]. In one experimental set up the plant medium was adjusted to pH 5, 6, 7 or 8 using NaOH (Fig. S2). Ten strains of A. thaliana Col-0 and 50 µl S. odorifera 4Rx13 (107 cell ml−1) were co-cultivated in bipartite Petri dishes as described by Wenke and colleagues [17] (Fig. 1 and S1). To evaluate the influence of nutrients, NBII was supplemented with 10 mM, 50 mM and 100 mM glucose (Carl Roth, Karlsruhe, Germany; Fig. S1). For root analysis, bipartite Petri dishes were positioned vertically in the growth chamber to allow plant roots to grow without restriction. Plant growth was determined according to i) the primary root length after 5 days and ii) the fresh weight of shoots after 10 days of co-cultivation. The results were compared to control plants that were grown without the co-cultivation of bacteria.
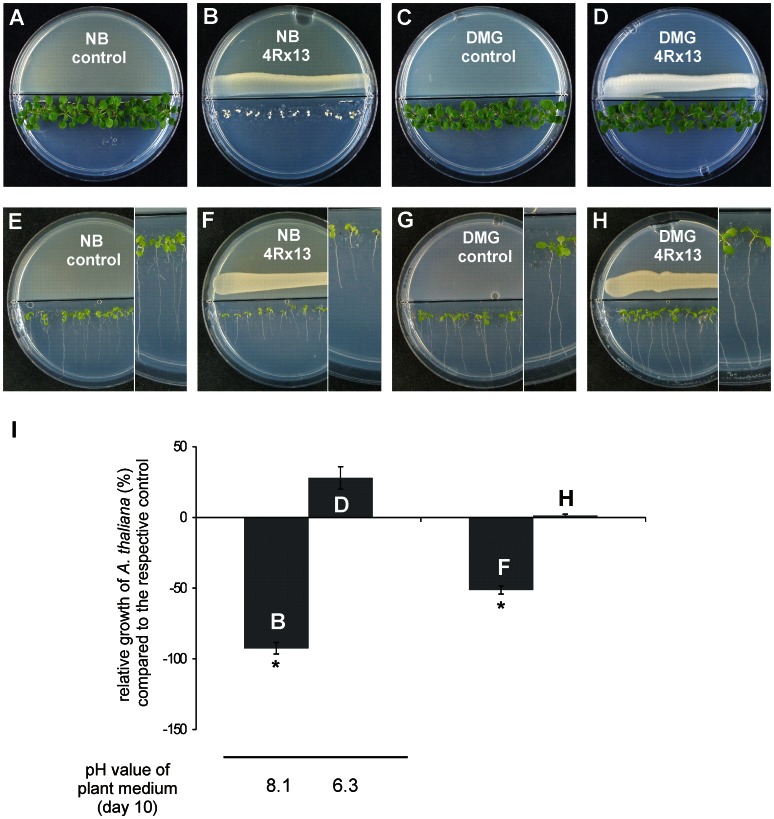
Figure 1. Growth of Arabidopsis thaliana Col-0 co-cultivated with Serratia odorifera 4Rx13.
(a–d) Determination of shoot fresh weight of A. thaliana co-cultivated with S. odorifera 4Rx13. A. thaliana seedlings were placed on MS medium and S. odorifera 4Rx13 was applied near the plastic barrier on NB II (b) or DMG (d). (e–h) Determination of root fresh weight of A. thaliana co-cultivated with S. odorifera 4Rx13. Petri dishes were incubated vertically to allow better exploration of root growth. (a, e) and (c, g) were inoculated without bacteria. (i) Quantitative determination of the growth of A. thaliana after 10 days of co-cultivation. Relative increase/decrease of fresh weights and root lengths was calculated in comparison to plants that were not co-cultivated with bacteria (a, c, e, g = controls). Lower panel indicates the pH of the medium at the end of the experiment. Arithmetic means and standard deviations were calculated based on three experiments with five replicates. Significance (*) was calculated using Students t-test (p≤0.01). NB II: nutrient broth II; DMG: Davis-Mingioli+glucose = minimal medium with 55 mM glucose; MS: half strength of Murashige-Skoog plant medium.
Determination of pH Values in the Agar and NH3 Emission of Different Bacteria
The pH values of the media were determined by placing pH paper on the agar (Carl Roth, Karlsruhe, Germany) at different time points during cultivation (Fig. 2a, b). The ammonia emission was determined using Quantofix® ammonium test sticks (Macherey & Nagel, Düren, Germany) as described [22]. 50 µl of a bacterial culture (107 cell ml−1) was applied as a line on NBII agar in one compartment of bipartite Petri dishes. After 72 hours of cultivation, a slit was cut into the wall of the empty compartment and the ammonium test stick was deposited opposite to the bacterial culture. The slit was sealed with Nescofilm® (Carl Roth, Karlsruhe, Germany) to avoid any loss of volatiles and contaminations. After two hours, a microliter syringe was inserted through the slit and Nessler reaction was initiated with 10 µl dH2O. After 30 sec, the chemical reaction was stopped by adding 10 µl of NaOH (32%). The color changes were documented and compared with calibrated standards of 0.5 µmol, 1 µmol, 2.5 µmol, 5 µmol, 10 µmol and 50 µmol ammonia solutions (Carl Roth, Karlsruhe, Germany) [22]. The NH3 emission of S. odorifera 4Rx13 was analyzed after 3 h, 6 h, 12 h, 24 h, and 48 h, and every 24 h after until 240 h (Fig. 2a). The NH3 production of nine rhizobacteria was determined after 72 h (Fig. 2b). To check whether NH3 might be responsible for pH-value changes of the agar and negative growth effects on A. thaliana, plants were cultivated with concentrations exchanged on a daily basis (see calibration standard) of ammonia solution (Fig. S3).
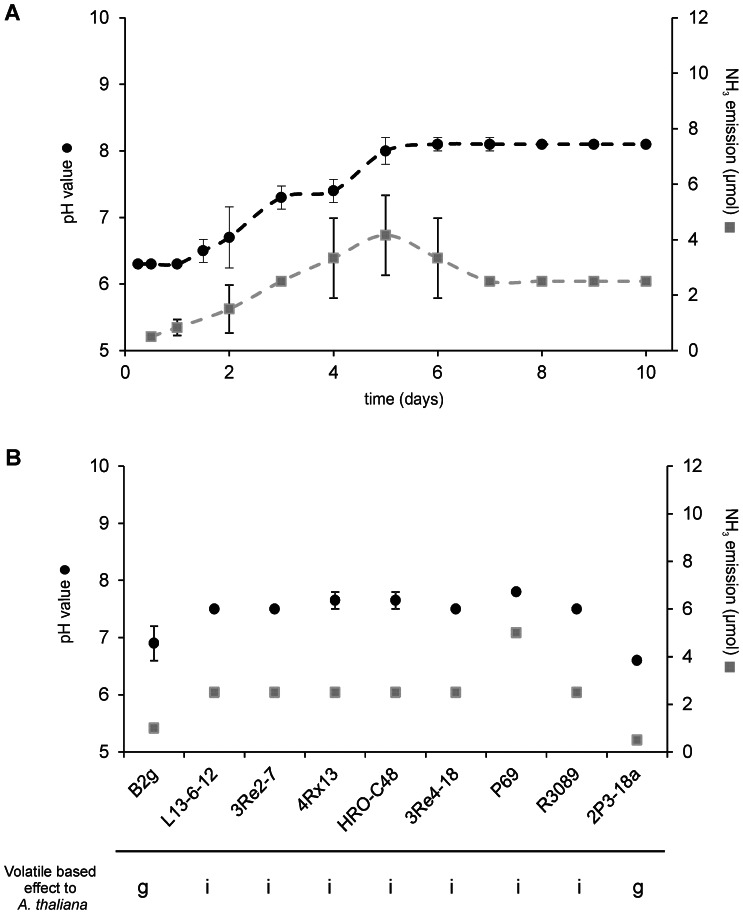
Figure 2. Emission of NH3 by Serratia odorifera 4Rx13 and pH shift of plant MS medium.
(a) S. odorifera 4Rx13 was applied on NB II medium along the plastic barrier of the bipartite Petri dish. At indicated time points, ammonia (gray, dashed line) was quantified in the headspace of the second compartment using Quantofix test paper. The color changes were documented and compared with calibrated standard curve of 0.5 µmol, 1 µmol, 2.5 µmol, 5 µmol, 10 µmol and 50 µmol ammonia solutions [22]. The pH value of the plant MS medium in the second compartment was also determined (black, dashed line). NH3 emissions and pH values were determined during a time course of 10 days. Arithmetic means and standard deviations were calculated based on three experiments each with two replicates. NB II: nutrient broth II; MS: half strength of Murashige-Skoog plant medium. (b) NH3 emissions (gray square) and pH values in the MS medium (black square) were determined after 72 hours of growth of the following rhizobacteria: B2g - Bacillus subtilis, L13-6-12 - Pseudomonas fluorescens, 3Re2-7– Pseudomonas trivialis, 4Rx13 - Serratia odorifera, HRO-C48– Serratia plymuthica, 3Re4-18– Serratia plymuthica, P69– Stenotrophomonas rhizophila, R3089– Stenotrophomonas maltophilia, 2P3-18a - Staphylococcus epidermidis. Lower panel indicates A. thaliana inhibition (i) or growth (g) during co-cultivation with respective bacterial isolates.
Co-cultivation of Plants and Bacteria in the Presence of Phosphoric Acid (H3PO4)
To further confirm the hypothesis, we conducted co-cultivation experiments with phosphoric acid. Phosphoric acid reacts with ammonia to form ammonium phosphate salts [28]. 15 surface-sterilized and stratified A. thaliana seedlings were cultivated on MS agar for 72 h in the first compartment of tripartite Petri dishes (Fig. 3). In the second compartment, 20 µl bacterial culture of S. odorifera 4Rx13 (107 cell ml−1) was spotted and the third compartment was filled with 5 ml 0.74 mM H3PO4 (Carl Roth, Karlsruhe, Germany). The fresh weight of shoots was documented after 10 days and compared with that of controls (Fig. 3a).
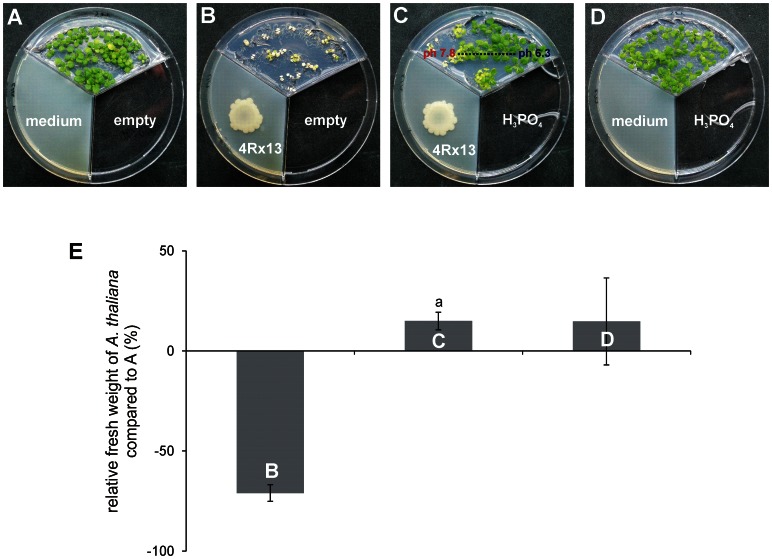
Figure 3. Co-cultivation of Arabidopsis thaliana Col-0 and Serratia odorifera 4Rx13 in the presence of phosphoric acid.
Growth of A. thaliana cultivated on MS medium in tripartite Petri dishes for 10 days: (a) cultivation without bacteria and without phosphoric acid (control 1); (b) co-cultivation with bacteria and without phosphoric acid; (c) co-cultivation with bacteria and with phosphoric acid; (d) cultivation without bacteria and with phosphoric acid (control 2). (e) Quantitative determination of the growth of A. thaliana. Relative increase/decrease of fresh weights of shoots was calculated in comparison to shoots cultivated without bacteria and without phosphoric acid (a). Arithmetic means and standard deviations were calculated based on three experiments each with five replicates. (a) indicates significances of p≤0.05 (Student’s t-test) in comparison to the co-cultivation of plants and bacteria without phosphoric acid. NB II: nutrient broth II; MS: half strength of Murashige-Skoog plant medium.
Statistics
Arithmetic means were calculated based on three repeated experiments, each performed with two to ten replicates. Significances were evaluated using Student’s t-test. P-values are indicated in the figure captions.
Results
It was previously documented that growth of Arabidopsis thaliana is inhibited by bacterial volatiles [12], [17], [23]. Chlorosis as well as the suppressed leaf and root development of A. thaliana were observed when the plants were co-cultivated with S. odorifera 4Rx13 on complex medium (NB II) (Fig. 1b and f, respectively), but no growth reduction was detected when the bacteria were cultivated on minimal medium supplemented with glucose (DMG) (Fig. 1d and h). This ‘glucose effect’ was corroborated by adding 10 to 100 mM glucose to NB II medium (Fig. S1); whereas strong inhibitory effects were obvious at low glucose concentrations, as glucose concentrations increased, growth retardation became less severe. It was concluded that S. odorifera 4Rx13 produced either different quantities or qualities of inhibitory or growth-promoting volatiles while growing on different media. In fact, the headspace volatiles of NBII were more complex and the profile was dominated by sodorifen [29], while only sparse amounts of sodorifen were present in the headspace of DMG (data not shown). Although contributing ca. 45% to Serratia’s blend, no plant growth alterations could be attributed to sodorifen, since the application of 0.2 µmol of pure sodorifen did not influence the growth of A. thaliana [22]. Interestingly, the growth inhibition of A. thaliana turned out to be only partially due to dimethyl disulfide and 2-phenylethanol [22], [17], and therefore it was concluded that other compounds of the bacterial blend must have inhibitory capabilities as well.
Routine determination of the pH of the plant medium gave the first hint of the identification of such a compound. Surprisingly, the pH of the plant medium increased from 6.3 to 8.1 upon volatile-mediated co-cultivation with S. odorifera 4Rx13 (Fig. S1g, lower panel). Furthermore, a correlation between plant growth inhibition and the alkalization of the plant medium was noticed. The plants grew well on media with pH of 5 and 6, while chlorotic phenotypes appeared when the plants were grown on media with elevated pH (Fig. S2). Consequently, it was hypothesized that bacterial volatiles may indirectly inhibit plant growth via the alkalization of the plant medium.
Ammonia was suspected to be responsible for this alkalization. In a time course experiment, bacterial ammonia and amine emissions were determined using Nessler’s reaction (Fig. 2a). A strong positive correlation between ammonia emission and pH alteration of the plant medium was observed between day 1 and day 5, while between day 5 and 10, ammonia emission slightly decreased and the pH of the medium remained at elevated levels. This decrease of ammonia emission is most likely due to growth cessation due to nutrient limitations. To further verify that the ammonia emanation of S. odorifera 4Rx13 was responsible for a pH shift of the plant medium, ammonia was scavenged by phosphoric acid to form ammonium phosphate salts [28]. Indeed, when A. thaliana was co-cultured with S. odorifera 4Rx13, and phosphoric acid was applied into the third compartment, the growth of the plant was restored (Fig. 3). Furthermore, the application of 1 µmol or higher amounts of commercially available ammonia to the test system retarded plant growth significantly (Fig. S3). The chlorotic phenotype, the decrease of fresh weight and the increased pH values of the plant medium in combination with the neutralization of these effects by ammonia removal indicate that ammonia caused plant growth inhibition indirectly via the alkalization of the plant medium.
In light of these results ammonia emissions of nine bacteria and the subsequent pH shifts in the plant media were determined (S. odorifera 4Rx13, S. plymuthica HRO-C48, S. plymuthica 3Re4-18, Pseudomonas fluorescens L13-6-12, P. trivialis 3Re2-7, Stenotrophomonas rhizophila P69 and Stenotrophomonas maltophilia R3089, Staphylococcus epidermidis 2P3-18a and Bacillus subtilis B2g). All tested bacterial strains except B. subtilis and S. epidermidis emitted ammonia at substantial levels; the emission by S. rhizophila P69 was especially pronounced (Fig. 2b). The high ammonia release generated a pH shift in the plant medium, which correlated with the negative growth effects of A. thaliana, while the low ammonia emission of B. subtilis B2g and S. epidermidis 2P3-18a resulted in small or no pH shifts and had no effect on plant growth. These results substantiated the observation that bacteria growing on peptone-rich media released ammonia in concentrations that were sufficient to alkalize the MS medium which in turn retarded plant growth.
Discussion
This paper demonstrates i) the potential of ammonia emission by rhizobacteria and ii) its consequences for the growth and development of Arabidopsis thaliana in volatile-mediated co-cultivations with bacteria.
Ammonia can be produced by nitrite ammonification [30], by the degradation of various amino acids utilized from proteins of food or of complex media [31], by the decarboxylation of amino acids to produce biogenic amines as well as ammonia [32], by deamination, and by the urease-mediated hydrolytic degradation of urea [33]. The genome of S. odorifera 4Rx13 encodes more than 55 putative ammonia-producing enzymes, including ammonia lyases, amino acid and nucleotide deaminases, nitrilases, nitrite reductases, pyridoxamine phosphate oxidases, and amino acid deaminases, which strongly support the process of ammonia synthesis. Similar enzyme activities are also expected to be present in the other eight bacterial species investigated here.
Ammonia fulfills several biological roles. In addition to its important metabolic role in many organisms, ammonia’s toxicity is well known. Ammonia seems not to be toxic for non-phototrophic bacteria even at rather high levels (over 100 mM); in contrast cyanobacteria and plants tolerate only low levels [34]. One prerequisite for toxic functionality appears to be its rapid diffusion through the majority of biological membranes [33]. Due to the rather lipophilic character of the uncharged NH3 molecule, the rapid permeation is biologically significant even at small concentration differences across the membrane. When ammonia accumulates in the plant cells at levels higher than 0.1 mM, plants showed symptoms such as the chlorosis of leaves, a lowered root/shoot ratio, stimulated root branching, declined mycorrhizal associations, and inhibited seed germination and seedling establishment [35].
The mechanisms that underlie these phenotypic aberrations are manifold; among them are biochemical pH-stat systems that account for differences in the internal H+ balance and decreasing external pH due to NH4 + acquisition. In contrast to the acidification of the rhizosphere, the co-cultivation experiments presented here demonstrated significant plant damage due to the alkalization of the medium (ca. pH 8) as a consequence of bacterial ammonia emission. It was previously shown that NH3 entered plant cells very rapidly in the presence of a high external pH, inducing transient elevations of cytoplasmic and vacuolar pH [36], [37]. Furthermore, NH3 treatment at high pH levels stimulated the increase of cytoplasmic calcium concentrations due to Ca2+ influx through the plasma membrane or Ca2+ release from internal stores; subsequently altering the calcium homeostasis [38]. It seems very likely that due to the continuous production and emission of NH3 into the headspace, similar events occurred in A. thaliana during its volatile-mediated co-cultivation with S. odorifera 4Rx13.
The volatile-based bacterial-plant interactions are, however, more complex and cannot only be explained by the emission of ammonia. Stenotrophomonas rhizophila P69 for example, emits much more ammonia compared to S. odorifera 4Rx13 (Fig. 2b), but the reaction of A. thaliana to the volatiles of both bacteria were almost the same [12], suggesting that other volatiles of S. rhizophila may compensate the effects of ammonia. Another discrepancy was detected: Bacillus subtilis B2g emits approximately 1 µmol of ammonia at the third day which shifted the plant medium to pH 7 (Fig. 2b) and the daily application of 1 µmol ammonia in a bipartite Petri dish resulted in 95% plant growth reduction (Fig. S3), while according to Vespermann and colleagues [12] the growth of A. thaliana was not affected by the volatiles of B. subtilis B2g at day 10. These apparent inconsistencies may partially be explained by the different experimental set ups, e.g. i) time points of examination were different (3 days vs. 10 days), ii) daily ammonia applications are different to continuous production of volatiles by the bacteria and iii) the application of a single volatile ammonia most likely generates different reactions in the plant compared to a complex volatile mixture emitted by bacteria.
In addition to the above mentioned indirect action mode, bacterial ammonia may also operate directly on the roots and/or leaves. Uptake must be facilitated by ammonium transporters. Six members of an AMT family are known in A. thaliana: five genes are expressed in roots and one gene is expressed in pollen [39], [40], [41]. Each of these transporters has its own affinity profile to ammonia, suggesting specific physiological functions [42]. A working model hypothesized that the external ammonium signal is conferred to the cytosolic side, via either a membrane-anchored receptor-like kinase or a transceptor (a protein that acts as a transporter and receptor at the same time). Consequently, the root length of A. thaliana was reduced during volatile-mediated co-cultivation with bacteria (Fig. 1) [17], [23], which correlated well with observations of stunted roots due to NH4 + contact with the primary root tip; such contact arrested root growth by inhibiting cell elongation rather than cell division [43].
Organismal diversity and plant-microbe interactions may depend on bacterial ammonia emission. Experiments have to be conducted which monitor bacterial derived ammonia in the rhizosphere to elucidate ammonia depending processes in the subterranean zone of plants.
Supporting Information
Growth of Arabidopsis thaliana Col-0 volatile-mediated co-cultivated with Serratia odorifera 4Rx13 growing on different glucose concentrations. A. thaliana was co-cultivated with S. odorifera 4Rx13. A. thaliana seedlings were placed on MS medium and S. odorifera 4Rx13 was applied near the plastic barrier on NB II (b–e) or DMG (f). NB II was supplemented with glucose at indicated concentrations (c–e). Fresh weights of the shoots were determined after 10 days of co-cultivation. (g) Quantitative determination of the growth of A. thaliana. Relative increase/decrease of fresh weights of shoots was calculated in comparison to shoots which were cultivated without bacteria (a). Arithmetic means and standard deviations were calculated based on three experiments each with five replicates. A–f indicate significances of at least p≤0.05 (Student’s t-test) in comparison (a) to respective control plants, (b) to NB II, (c) to NB II +10 mM glucose, (d) to NB II +50 mM glucose, (e) to NB II +100 mM glucose and (f) to DMG. Lower panel: pH values of MS medium after 10 days of co-cultivation (b–f). pH values were analyzed by placing pH indicator paper on agar. The pH of the MS medium in the control experiment (a) remained at 6.3 throughout the experiment. NB II: nutrient broth II; DMG: Davis-Mingioli+glucose = minimal medium with 55 mM glucose; MS: half strength of Murashige-Skoog plant medium.
(TIF)
Growth of Arabidopsis thaliana Col-0 on MS medium of different pH. (a–e) A. thaliana was cultivated on MS medium of pH = 5, pH = 6, pH = 7, pH = 8 and pH = 9 for 10 days. (f) Quantitative determination of growth of A. thaliana. Relative fresh weights of shoots were calculated in comparison to the weight of plants grown at pH 6 (b = 100%). Arithmetic means and standard deviations were calculated based on three experiments each with seven replicates. Significances were calculated using Student’s t-test in comparison to b (a: p≤0.001; b: p≤0.005). MS: half strength of Murashige-Skoog plant medium.
(TIF)
Growth of Arabidopsis thaliana Col-0 in the presence of NH3. (a–g) Growth of ten surface-sterilized and stratified A. thaliana in the presence of different amounts of ammonia (0 to 50 µmol NH3 solved in 10 ml water). A. thaliana seedlings were placed on MS medium and ammonia was filled into the upper compartment of a bipartite Petri dish. Every 24 h the solution was exchanged and freshly prepared ammonia solution was applied. (h) Quantitative determination of the growth of A. thaliana after 10 days of co-cultivation. Relative increase/decrease of fresh weights of shoots was calculated in comparison to the weight of shoots cultivated without ammonia (a). Arithmetic means and standard deviations were calculated based on three experiments each with five replicates. Significances (*) were calculated using Student’s t-test in comparison to control (a) and 0.5 µmol NH3 (b), p>0.01. Lower panel: pH values of the MS medium 10 days after cultivation (b–g). pH values were determined by placing pH indicator paper on agar. The pH of the MS medium in the control experiment (a) remained at 6.3 throughout the experiment. MS: half strength of Murashige–Skoog plant medium.
(TIF)
Acknowledgments
The authors thank Sarah Härtl for technical assistance, and the University of Rostock and DFG for financial support to BP. We thank Emily Wheeler, Boston, Massachusetts, for improving the English of the manuscript.
Funding Statement
The author Birgit Piechulla was supported by DFG (Pi 153/26-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Egamberdieva D, Kamilova F, Validov L, Kucharova Z, Lugtenberg B (2008) High incidence of plant growth stimulating bacteria associated with the rhizosphere of wheat grown on salinated soil in Uzbekistan. Environ Microbial 10: 1–9. [DOI] [PubMed] [Google Scholar]
- 2. Lynch JM, Whipps JM (1990) Substrate flow in the rhizosphere. Plant and Soil 129: 1–10. [Google Scholar]
- 3.Uren NC (2001) Types, amounts, and possible functions of compounds released into the rhizosphere by soil-grown plants. In: Pinton R, Varanini Z, Nannipieri P (eds) The Rhizosphere – Biochemistry and Organic Substances at the Soil–Plant Interface, Marcel Dekker Inc, New York, 1–22.
- 4. Nguyen C (2003) Rhizodeposition of organic C by plants: Mechanisms and controls. Agronomie 23: 375–396. [Google Scholar]
- 5. Dennis PG, Miller AJ, Hirsch PR (2010) Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol Ecol 72: 313–327. [DOI] [PubMed] [Google Scholar]
- 6. Haas D, Defago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nature Reviews Microbiol 3: 307–319. [DOI] [PubMed] [Google Scholar]
- 7. Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Ann Rev Microbiol 63: 541–56. [DOI] [PubMed] [Google Scholar]
- 8. Effmert U, Kalderas J, Warnke R, Piechulla B (2012) Volatile mediated interactions between bacteria and fungi in the soil. J Chem Ecol 38: 665–703. [DOI] [PubMed] [Google Scholar]
- 9. Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, et al. (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci USA 100: 4927–4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernando WGD, Ramarathnam R, Krishnamoorthy AS, Savchuk SC (2005) Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol Biochem 37: 955–964. [Google Scholar]
- 11. Kai M, Effmert U, Berg G, Piechulla B (2007) Volatiles of bacterial antagonists inhibit mycelial growth of the plant pathogen Rhizoctonia solani . Arch Microbiol 187: 351–360. [DOI] [PubMed] [Google Scholar]
- 12. Vespermann A, Kai M, Piechulla B (2007) Rhizobacterial volatiles affect the growth of fungi and Arabidopsis thaliana . Appl Environ Microbiol 73: 5639–5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zou CS, Mo MH, Gu YQ, Zhou JP, Zhang KQ (2007) Possible contribution of volatile-producing bacteria in soil fungistasis. Soil Biol Biochem 39: 2371–2379. [Google Scholar]
- 14. Kai M, Vespermann A, Piechulla B (2008) The growth of fungi and Arabidopsis thaliana is influenced by bacterial volatiles. Plant Signal Behav 3: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kai M, Haustein M, Molina F, Petri A, Scholz B, et al. (2009) Bacterial volatiles and their action potential. Appl Microbiol Biotechnol 81: 1001–1012. [DOI] [PubMed] [Google Scholar]
- 16. Wenke K, Kai M, Piechulla B (2010) Belowground volatiles facilitate interactions between plant roots and soil organisms. Planta 231: 499–506. [DOI] [PubMed] [Google Scholar]
- 17. Wenke K, Wanke D, Kilian J, Berendzen K, Harter K, et al. (2012 b) Volatiles of two growth-inhibiting rhizobacteria commonly enroll AtWRKY1 function. The Plant J 70: 445–459. [DOI] [PubMed] [Google Scholar]
- 18. Kai M, Piechulla B (2010) Impact of volatiles of the rhizobacteria Serratia odorifera on the moss Physcomitrella patens . Plant Signal Behav 5: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blom D, Fabbri C, Eberl L, Weisskopf L (2011) Volatile-mediated killing of Arabidopsis thaliana by bacteria is mainly due to hydrogen cyanide. Appl Environ Microbiol 77: 1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kai M, Piechulla B (2009) Plant growth promotions due to rhizobacterial volatiles - an effect of CO2? FEBS Lett 583: 3473–3477. [DOI] [PubMed] [Google Scholar]
- 21. Zou CS, Li Z, Yu D (2010) Bacillus megaterium strain XTBG34 promotes plant growth by producing 2-pentylfuran. The Journal of Microbiology 48: 460–466. [DOI] [PubMed] [Google Scholar]
- 22. Kai M, Crespo E, Cristescu SM, Harren FJM, Piechulla B (2010) Serratia odorifera: analysis of volatile emission and biological impact of volatile compounds on Arabidopsis thaliana . Appl Microbiol Biotechnol 88: 965–976. [DOI] [PubMed] [Google Scholar]
- 23.Wenke K, Weise T, Warnke R, Valverde C, Wanke D, et al. (2012 a) Bacterial Volatiles Mediating Information Between Bacteria and Plants. In: Witzany G (ed) Biocommunication, Signaling and Communication in Plants, Springer Verlag, Berlin, Heidelberg, 327–347.
- 24. Knowles CJ (1976) Microorganisms and cyanide. Bacteriol Rev 40: 652–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berg G, Roskot N, Steidle A, Eberl L, Zock A, et al. (2002) Plant-dependent genotypic and phenotypic diversity of antagonistic rhizobacteria isolated from different Verticillium host plants. Appl Environ Microbiol 68: 3328–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davis BD, Mingioli E (1950) Mutants of Escherichia coli requiring methionine or vitamine B12. J Bacteriol 60: 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497. [Google Scholar]
- 28. Ryden JC, Mc Neil JE (1984) Application of the micrometeorological mass balance method to the determination of ammonia loss from a grazed sward. J Sci Food Agr 35: 1297–1310. [Google Scholar]
- 29. von Reuß SH, Kai M, Piechulla B, Francke W (2010) Octamethylbicyclo[3.2.1.]octadienes from the rhizobacterium Serratia odorifera 4Rx13. Angew Chem Int Ed 49: 2009–2010. [DOI] [PubMed] [Google Scholar]
- 30. Simon J (2002) Enzymology and bioenergetics of respiratory nitrite ammonification. FEMS Microbiol Rev 26: 285–309. [DOI] [PubMed] [Google Scholar]
- 31. Kanapka JA, Kleinberg I (1983) Catabolism of arginine by the mixed bacteria in human salivary sediment under conditions of low and high glucose concentration. Arch Oral Biol 11: 1007–1015. [DOI] [PubMed] [Google Scholar]
- 32. Özugul F, Özugul Y (2007) The ability of biogenic amines and ammonia production by single bacterial cultures. Eur Food Res Technol 225: 385–394. [Google Scholar]
- 33. Kleiner D, Traglauer A, Domm S (1998) Does ammonia production by Klebsiella contribute to pathogenesis? Bull Inst Pasteur 96: 257–265. [Google Scholar]
- 34. Henderson PJF (1971) Ion transport by energy-conserving biological membranes. Ann Rev Microbiol 25: 393–428. [DOI] [PubMed] [Google Scholar]
- 35. Britto DT, Kronzucker HJ (2002) NH4 + toxicity in higher plants: a critical review. J Plant Physiol 159: 567–584. [Google Scholar]
- 36. Kosegarten H, Grolig F, Wieneke J, Wilson G, Hoffmann B (1997) Differential ammonia-elicited changes of cytosolic pH in root hair cells of rice and maize as monitored by 2′,7′-bis-(2-car-boxyethyl)-5 (and -6)-carboxyfluorescein-fluorescence ratio. Plant Physiol 113: 451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilson GH, Grolig F, Kosegarten H (1998) Differential pH restoration after ammonia-elicited vacuolar alkalization in rise and maize root hairs as measured by fluorescence ratio. Planta 206: 154–161. [Google Scholar]
- 38. Plieth C, Sattelmacher B, Knight MR (2000) Ammonium uptake and cellular alkalization in roots of Arabidopsis thaliana: The involvement of cytoplasmic calcium. Physiol Plant 110: 518–523. [Google Scholar]
- 39. Loque D, von Wiren N (2004) Regulatory levels for the transport of ammonium in plant roots. J Exp Bot 55: 1293–1305. [DOI] [PubMed] [Google Scholar]
- 40. Yuan L, Loque D, Kojima S, Rauch S, Ishiyama K, et al. (2007) The organization of high affinity ammonium uptake in Arabidopsis roots depends on the spatial arrangement and biochemical properties of AMT1-type transporters. Plant Cell 19: 2636–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yuan L, Graff L, Loque D, Kojima S, Tsuchuya YN, et al. (2009) AtAMT1;4, a pollen-specific high affinity ammonium transporter of the plasma membrane in Arabidopsis. Plant Cell Physiol 50: 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lima JE, Kojima S, Takahashi H, von Wiren N (2010) Ammonium triggers lateral root branching in Arabidopsis in an ammonium transporter 1;3-dependent manner. Plant Cell 22: 3621–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li Q, Li BH, Kronzucker HJ, Shi WM (2010) Root growth inhibition by NH4 + in Arabidopsis is mediated by the root tip and is linked to NH4 + efflux and GMPase activity. Plant Cell Environ 9: 1529–1542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Growth of Arabidopsis thaliana Col-0 volatile-mediated co-cultivated with Serratia odorifera 4Rx13 growing on different glucose concentrations. A. thaliana was co-cultivated with S. odorifera 4Rx13. A. thaliana seedlings were placed on MS medium and S. odorifera 4Rx13 was applied near the plastic barrier on NB II (b–e) or DMG (f). NB II was supplemented with glucose at indicated concentrations (c–e). Fresh weights of the shoots were determined after 10 days of co-cultivation. (g) Quantitative determination of the growth of A. thaliana. Relative increase/decrease of fresh weights of shoots was calculated in comparison to shoots which were cultivated without bacteria (a). Arithmetic means and standard deviations were calculated based on three experiments each with five replicates. A–f indicate significances of at least p≤0.05 (Student’s t-test) in comparison (a) to respective control plants, (b) to NB II, (c) to NB II +10 mM glucose, (d) to NB II +50 mM glucose, (e) to NB II +100 mM glucose and (f) to DMG. Lower panel: pH values of MS medium after 10 days of co-cultivation (b–f). pH values were analyzed by placing pH indicator paper on agar. The pH of the MS medium in the control experiment (a) remained at 6.3 throughout the experiment. NB II: nutrient broth II; DMG: Davis-Mingioli+glucose = minimal medium with 55 mM glucose; MS: half strength of Murashige-Skoog plant medium.
(TIF)
Growth of Arabidopsis thaliana Col-0 on MS medium of different pH. (a–e) A. thaliana was cultivated on MS medium of pH = 5, pH = 6, pH = 7, pH = 8 and pH = 9 for 10 days. (f) Quantitative determination of growth of A. thaliana. Relative fresh weights of shoots were calculated in comparison to the weight of plants grown at pH 6 (b = 100%). Arithmetic means and standard deviations were calculated based on three experiments each with seven replicates. Significances were calculated using Student’s t-test in comparison to b (a: p≤0.001; b: p≤0.005). MS: half strength of Murashige-Skoog plant medium.
(TIF)
Growth of Arabidopsis thaliana Col-0 in the presence of NH3. (a–g) Growth of ten surface-sterilized and stratified A. thaliana in the presence of different amounts of ammonia (0 to 50 µmol NH3 solved in 10 ml water). A. thaliana seedlings were placed on MS medium and ammonia was filled into the upper compartment of a bipartite Petri dish. Every 24 h the solution was exchanged and freshly prepared ammonia solution was applied. (h) Quantitative determination of the growth of A. thaliana after 10 days of co-cultivation. Relative increase/decrease of fresh weights of shoots was calculated in comparison to the weight of shoots cultivated without ammonia (a). Arithmetic means and standard deviations were calculated based on three experiments each with five replicates. Significances (*) were calculated using Student’s t-test in comparison to control (a) and 0.5 µmol NH3 (b), p>0.01. Lower panel: pH values of the MS medium 10 days after cultivation (b–g). pH values were determined by placing pH indicator paper on agar. The pH of the MS medium in the control experiment (a) remained at 6.3 throughout the experiment. MS: half strength of Murashige–Skoog plant medium.
(TIF)