Abstract
We have previously reported that intrarenal angiotensin II (Ang II) levels are increased long before diabetes becomes apparent in obese Otsuka-Long-Evans-Tokushima-Fatty (OLETF) rats, a model of type 2 diabetes. In this study, we examined the changes in intrarenal renin-angiotensin system (RAS) activity in the developing kidneys of OLETF rats. Ang II contents and mRNA levels of RAS components were measured in male OLETF and control Long-Evans Tokushima (LETO) rats at postnatal days (PND) 1, 5, and 15, and at 4–30 weeks of age. In both LETO and OLETF rats, kidney Ang II levels peaked at PND 1, then decreased during the pre- and post-weaning periods. However, Ang II levels and gene expression of RAS components, including angiotensinogen (AGT), renin, and angiotensin-converting enzyme (ACE), were not significantly different between LETO and OLETF rats. Intrarenal Ang II contents further decreased during puberty (from 7 to 11 weeks of age) in LETO rats, bur not in OLETF rats. At 11 weeks of age, kidney Ang II levels, urinary AGT excretion, and mRNA levels of AGT and renin were higher in OLETF rats than in LETO rats, while blood glucose levels were not significantly different between these groups of rats. These data indicate that continued intrarenal expression of Ang II during pubescence contributes to the increases in intrarenal Ang II levels in prediabetic OLETF rats, and is associated with increased intrarenal AGT and renin expression. Inappropriate activation of the intrarenal RAS in the prediabetic stage may facilitate the onset and development of diabetic nephropathy in later life.
Keywords: angiotensin II, developing kidney, diabetes, angiotensinogen
Introduction
Recent clinical studies have identified potential role of the renin-angiotensin system (RAS) in the onset and development of diabetic nephropathy, which has blood pressure-independent anti-albuminuric effects in both hypertensive and normotensive patients with diabetic nephropathy [1, 2]. It has been also recently reported that early treatment with an angiotensin receptor blocker (ARB) significantly reduced the prevalence of microalbuminuria in patients and animal models with type 2 diabetes [3–6]. These data indicate that local activation of the intrarenal RAS is fundamental to the onset of diabetic nephropathy.
Preclinical studies have indicated that the intrarenal RAS in diabetic individuals is inappropriately activated, causing local Ang II over production in proximal tubular epithelia cells [7], mesangial cells [8], and podocytes [9], although the systemic RAS is generally suppressed. We previously reported that kidney Ang II levels are already increased in prediabetic state in obese Otsuka-Long-Evans-Tokushima-Fatty (OLETF) rats, a model of type 2 diabetes [10]. However, currently there are no data showing when the intrarenal RAS is actually activated in prediabetic obese animals.
In growing animals, postnatal maturation is accompanied by time-dependent changes in the expression of components of the intrarenal RAS [11–13]. For example, Yosipiv [13] et al. reported that kidney Ang II levels, which are related to the temporal changes in renin gene expression, were much higher in newborn than in adult kidneys. These data suggest that RAS is activated in the newborn period and then rapidly decreases during maturation in normal animals. However, no studies have compared the activity of the intrarenal RAS in type 2 diabetic animals from birth to adulthood compared with that in normal animals, whose RAS activity progressively decreases from birth to adulthood.
In the present study, we investigated whether intrarenal Ang II is inappropriately regulated in type 2 diabetic rats during maturation. In particular, we focused on kidney Ang II levels and gene expression of RAS components in the developing kidneys of OLETF rats at postnatal day (PND) 1 and during the pre- and post-weaning periods. We also examined whether the changes in these parameters that occurred during these earlier periods tracked with the development of diabetic nephropathy from week 11 to week 30.
Materials and Methods
Animal preparation
All experimental procedures were performed under the guidelines for the care and use of animals as established by Kagawa University Medical School. Female (late-gestation pregnant) OLETF and control Long-Evans Tokushima Otsuka (LETO) rats were supplied by Otsuka Pharmaceutical Co. Ltd. (Tokushima, Japan). All rats were supplied regular rat chow and tap water until the first day of the study. After spontaneous delivery, studies were performed in male rats at PND 1, 5, and 14, and at 4–30 weeks of age.
Blood pressure was measured in conscious rats by tail-cuff plethysmography (BP-98A; Softron Co., Tokyo, Japan) and mean blood pressure (MBP) was calculated. Twenty-four-hour urine samples were collected at 6–30 weeks of age. After decapitation, the individual kidneys were collected from 8–12 rats per group. One third of the left kidney of each animal was fixed in 10 % buffered paraformaldehyde for histological examination. The renal cortex was separated from the remaining left kidneys and used for mRNA extraction. The right kidney of each rat was homogenized in cold methanol and processed to measure intrarenal Ang II contents as previously described [13].
Real-time reverse transcription-PCR
The mRNA expression of glyceraldehydes-3-phosphate dehydrogenase (GAPDH), AGT, renin, ACE, Ang II type 1a receptor (AT1a), AT1b, AT2, transforming growth factor-β (TGF-β), connective tissue growth factor (CTGF), type I collagen, and type IV collagen was determined by real-time PCR using a Light Cycler Fast Start DNA Master SYBR Green I kits or TaqMan Gene Expression Assay kits (Applied Biosystems, Foster City, CA), as previously described [14, 15]. All data are expressed as the relative differences between LETO and OLETF rats after normalization for GAPDH expression.
Renal Ang II measurement
Renal AngII was measured using a radioimmunoassay as previously described [13]. Briefly, kidney samples were first extracted for angiotensin peptides by homogenization (ice-cold 100 % methanol), evaporation (overnight in a vacuum centrifuge), dissolution (50 mM sodium phosphate, 1 mM EDTA, 0.25 mM thimerosal, 2.5 mg/ml BSA, pH 7.4), and extraction (water, hexane, chloroform, and 90 % methanol). Finally, the angiotensin peptides were incubated with antiserum and 125 I-radiolabeled Ang II for 48 h at 4 °C. Bound and free angiotensin peptides were separated by dextran-coated charcoal, and the supernatants were counted by a computer-linked gamma counter for 5 min. Results were reported in femtomoles per g kidney weight.
Other analytical procedures
Urinary albumin excretion was determined using a rat albumin ELISA kit (Shibayagi Co., Ltd., Gunma, Japan) [15]. Postprandial blood glucose levels were measured with a glucose analyzer (microTP-test; Wako Co., Osaka, Japan) [10]. The urine creatinine concentration was measured using a Jaffe-method kit (microTPtest, Wako Co.) [14]. The urinary AGT concentration was determined using a rat AGT sandwich enzyme-linked immunosorbent assay as previously described [16].
Kidneys were fixed with 10 % formalin (pH 7.4), embedded in paraffin, sectioned into 3 µm thick slices, and were stained with periodic acid-Schiff (PAS; Mass Histology Service, Worcester, MA, USA). The extent of glomerular sclerosis was quantitatively evaluated by an automatic image analysis system using the stained tissue sections, as previously described [14–18]. The severity of glomerular sclerosis in the PAS-stained sections was determined using a semiquantitative score from 0 to 4: 0, no matrix expansion; 1, minor; 2, weak; 3, moderate; and 4, strong [6, 10, 14, 18].
Statistical analysis
Values are presented as means ± standard error. Differences between groups/times were evaluated using 1 or 2-way analysis of variance with repeated measures followed by the Newman-Keuls post hoc test. Values of p < 0.05 were considered statistically significant.
Results
MBP, blood glucose, body weight, left kidney/body weight ratio, and albumin/creatinine ratio
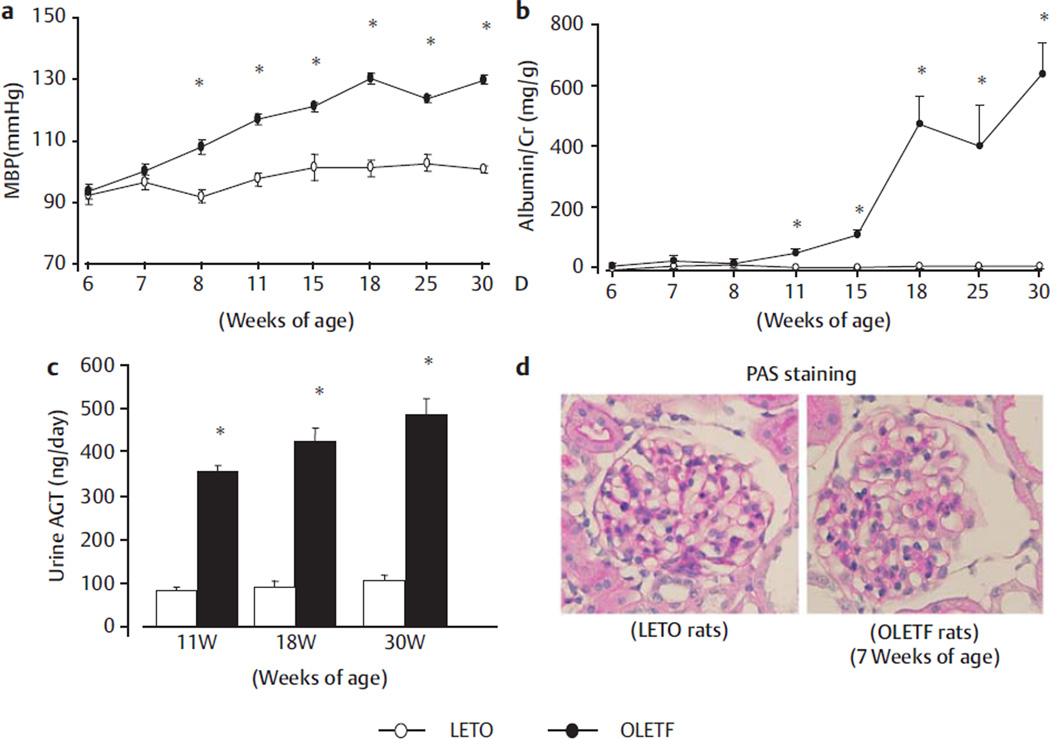
The temporal profiles of MBP, postprandial blood glucose, body weight and left kidney/body weight ratio are summarized in Fig. 1a and in Table 1. As shown in Fig. 1a MBP did not change between 6 and 30 weeks of age in LETO rats. On the other hand, progressively worsening hypertension was observed in OLETF rats (Fig. 1a). The body weight of OLETF rats was significantly higher than that of LETO rats from 4 weeks of age. The kidney/body weight ratio was also significantly higher in OLETF rats than in LETO rats after weaning. Postprandial blood glucose levels were significantly higher in OLETF rats than in LETO rats at 18 and 30 weeks of age (Table 1).
Fig. 1.
Time profiles of Mean blood pressure (MBP) a and urine albumin/creatinine ratio b and urinary excretion rate of AGT c in OLETF rats (number of 2 weeks: 22; 4 weeks: 10; 7 weeks: 10; 11 weeks: 10; 18 weeks: 8; 30 weeks: 6) and LETO rats (number of 2 weeks: 10; 4 weeks: 10; 7 weeks: 10; 11 weeks: 9; 18 weeks: 10; 30 weeks: 12). Photomicrographs of glomeruli with periodic acid-Schiff (PAS; d) reagents in 7-week-old OLETF rats and LETO rats. Original magnification, ×200 in d. *p < 0.05 vs. LETO rats at the same age.
Table 1.
Characteristics of OLETF and LETO rats at 2–30 weeks of age.
| Age (weeks) | 2 | 4 | 7 | 11 | 18 | 30 | |
|---|---|---|---|---|---|---|---|
| Number | OLETF | 22 | 10 | 10 | 10 | 8 | 6 |
| LETO | 10 | 10 | 10 | 9 | 10 | 12 | |
| BW (g) | OLETF | 32 ± 1 * | 95 ± 2 * | 240 ± 4 * | 352 ± 5 * | 533 ± 11 * | 573 ± 8 * |
| LETO | 26 ± 1 | 89 ± 2 | 189 ± 5 | 305 ± 4 | 441 ± 12 | 473 ± 10 | |
| LKW (g) | OLETF | 0.19 ± 0.01 * | 0.54 ± 0.02 * | 1.11 ± 0.03 * | 1.13 ± 0.05 | 1.62 ± 0.04 * | 1.54 ± 0.05 * |
| LETO | 0.15 ± 0.01 | 0.42 ± 0.01 | 0.79 ± 0.02 | 0.92 ± 0.03 | 1.13 ± 0.03 | 1.25 ± 0.01 | |
| LKW/BW (* 1000) | OLETF | 5.8 ± 0.1 | 5.8 ± 0.2 * | 4.7 ± 0.2 * | 3.2 ± 0.2 | 3.0 ± 0.1 * | 2.7 ± 0.1 * |
| LETO | 6.0 ± 0.1 | 4.6 ± 0.1 | 4.2 ± 0.2 | 3.0 ± 0.1 | 2.6 ± 0.1 | 2.3 ± 0.1 | |
| PPBG (mmol/l) | OLETF | 8.1 ± 0.3 | 8.1 ± 0.3 | 8.0 ± 0.1 | 10.7 ± 0.7* | 10.7 ± 0.9* | |
| LETO | 7.2 ± 0.2 | 7.6 ± 0.2 | 7.9 ± 0.3 | 8.4 ± 0.4 | 8.4 ± 0.2 |
Values are means ± standard error
p < 0.05 vs. LETO rats at the same age. BW: body weight; LKW: left kidney weight; PPBG: postprandial blood glucose
The temporal profile of the albumin/creatinine ratio is shown in Fig. 1b. The albumin/creatinine ratio was generally stable from 6 to 30 weeks of age in LETO rats. By contrast, the albumin/ creatinine ratio of OLETF rats was significantly higher than that of LETO rats from 7 to 30 weeks of age, increasing from 30 ± 2 to 646 ± 103 mg/g between these times (Fig. 1b).
AGT, renin, ACE, Ang II, AT1a, AT1b, and AT2 expression
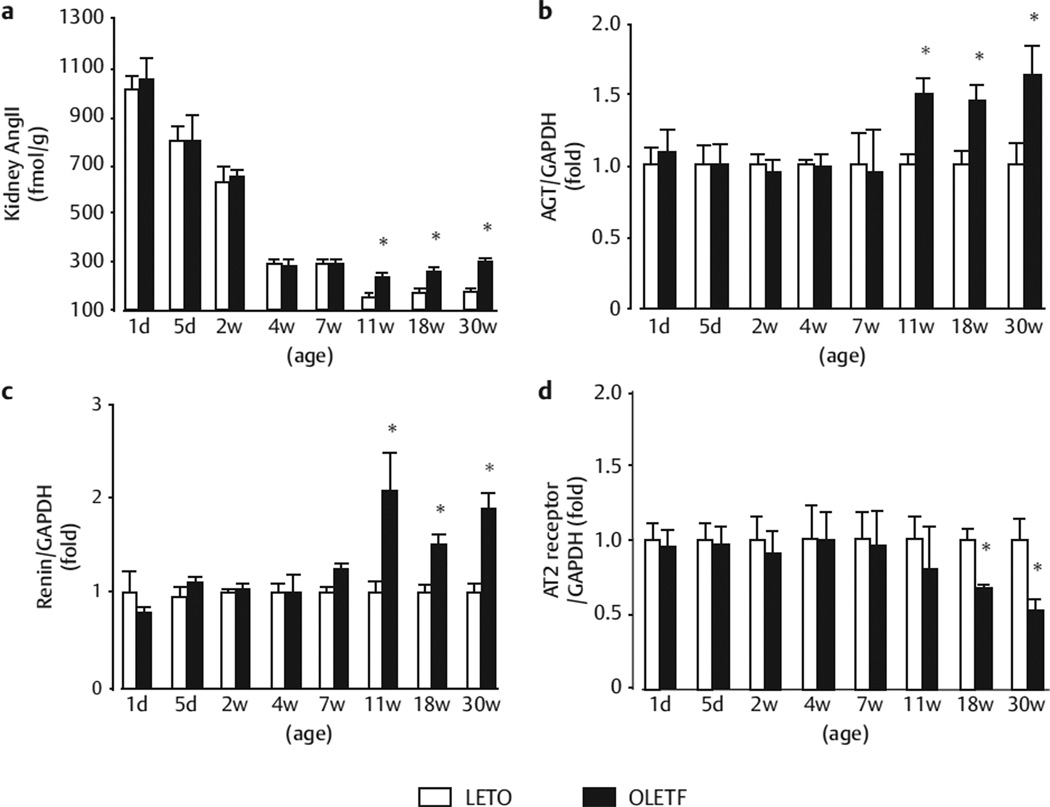
Ang II contents peaked at PND 1 in LETO and OLETF rats (1053 ± 92 and 1011 ± 54 pmol/g), (Fig. 2a) and decreased with age during the pre- and post-weaning periods (i. e., from PND 1 to 4 weeks of age) in both types of rats (Fig. 2a). There were no significant differences in Ang II contents or mRNA levels of AGT, renin between OLETF and LETO rats from PND 1 to 7 weeks of age (Fig. 2a–c). At 11 weeks of age, intrarenal Ang II content was significantly higher in OLETF rats than in LETO rats (241 ± 17 vs. 152 ± 11 pmol/g, Fig. 2a). At 11–30 weeks of age, AGT and renin mRNA levels were significantly higher in OLETF rats than in LETO rats (Fig. 2b, c). The urinary AGT excretion was also significantly greater in OLETF rats than in LETO rats from 11 weeks of age onwards (358 ± 14, 426 ± 28, and 487 ± 35 vs. 80 ± 11, 92 ± 11, and 103 ± 14 ng/day, at 11, 18, and 30 weeks of age; all p < 0.05) (Fig. 1c).
Fig. 2.
Time profiles of intrarenal cortical tissue Ang II content agene expression levels of AGT b renin c, and AT2 d. The mRNA expression levels are expressed as the relative differences between OLETF rats (number of 2 weeks: 22; 4 weeks: 10; 7 weeks: 10; 11 weeks: 10; 18 weeks: 8; 30 weeks: 6) and LETO rats (number of 2 weeks: 10; 4 weeks: 10; 7 weeks: 10; 11 weeks: 9; 18 weeks: 10; 30 weeks: 12) after normalization for the expression of GAPDH. *p < 0.05 vs. LETO rats at the same age.
The mRNA levels of ACE, AT1a, and AT1b were not significantly different during this time between OLETF and LETO rats (data not shown). On the other hand, AT2 mRNA levels were 0.8 ± 0.3- and 0.5 ± 0.1-fold lower in OLETF rats than in LETO rats at 11 and 30 weeks of age (Fig. 2d).
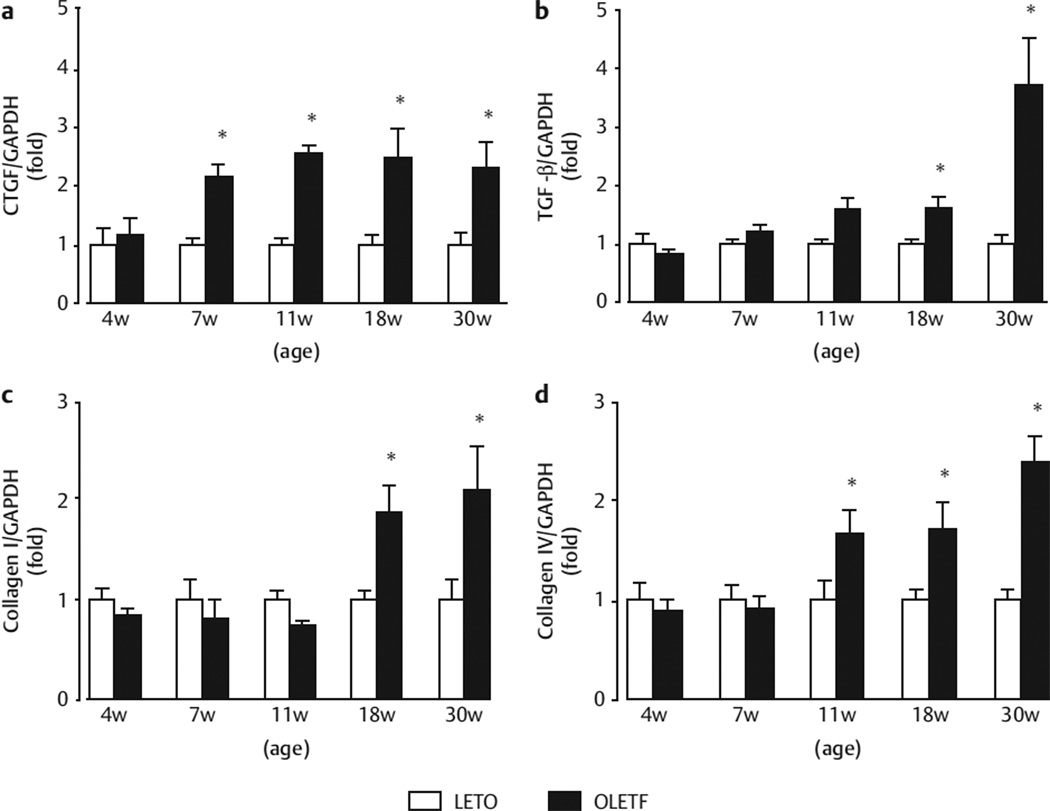
Renal cortical collagen, TGF-β, CTGF
The temporal expression profiles of CTGF, TGF-β, type I collagen, and type IV collagen are shown in Fig. 3. Renal cortical CTGF mRNA expression in OLETF rats was more higher than that of LETO rats at 7 and 30 weeks of age (Fig. 3a). Renal cortical mRNA expression of TGF-β (Fig. 3b) and type I collagen (Fig. 3c) in OLETF rats increased gradually until 18 weeks of age and then massively by 30 weeks of age compared with LETO rats. Similarly, the renal cortical tissue mRNA expression levels of type IV collagen (Fig. 3d) were higher in OLETF rats than in LETO rats from 11 to 30 weeks of age.
Fig. 3.
Gene expression levels of CTGF a TGF-β b and type I collagen c and type IV collagen d. The mRNA expression levels are expressed as the relative differences between OLETF rats (number of 2 weeks: 22; 4 weeks: 10; 7 weeks: 10; 11 weeks: 10; 18 weeks: 8; 30 weeks: 6) and LETO rats (number of 2 weeks: 10; 4 weeks: 10; 7 weeks: 10; 11 weeks: 9; 18 weeks: 10; 30 weeks: 12) after normalization for the expression of GAPDH. *p < 0.05 vs. LETO rats at the same age.
In terms of renal histology, glomerular histologic findings with PAS staining were shown in 7-week-old OLETF rats (Fig. 1d) and as previously reported [6, 10, 15]. For 7–18-week-old OLETF rats there were no clear PAS-positive area in glomeruli and tubulointerstitium. However, for 30-week-old OLETF rats there are minor alterations of the size of glomeruli and mesangial expansion accompanied in glomeruli but it is no significance by quantitative analyses compared with LETO rats (n = 12).
Discussion
We previously reported that intrarenal Ang II content is augmented in 11-week-old obese OLETF rats, before the onset of overt diabetes. However, it has not been reported whether intrarenal Ang II content is inappropriately increased during maturation in prediabetic OLETF rats. Consistent with the observations in normal Sprague-Dawley rats [11], the present study showed that kidney Ang II content peaked at PND 1 and decreased age-dependently during the pre- and post-weaning periods (i. e., from PND 1 to 4 weeks of age) in both LETO and OLETF rats. During this time, there were no significant differences in intrarenal Ang II content between LETO and OLETF rats. Likewise, intrarenal Ang II content at the start of puberty (7 weeks of age) was not different between LETO and OLETF rats. Unlike the progressive decrease in intrarenal Ang II levels during puberty in LETO rats (from 7 to 11 weeks of age), no reductions were observed during this time in obese prediabetic OLETF rats. These data indicate that the lack of a reduction in intrarenal Ang II during pubescence contributes to the inappropriately elevated intrarenal Ang II content in obese prediabetic OLETF rats.
Preclinical studies have already shown that short-term treatment with an ARB during the prediabetic stage attenuates the progression of renal injury in type 2 diabetic OLETF rats [8]. Recently, the Randomized Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) study was conducted to examine whether an ARB (olmesartan) can prevent the onset of microalbuminuria in humans. In that study, early treatment with olmesartan significantly reduced the incidence of micro-albuminuria in patients with type 2 diabetes [3, 4]. Our present study showed that the inappropriate elevation in intrarenal Ang II content during pubescence was maintained even after the OLETF rats reached adolescence (i. e., at 18 and 30 weeks of age). Taken together, these data support the hypothesis that inappropriate regulation of intrarenal Ang II content precedes the onset of type 2 diabetic nephropathy, and that early treatment with an ARB could help to prevent the onset and development of diabetic nephropathy in later life.
The mRNA expression levels of AGT, renin, and ACE in renal cortical tissues did not differ between LETO and OLETF rats from birth to weaning (from PND 1 to 4 weeks of age). Similarly, there was no difference in the gene expression of these RAS components between LETO and OLETF rats at the start of puberty (7 week of age). However, AGT and renin mRNA levels remained significantly higher in OLETF rats than in LETO rats later in puberty (11 weeks of age). Interestingly, this period overlaps with the period when intrarenal Ang II content in OLETF rats exceeded that of LETO rats. These data suggest that the inappropriately high expression levels of AGT and renin in the kidney contribute to the inadequate reduction in intrarenal Ang II content during pubescence. Unfortunately, because the precise mechanisms responsible for the augmentation of intrarenal AGT and renin expression in obese OLETF rats during pubescence remain unclear, we cannot simply attribute it to the increases in blood glucose levels, because these changes were evident in OLETF rats at 11 weeks of age before the onset of overt diabetes. The gene expression levels of AT1a and AT1b were not significantly different between LETO and OLETF rats during the observation period. On the other hand, renal cortical AT2 mRNA expression was significantly lower in OLETF rats than in LETO rats at 18 and 30 weeks of age. At this time, we cannot address the pathophysiological effects of these changes, and further studies are needed.
The TGF-β/CTGF-dependent pathway stimulates extracellular matrix protein synthesis, including collagen [19–21]. We found that the renal cortical mRNA levels of CTGF and type IV collagen were markedly increased in OLETF rats during puberty (at 7 or 11 weeks of age). On the other hand, TGF-β and type I collagen expression was increased at 18 and 30 weeks of age. These data with those of a previous study show that renal CTGF and type IV collagen gene expression were increased in the prediabetic stage in 11-week-old OLETF rats [10]. In the present study, we did not observe any significant histological renal injury in prediabetic OLETF rats at 7–30 weeks of age. These results were verified by our latest studies exploring that there were no significant changes of PAS and Masson’s trichrome between LETO and OLETF rats at 7–35 weeks age [6, 10, 15]. However, from 15-week-old later, significant desmin-positive areas were gradually displayed in juxtamedullary glomeruli by desmin staining [6]. Nevertheless, minor renal abnormalities (i. e., increases in CTGF and type IV collagen) had already occurred at the prediabetic stage, which may contribute to the progression of albuminuria as well as podocyte injury and then massively by diabetic nephropathy in later life.
In conclusion, the results of the present study indicate an inadequate reduction in intrarenal Ang II content during pubescence contributes to the high intrarenal Ang II content in prediabetic OLETF rats, which is the result of inappropriately elevated intrarenal AGT and renin expression. Therefore, inappropriate activation of the intrarenal RAS in the prediabetic stage may contribute to the onset and development of diabetic nephropathy in later life.
Acknowledgements
We are grateful to the Ministry of Education, Culture, Sports, Science and Technology of Japan (20590253 to Akira Nishiyama) and Nation Natural Science Foundation of China/81141040 to Yu-Yan Fan for supporting our research, and Otsuka Pharmaceutical Co. Ltd. (Tokushima, Japan) for supplying OLETF and LETO rats.
Abbreviations
- RAS
renin-angiotensin system
- AII
angiotensin II
- AI
angiotensin I
- OLETF
Otsuka-Long-Evans-Tokushima-Fatty
- LETO
Long-Evans Tokushima
- AGT
angiotensinogen
- ACE
angiotensin-converting enzyme
- PND
postnatal days
- MBP
mean blood pressure
Footnotes
Conflict of Interest
The authors have no conflict of interest.
References
- 1.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 2.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 3.Ihara G, Kiyomoto H, Kobori H, Nagai Y, Ohashi N, Hitomi H, Nakano D, Pelisch N, Hara T, Mori T, Ito S, Kohno M, Nishiyama A. Regression of superficial glomerular podocyte injury in type 2 diabetic rats with overt albuminuria: effect of angiotensin II blockade. J Hypertens. 2010;28:2289–2298. doi: 10.1097/HJH.0b013e32833dfcda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grassi G. The ROADMAP trial: olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. Expert Opin Pharmacother. 2011;12:2421–2424. doi: 10.1517/14656566.2011.602068. [DOI] [PubMed] [Google Scholar]
- 5.Haller H, Ito S, Izzo JL, Jr, Januszewicz A, Katayama S, Menne J, Mimran A, Rabelink TJ, Ritz E, Ruilope LM, Rump LC, Viberti G ROADMAP Trial Investigators. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364:907–917. doi: 10.1056/NEJMoa1007994. [DOI] [PubMed] [Google Scholar]
- 6.Sofue T, Kiyomoto H, Kobori H, Urushihara M, Nishijima Y, Kaifu K, Hara T, Matsumoto S, Ichimura A, Ohsaki H, Hitomi H, Kawachi H, Hayden MR, Whaley-Connell A, Sowers JR, Ito S, Kohno M, Nishiyama A. Early treatment with olmersartan prevents juxtamedullary glomerular podocyte injury and the onset of microalbuminuria in type 2 diabetic rats. Am J Hypertens. 2012;25:604–611. doi: 10.1038/ajh.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feliers D, Kasinath BS. Mechanism of VEGF expression by high glucose in proximal tubule epithelial cells. Mol Cell Endocrinol. 2010;314:136–142. doi: 10.1016/j.mce.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon JY, Tanimoto M, Gohda T, Hagiwara S, Yamazaki T, Ohara I, Murakoshi M, Aoki T, Ishikawa Y, Lee SH, Jeong KH, Lee TW, Ihm CG, Lim SJ, Tomino Y. Attenuating effect of angiotensin-(1–7) on angiotensin II-mediated NAD(P)H oxidase activation in type 2 diabetic nephropathy of KK-A(y)/Ta mice. Am J Physiol Renal Physiol. 2011;300:1271–1282. doi: 10.1152/ajprenal.00065.2010. [DOI] [PubMed] [Google Scholar]
- 9.Durvasula RV, Shankland SJ. Activation of a local renin angiotensin system in podocytes by glucose. Am J Physiol Renal Physiol. 2008;294:830–839. doi: 10.1152/ajprenal.00266.2007. [DOI] [PubMed] [Google Scholar]
- 10.Nagai Y, Yao L, Kobori H, Miyata K, Ozawa Y, Miyatake A, Yukimura T, Shokoji T, Kimura S, Kiyomoto H, Kohno M, Abe Y, Nishiyama A. Temporary angiotensin II blockade at the prediabetic stage attenuates the development of renal injury in type 2 diabetic rats. J Am Soc Nephrol. 2005;16:703–711. doi: 10.1681/ASN.2004080649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez RA, Lynch KR, Sturgill BC, Elwood JP, Chevalier RL, Carey RM, Peach MJ. Distribution of renin mRNA and its protein in the developing kidney. Am J Physiol. 1989;257(5 Pt 2):850–858. doi: 10.1152/ajprenal.1989.257.5.F850. [DOI] [PubMed] [Google Scholar]
- 12.Yosipiv IV, Dipp S, el-Dahr SS. Ontogeny of somatic angiotensin-converting enzyme. Hypertension. 1994;23:369–374. doi: 10.1161/01.hyp.23.3.369. [DOI] [PubMed] [Google Scholar]
- 13.Yosipiv IV, el-Dahr SS. Activation of angiotensin-generating systems in the developing rat kidney. Hypertension. 1996;27:281–286. doi: 10.1161/01.hyp.27.2.281. [DOI] [PubMed] [Google Scholar]
- 14.Fan YY, Baba R, Nagai Y, Miyatake A, Hosomi N, Kimura S, Sun GP, Kohno M, Fujita M, Abe Y, Nishiyama A. Augmentation of intrarenal angiotensin II levels in uninephrectomized aldosterone/salt-treated hypertensive rats, renoprotective effects of an ultrahigh dose of olmesartan. Hypertens Res. 2006;29:169–178. doi: 10.1291/hypres.29.169. [DOI] [PubMed] [Google Scholar]
- 15.Nishiyama A, Nakagawa T, Kobori H, Nagai Y, Okada N, Konishi Y, Morikawa T, Okumura M, Meda I, Kiyomoto H, Hosomi N, Mori T, Ito S, Imanishi M. Strict angiotensin blockade prevents the augmentation of intrarenal angiotensin II and podocyte abnormalities in type 2 diabetic rats with microalbuminuria. J Hypertens. 2008;26:1849–1859. doi: 10.1097/HJH.0b013e3283060efa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobori H, Katsurada A, Miyata K, Ohashi N, Satou R, Saito T, Hagiwara Y, Miyashita K, Navar LG. Determination of plasma and urinary angiotensinogen levels in rodents by newly developed ELISA. Am J Physiol Renal Physiol. 2008;294:1257–12631. doi: 10.1152/ajprenal.00588.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han SY, So GA, Jee YH, Han KH, Kang YS, Kim HK, Kang SW, Han DS, Han JY, Cha DR. Effect of retinoic acid in experimental diabetic nephropathy. Immunol Cell Biol. 2004;82:568–576. doi: 10.1111/j.1440-1711.2004.01287.x. [DOI] [PubMed] [Google Scholar]
- 18.Sun GP, Kohno M, Guo P, Nagai Y, Miyata K, Fan YY, Kimura S, Kiyomoto H, Ohmori K, Li DT, Abe Y, Nishiyama A. Involvements of Rho-kinase and TGF-beta pathways in aldosterone-induced renal injury. J Am Soc Nephrol. 2006;17:2193–2201. doi: 10.1681/ASN.2005121375. [DOI] [PubMed] [Google Scholar]
- 19.Riser BL, Denichilo M, Cortes P, Baker C, Grondin JM, Yee J, Narins RG. Regulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J Am Soc Nephrol. 2000;11:25–38. doi: 10.1681/ASN.V11125. [DOI] [PubMed] [Google Scholar]
- 20.Yokoi H, Mukoyama M, Nagae T, Mori K, Suganami T, Sawai K, Yoshioka T, Koshikawa M, Nishida T, Takigawa M, Sugawara A, Nakao K. Reduction in connective tissue growth factor by antisense treatment ameliorates renal tubulointerstitial fibrosis. J Am Soc Nephrol. 2004;15:1430–1440. doi: 10.1097/01.asn.0000130565.69170.85. [DOI] [PubMed] [Google Scholar]
- 21.Weston BS, Wahab NA, Mason RM. CTGF mediates TGF-beta-induced fibronectin matrix deposition by upregulating active alpha5beta1 integrin in human mesangial cells. J Am Soc Nephrol. 2003;14:601–610. doi: 10.1097/01.asn.0000051600.53134.b9. [DOI] [PubMed] [Google Scholar]