Abstract
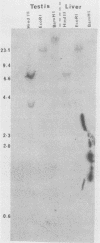
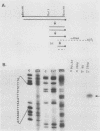
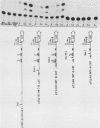
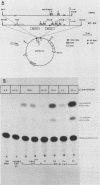
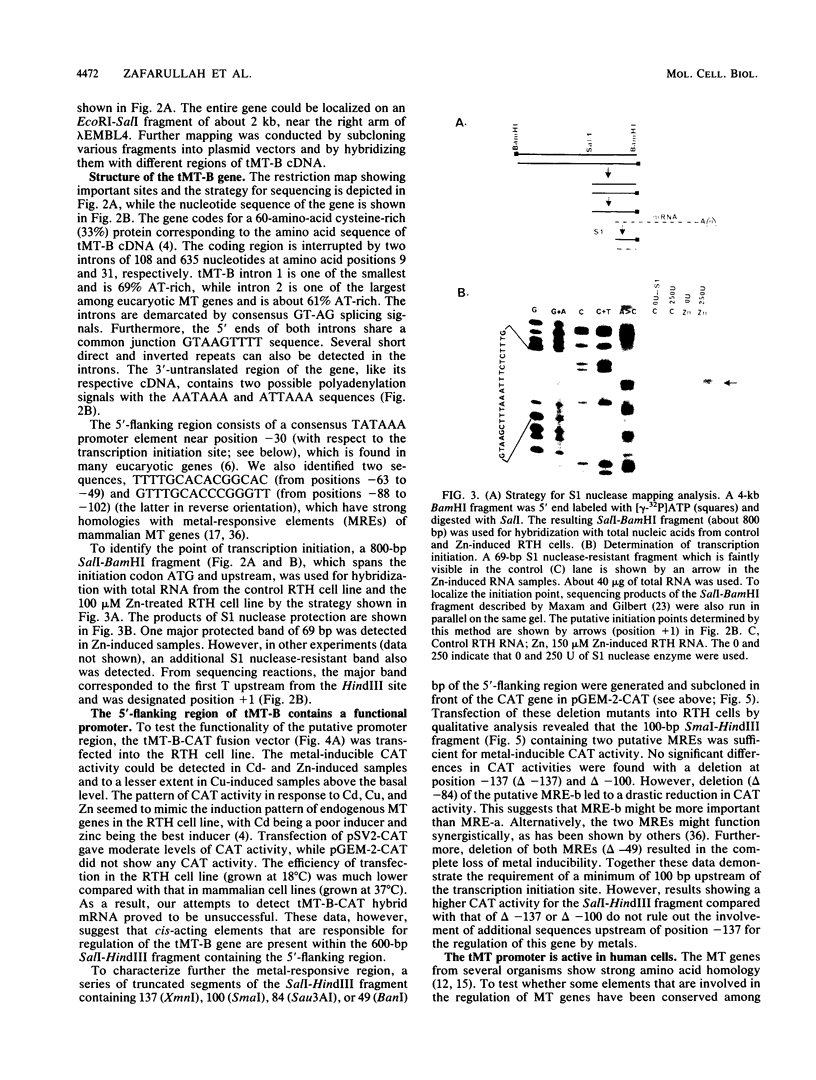
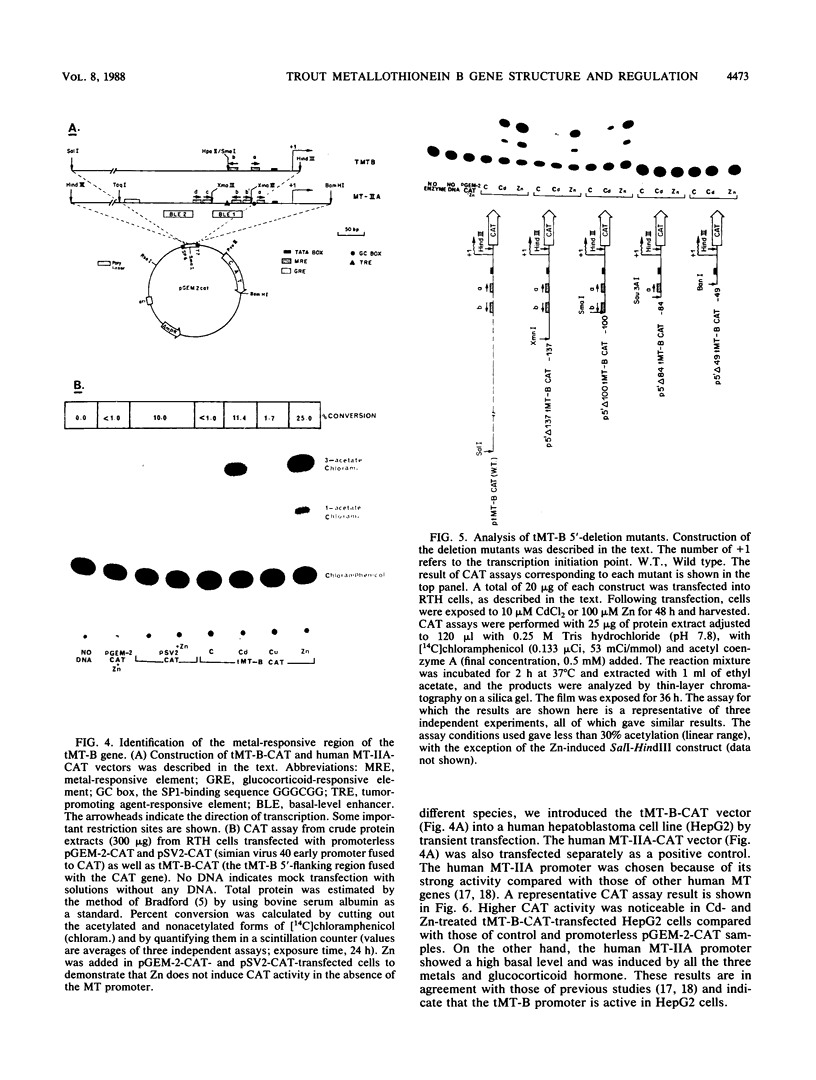
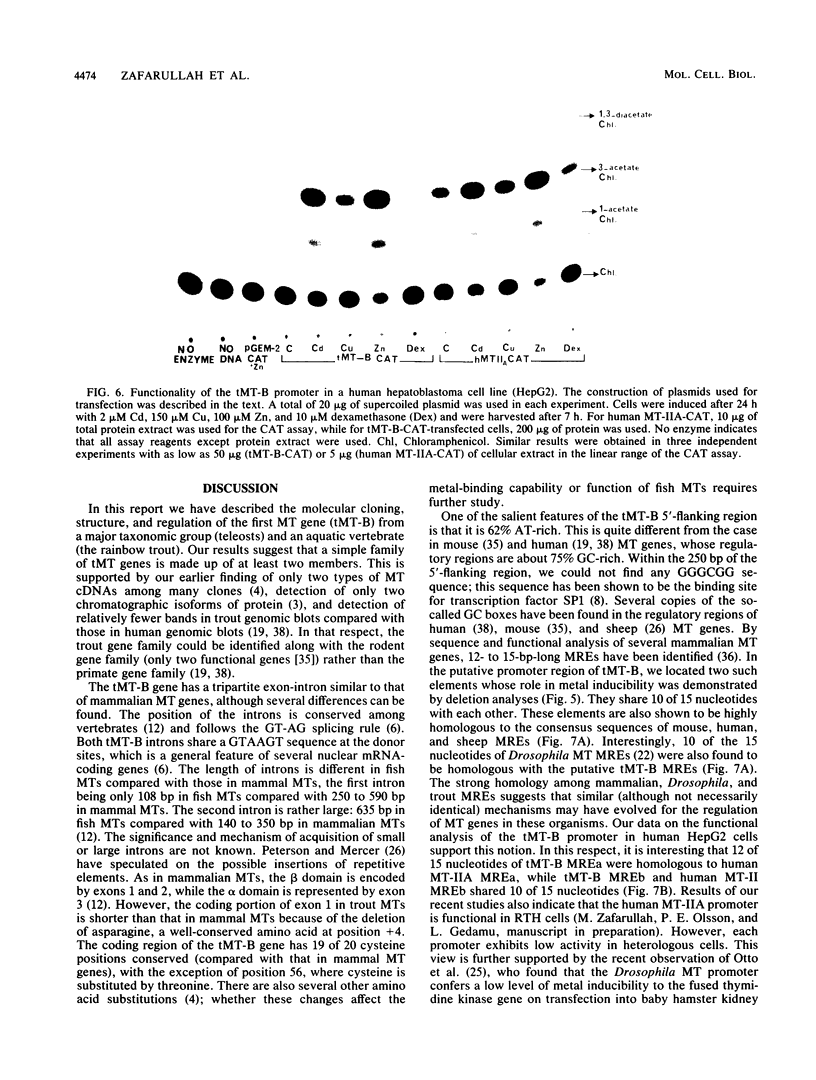
The trout metallothionein (MT) genes consist of two members. We describe the structure of the first fish MT (tMT-B) gene which shows an overall resemblance but some remarkable differences with mammalian MT genes. The similarities included (i) tripartite structure of the gene, (ii) conservation of cysteine residues, and (iii) a TATAAA signal and two copies of metal-responsive elements (MREs). The differences consisted of (i) an AT-rich tMT-B promoter compared with highly GC-rich mammalian MT promoters and (ii) a lack of SP1-binding sites in the tMT-B promoter. Functional analysis of the tMT-B 5'-flanking region following fusion with the bacterial chloramphenicol acetyltransferase gene and its transfection into the rainbow trout hepatoma cell line revealed that sequences from positions -600 to +8 are sufficient for regulation by metals. Further deletion analyses of this fragment suggested that a minimum of 100 nucleotides upstream of the transcription initiation site are required for induction by cadmium and zinc. The tMT-B promoter was also functional in the human hepatoblastoma cell line, suggesting that an MT regulatory factor(s) is conserved in phylogenetically distant species like humans and fish.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham K., Gedamu L. Induction of metallothionein and metallothionein mRNA in rainbow-trout liver following cadmium treatment. Biosci Rep. 1984 Aug;4(8):633–642. doi: 10.1007/BF01121016. [DOI] [PubMed] [Google Scholar]
- Bonham K., Zafarullah M., Gedamu L. The rainbow trout metallothioneins: molecular cloning and characterization of two distinct cDNA sequences. DNA. 1987 Dec;6(6):519–528. doi: 10.1089/dna.1987.6.519. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Butt T. R., Sternberg E. J., Gorman J. A., Clark P., Hamer D., Rosenberg M., Crooke S. T. Copper metallothionein of yeast, structure of the gene, and regulation of expression. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3332–3336. doi: 10.1073/pnas.81.11.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Heguy A., Dietlin T., Cooke T. Metal-responsive elements act as positive modulators of human metallothionein-IIA enhancer activity. Mol Cell Biol. 1987 Feb;7(2):606–613. doi: 10.1128/mcb.7.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Karin M. Metallothioneins: proteins in search of function. Cell. 1985 May;41(1):9–10. doi: 10.1016/0092-8674(85)90051-0. [DOI] [PubMed] [Google Scholar]
- Maroni G., Otto E., Lastowski-Perry D. Molecular and cytogenetic characterization of a metallothionein gene of Drosophila. Genetics. 1986 Mar;112(3):493–504. doi: 10.1093/genetics/112.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Münger K., Germann U. A., Lerch K. Isolation and structural organization of the Neurospora crassa copper metallothionein gene. EMBO J. 1985 Oct;4(10):2665–2668. doi: 10.1002/j.1460-2075.1985.tb03985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto E., Allen J. M., Young J. E., Palmiter R. D., Maroni G. A DNA segment controlling metal-regulated expression of the Drosophila melanogaster metallothionein gene Mtn. Mol Cell Biol. 1987 May;7(5):1710–1715. doi: 10.1128/mcb.7.5.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. G., Mercer J. F. Structure and regulation of the sheep metallothionein-Ia gene. Eur J Biochem. 1986 Nov 3;160(3):579–585. doi: 10.1111/j.1432-1033.1986.tb10077.x. [DOI] [PubMed] [Google Scholar]
- Price-Haughey J., Bonham K., Gedamu L. Heavy metal-induced gene expression in fish and fish cell lines. Environ Health Perspect. 1986 Mar;65:141–147. doi: 10.1289/ehp.8665141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price-Haughey J., Bonham K., Gedamu L. Metallothionein gene expression in fish cell lines: its activation in embryonic cells by 5-azacytidine. Biochim Biophys Acta. 1987 Feb 27;908(2):158–168. doi: 10.1016/0167-4781(87)90055-8. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadhu C., Gedamu L. Regulation of human metallothionein (MT) genes. Differential expression of MTI-F, MTI-G, and MTII-A genes in the hepatoblastoma cell line (HepG2). J Biol Chem. 1988 Feb 25;263(6):2679–2684. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle P. F., Davison B. L., Stuart G. W., Wilkie T. M., Norstedt G., Palmiter R. D. Regulation, linkage, and sequence of mouse metallothionein I and II genes. Mol Cell Biol. 1984 Jul;4(7):1221–1230. doi: 10.1128/mcb.4.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G. W., Searle P. F., Palmiter R. D. Identification of multiple metal regulatory elements in mouse metallothionein-I promoter by assaying synthetic sequences. 1985 Oct 31-Nov 6Nature. 317(6040):828–831. doi: 10.1038/317828a0. [DOI] [PubMed] [Google Scholar]
- Varshney U., Gedamu L. Human metallothionein MT-I and MT-II processed genes. Gene. 1984 Nov;31(1-3):135–145. doi: 10.1016/0378-1119(84)90204-x. [DOI] [PubMed] [Google Scholar]
- Varshney U., Jahroudi N., Foster R., Gedamu L. Structure, organization, and regulation of human metallothionein IF gene: differential and cell-type-specific expression in response to heavy metals and glucocorticoids. Mol Cell Biol. 1986 Jan;6(1):26–37. doi: 10.1128/mcb.6.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D. G., Nemer M. Metallothionein genes MTa and MTb expressed under distinct quantitative and tissue-specific regulation in sea urchin embryos. Mol Cell Biol. 1987 Jan;7(1):48–58. doi: 10.1128/mcb.7.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]