Abstract
Background
The aim of the study was to determine the helminthic species occurring in great gerbil Rhombomys opimus collected from Maraveh Tappeh, Golestan Province, northeast Iran.
Methods
During 2010-2011, a total of 77 R. opimus were captured from rural areas of Maraveh Tappeh, Golestan Province, using Sherman live traps and examined for infectivity with any larva or adult stages of helminthic parasites.
Results
Overall, 63 R. opimus (81.8%) were found infected with different helminthic species. The rate of infectivity with each species was as follows: Trichuris rhombomidis 31.2%, Trichuris muris 32.5%, Trichuris spp. 10.4%, Syphacia muris 2.6%, Dipetalonema viteae (Acanthocheilonema viteae) 37.7%, Skrjabinotaenia lobata 15.6%, Hymenolepis (=Rodentolepis) nana fraterna 5.2%, and Taenia endothoracicus larva 1.3%.
Conclusion
R. opimus is host for several species of cestodes and nematodes in the study area. The high rate of infectivity with D. viteae indicates the susceptibility of these gerbils to this filarial nematode. Synchronous infections occurred up to four species of helminthes in one host.
Keywords: Rhombomis opimus, Helminths, Parasites, Iran
Introduction
The great gerbil Rhombomis opimus (Lichtenstein 1832) (Rodentia: gerbillinae) has a vast distribution range from Iran through Pakistan, Afghanistan via Kazakhstan to China and Mongolia (1). They are most abundant in sand and clay deserts (2), and in some areas they are widely regarded as a pest species (1). In Iran, they are distributed throughout south, central and northeast of the country, including Golestan Province (3) in north of the country. Since long time ago R. opimus is known as a principal natural reservoir of zoonotic cutaneous leishmaniasis (ZCL) in Iran. One of these natural foci is located in Golestan Province (4). In spite of distribution of R. opimus in this province, and adjacent of their colonies with rural housings, no attention has been paid on the other parasitic agents in this rodent. Actually, there are only rare reports on occurrence of helminthic parasites in this rodent in Iran; these reports are from central parts of the country, including Isfahan (5) and on a few numbers from Kashan (6).
Since this gerbil is capable to harbor a wide variety of helminthic parasites especially cestodes and nematodes species (1), as definitive or intermediate host, this study was aimed to determine the helminthic species occurring in R. opimus in rural area of this province, where this gerbil is in close association with human settlements.
Material & Methods
Study site
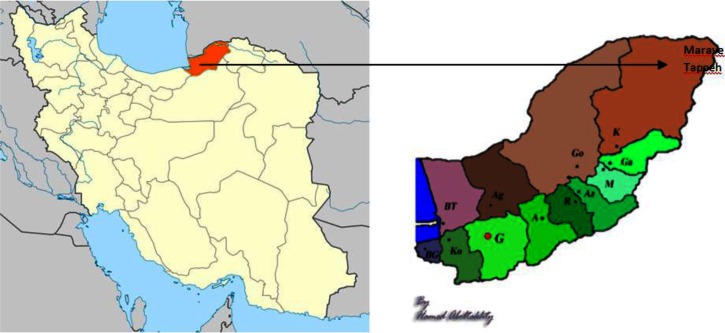
Golestan Province (36° 83’ 93" N and 54° 44’ 44" E) is located in the north east of Iran, south of the Caspian Sea, with Gorgan as capital. Golestan has an area of 20,380 km2. Three different climates exist in the region, including plain moderate, mountainous, and semi-arid. The study site, Maraveh Tappeh (Fig. 1), is a county in this province. It is located between two mountain ranges, having steppe vegetation. It is in adjacent to Turkmenistan Country from north and North Khorasan Province from east. The climate of the county is semi-arid (7).
Fig. 1.
Map of the study area; Left: Map of Iran, Right: Map of Golestan Province
Sampling
Samples of R. opimus were part of a collection for a molecular research on Leishmania species in this area. Samplings were carried out during 2010-2011, using Sherman live traps, baited with walnut, cucumber or tomato. Traps were placed near the burrows of R. opimus tunnels from early sunrise till evening, with an inspection around nine o'clock for collection of captured rodents and resetting the traps. Rodents were transported to the field laboratory in Maraveh Tappeh, keeping in cool and shaded place with sufficient food, prior to shipping to the Animal Unit of the School of Public Health, Tehran University of Medical Sciences, where animals where anesthetized and bled. This study was approved by Ethics Committee of Tehran University of Medical Sciences.
Rodents’ examination and Parasites identification
Thin and thick blood smears were prepared, for further Giemsa staining and microscopical observation. Soon after death, the carcass of each R. opimus was carefully dissected and every organ was examined separately under stereomicroscope for the presence of any helminthic larva or adult. Subcutaneous tissue was observed macroscopically for the presence of adult filaria. Recovered parasites were removed and relaxed in warm saline prior to gradual preservation in 70% ethanol. Samples of cestode parasites and Trichuris were tied between two proper glasses before immersing in the preservative. Before microscopical examination, parasites were either cleared in lactophenol or stained by carmine alum, dehydrating in graded series of ethanol alcohol, clearing in xylene and mounting in canada balsam. For each animal, formalin ether sedimentation technique was also performed on feces and examined for the presence of helminth egg or larva.
Morphological and morphometrical characteristics of helminthes were characterized with the aid of a calibrated microscope, equipped with camera lucida drawing tube. For species identification valid systematic keys including different volumes of K I Skrjabin et al., as well as Yamaguti S (8) were used. Data processing and statistical analysis were performed using SPSS version 11.5. A P-value of <0.05 was considered as significant difference.
Result
Overall, 77 R. opimus were examined for infectivity with helminth parasites. Table 1 represents the rate of infectivity with different species of helminthes according to the sex of R. opimus and the organ involved. If different species of Trichuris are pooled and considered altogether, the genus Trichuris would be the most prevalent one (62.3%).
Table 1.
Frequency and Prevalence of helminth parasites in Rhombomys opimus from Golestan Province according to the sex of the rodent and different organs
| Organ | Helminth species | Rodent sex | |||||
|---|---|---|---|---|---|---|---|
| Male (N = 65) | Female (N = 12) | Total (N = 77 | |||||
| No. | % | No. | % | No. | % | ||
| Omentum | Taenia endothoracicus larva | 1 | 1.5 | - | - | 1 | 1.3 |
| Small intestine | Hymenolepis nana fraterna | 1 | 1.5 | 3 | 25 | 4 | 5.2 |
| Skrjabinotaenia lobata | 10 | 15.4 | 2 | 16.7 | 12 | 15.6 | |
| Large intestine and cecum | Trichuris rhombomidis | 21 | 32.3 | 3 | 25 | 24 | 31.2 |
| Trichuris muris | 25 | 38.5 | - | - | 25 | 32.5 | |
| Trichuris sp. | 2 | 3.1 | 6 | 50 | 8 | 10.4 | |
| Syphacia muris | - | - | 2 | 16.7 | 2 | 2.6 | |
| Subcutaneous and blood | Dipetalonema viteae | 26 | 40 | 3 | 25 | 29 | 37.7 |
| Total Infection* | 53 | 81.5 | 10 | 83.3 | 63 | 81.8 | |
There were some cases of co-infection with two or more parasites.
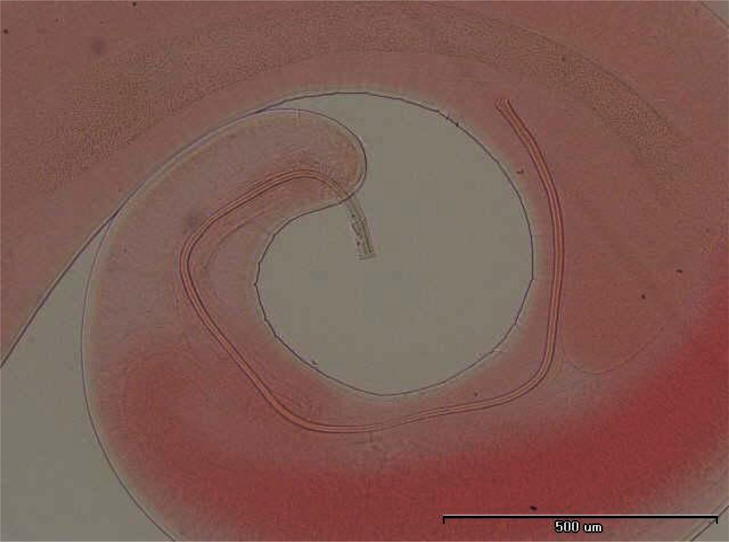
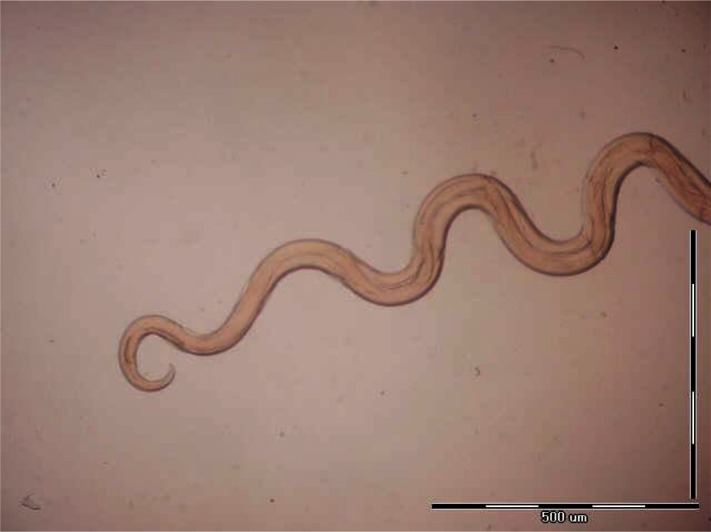
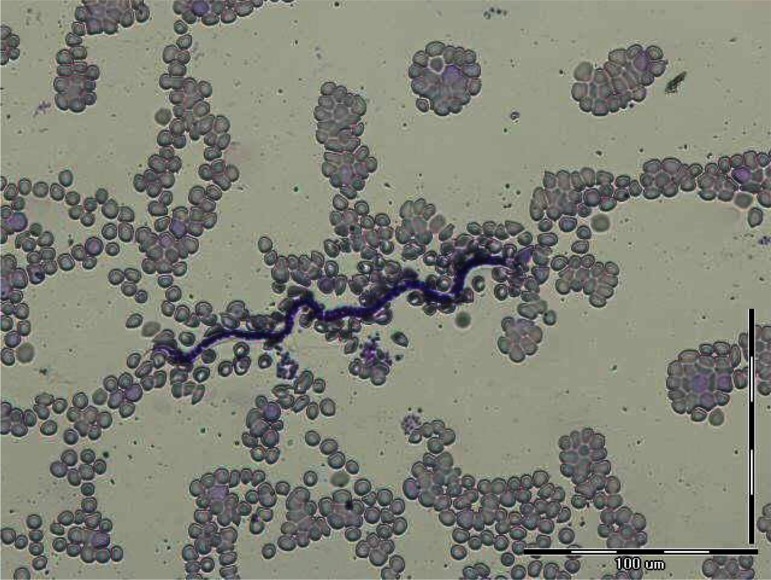
In some individuals, especially females of R. opimus only female nematodes were present and based on the morphological criteria species determination was not accurate; therefore, in such cases the species were registered as Trichuris sp. (Fig. 2). However, among those gerbils harboring male Trichuris, species identification was performed and accordingly 31.2% and 32.5% of the gerbils were found infected with Trichuris rhombomidis (Fig. 3) and Trichuris muris (Fig. 4), respectively. In 11.7% also co-infectivity with both species was found (not shown in the table).
Fig. 2.
Eggs of Trichuris sp
Fig. 3.
Trichuris rhombomidis posterior end of male
Fig. 4.
Trichuris muris posterior end of male
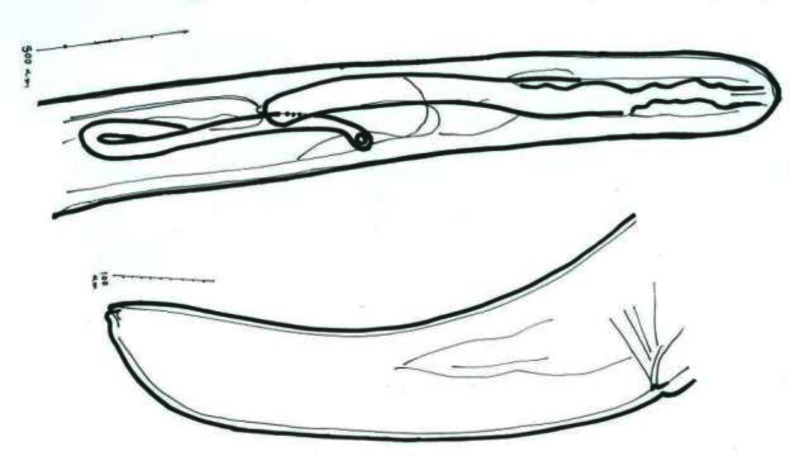
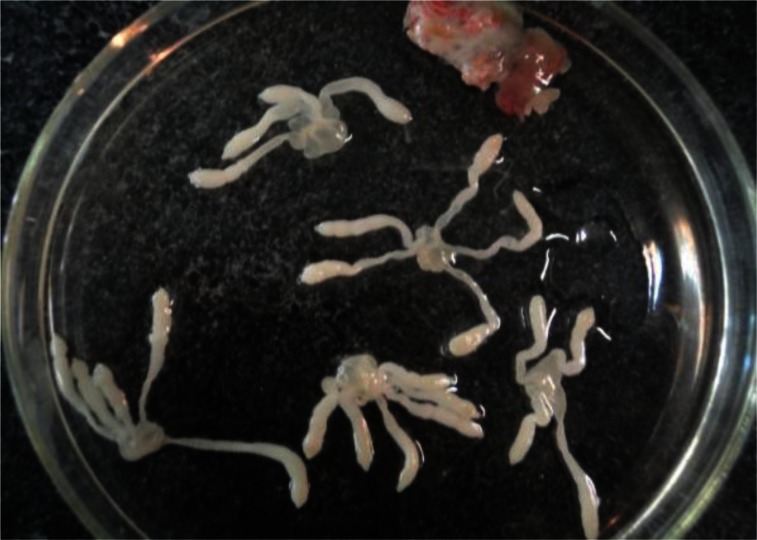
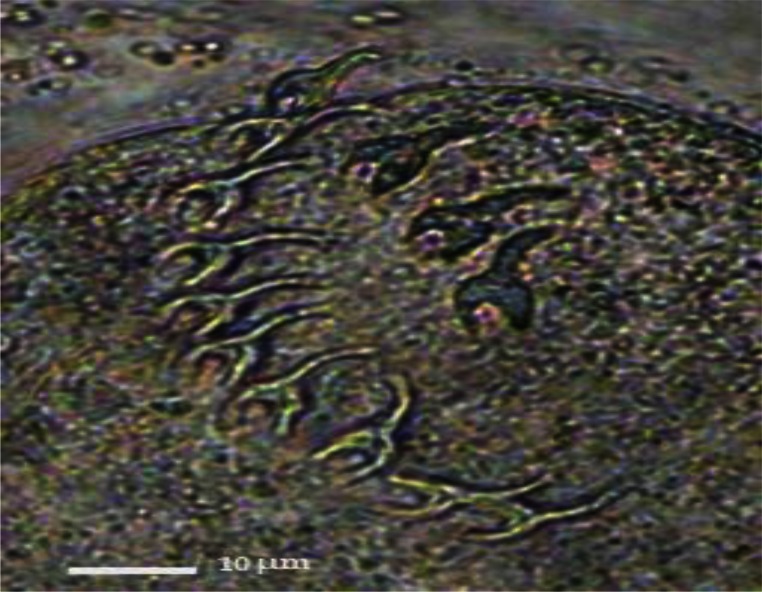
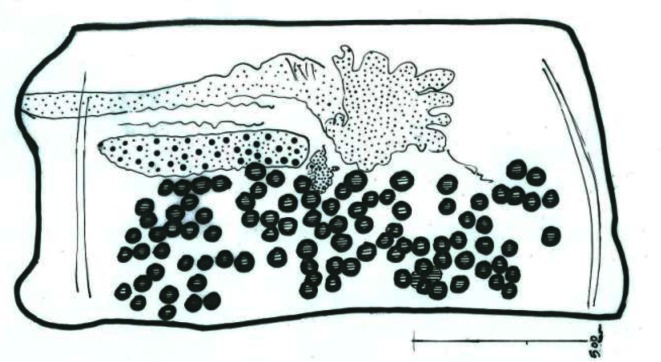
As Table 1 shows, the most prevalent helminth species was Dipetalonema (Acanthocheilonema) viteae (37.7%). In infected R. opimus, the adult D. viteae (Figs. 5 and 6) were found subcutaneously and microfilaria in the peripheral blood (Fig. 7). Respect to the cestodes occurring in R. opimus, three species were identified including Taenia endothoracicus larva (Figs. 8 and 9) in omentum (1.3%) and Hymenolepis (= Rodentolepis) nana fraterna (5.2%) (Fig. 10) and Skrjabinotaenia lobata (15.6%) (Fig. 11) in small intestine. Statistically, H. nana fraterna and T. muris were significantly more prevalent in female (P= 0.01) and male (P= 0.006), respectively. For, other species no sex ratio difference was found.
Fig. 5.
Posterior end of Dipetalonema viteae male
Fig. 6.
Camera lucida drawings of anterior and posterior ends of Dipetalonema viteae female
Fig. 7.
Dipetalonema viteae microfilariae in peripheral blood of Rhombomis opimus
Fig. 8.
Larvae of Taenia endothoracicus
Fig. 9.
Scolex of Taenia endothoracicus larva
Fig. 10.
Hooks of Hymenolepis nana fraterna
Fig. 11.
Camera lucida drawing of Skrjabinotaenia lobata mature proglotid
Synchronous infections occurred up to four species of helminthes in one rodent. Percentages of infectivity with one, two, three and four species of helminthes in infected gerbils were 50.6%, 20.8%, 9.1%, and 1.3%, respectively. No sex-related statistical difference was there respect to the variety of helminth species.
Discussion
Rhombomys opimus inhabits the desert of central Asia, from the Caspian Sea to southern Mongolia and north-central China (2). In Iran, it is known as the most important reservoir for ZCL (4). One of the provinces in Iran in which R. opimus is abundant is Golestan Province (3). In this study the helminth parasites of 77 R. opimus collected from rural areas of this province were identified. In general 81.8% of the gerbils were found infected with at least one species of helminthes. Genus Trichuris was found in 62.3% of R. opimus. Between the two identified Trichuris species, T. muris (32.5%) has been frequently reported from different species of rodents in the country (6, 9, 10); but T. rhombomidis (31.2%) has been rarely reported (11). In a study on helminth fauna of R. opimus in Uzbekistan, T. rhombomidis comprised the most frequent heminth parasite (in 78.4%) in Tashkent and Samarkand districts in this country (12).
One of the other prevalent helminth species in the current study was D. viteae with the rate of 37.7% infectivity. Although the males were more infected than females (40% vs. 25%), but statistically, the difference was not significant. This is probably due to low sample size of trapped females. There is only one previous documented record on occurrence of this parasite in Iran, back to 1967 from Meriones persicus in Tehran and Ghazvin (13). Those authors declared that soft tick Ornithodorus was the vector of this nematode. The occurrence of D. viteae from R. opimus in this study constitutes new host record in the country.
Among cestodes occurring in R. opimus, H. nana fraterna has been frequently reported from different species of rodents in the country (5, 6, 10, 11, 14–16), as well as laboratory animals (17), however, the reports on occurrence of the two other mentioned cestodes are rare in Iran. T. endothoracicus larva has been reported from Meriones species including Meriones lybicus (5, 18), and Meriones persicus (14); and S. lobata from M. persicus (15) , and Tatera indica (11). Therefore, occurrences of T. endothoracicus larva and S. lobata in R. opimus in this study constitute records in new host species in Iran.
Considering sex-related distribution, statistical analysis revealed significant differences between males and females of R. opimus and infectivity with H. nana fraterna and T. muris. For H. nana females were more infected than males (P=0.01). Similar result has also been observed in brown rats Rattus norvegicus infected with this cestode (19). This is most probably due to direct life cycle of this parasite and feasible transmission of eggs from adult females to their newborns during lactating and nursing. On the contrary, T. muris was significantly higher in males of R. opimus (P=0.006). This finding is coincident with the report on significantly higher prevalence of T. muris in males than females in R. norvegicus from urban habitats of Belgrade area (Serbia) (20). According to the results of this study, R. opimus is host for variety of cestodes (three species) and nematodes (at least five species) in the study area. Synchronous infections were found up to four species of helminthes in single host. This is coincidence with the result of Kataranovski et al. (20) who reported no more than four parasite species in R. norvegicus.
Conclusion
A high rate of infectivity with helminth parasites (81.8%) was found in R. opimus in the study area. The most prevalent parasites were genus Trichuris and D. viteae. The occurrence of D. viteae, T. endothoracicus larva and S. lobata in R. opimus constituted new host records in Iran. The high rate of infectivity with D. viteae indicates the susceptibility of these gerbils to this filarial nematode. Further studies on D. viteae are needed to determine its natural vector in the area, as well as the role of congenital transmission of this parasite to R. opimus.
Acknowledgement
This study was financially supported by the Deputy of Research, Tehran University of Medical Sciences, Project No: 90-01-27-13421.The authors would like to thank all people contributed to perform this study, especially Mrs. F. Zahabiun and Mr. A. Rahimi from the Department of Medical Parasitology and Mycology (TUMS). The authors declare that there is no conflict of interest.
References
- 1.Tinnin DS, Ganzorig S, Gardner SL. Faculty Publications from the Harold W. Manter Laboratory of Parasitology; 2011. Helminths of small mammals (Erinaceomorpha, Soricomorpha, Chiroptera, Rodentia, and Lagomorpha) of Mongolia. Paper 696. [Google Scholar]
- 2.Nowak R. Walker's Mammals of the World. 6th ed. Baltimore: Johns Hopkins University Press; 1999. [Google Scholar]
- 3.Misonnes X. 2001. Zoogeography of the Mammals of Iran (1959) Translator: Darvish, Ravagh mehr. [Google Scholar]
- 4.Mohebali M, Javadian E, Yaghoobi-Ershadi MR, Akhavan AA, Hajjaran H, Abaei MR. Characterization of Leishmania infection in rodents from endemic areas of the Islamic Republic of Iran. Eastern Mediterr Health J. 2004;10(4-5):591–599. [PubMed] [Google Scholar]
- 5.Fasihi-Harandi M. Study on the fauna of parasites of wild rodents in northern Isfahan; Tehran, Iran: Tehran University of Medical Sciences; 1992. MSc. Thesis. [Google Scholar]
- 6.Rasti S, Mobedi I, Dehghani R, Drodgar A. The survey of gastrointestinal helminths of mice in Kashan. Journal of the Faculty of Veterinary Medicine. 2000;55(4):57–59. [Google Scholar]
- 7. Availabla at: http://en.wikipedia.org.
- 8.Yamaguti S. Systema Helminthum. 1st ed. New York: Interscience Publishers; 1958. pp. 1958–1963. [Google Scholar]
- 9.Gholami Sh, Mobedi I, MotevaliHaghi F, Shahabi S. Study of helmintic intestinal parasites in the rodents from the rural and central regions of Mazandaran Province in the years 1997 to 1999. J of Mazandaran University of Med Sciences. 2002;12(35):67–75. [Google Scholar]
- 10.Mohebali M, Rezai H, Farahnak A, Nootash A. A survey on parasitic fauna (helminths and ectoparasites) of the rodents in Meshkinshahr district, north west Iran. J Facul Veter Med. 1997;52(3):23–25. [Google Scholar]
- 11.Sadjjadi S, Massoud J. Helminth parasites of wild rodents in Khuzestan province, south west of Iran. J Vet Parasitol. 1999;13(1):55–56. [Google Scholar]
- 12.Shleikher EI, Samsonova AV. The helminth fauna of Rhombomys opimus in Uzbekistan. In: Petrov A. M, editor. Moscow: Akademiya Nauk SSSR; 1953. pp. 770–773. Papers on helminthology presented to academician K. I. Skryabin on his 75th birthday. [Google Scholar]
- 13.Chamsa M, Weiss N. Epidemiological study of rodent filariasis (Dipetalonema viteae Krepkogorskaja 1933) in Iran) Acta Trop. 1969;26(4):370–375. [PubMed] [Google Scholar]
- 14.Kia E, Shahryary-Rad E, Mohebali M, et al. Endoparasites of rodents and their zoonotic importance in Germi, Dashte-Mogan, Ardabil Province, Iran. Iran J Parasitol. 2010;5(4):15–20. [PMC free article] [PubMed] [Google Scholar]
- 15.Najari J. Study on intestinal helminthic parasites of rodents in Moghan region, Ardabil Province; North-West Iran; Tehran, Iran: Tehran University of Medical Sciences; 1993. MSc. Thesis. [Google Scholar]
- 16.Kia EB, Homayouni MM, Farahnak A, Mohebali M, Shojai S. Study of Endoparasites of Rodents and their Zoonotic Importance In Ahvaz, South West Iran. Iranian J Publ Health. 2001;30(1-2):49–52. [Google Scholar]
- 17.Tanideh N, Sadjjadi SM, Mohammadzadeh T, Mehrabani D. Helminthic infections of laboratory animals in animal house of Shiraz University of Medical Sciences and the potential risks of zoonotic infections for researches. Iranian Red Crescent Medical Journal. 2010;12(2):151–157. [Google Scholar]
- 18.Mowlavi GR, Kia EB, Mobedi I. Natural infection of the gerbil Meriones lybicus with the metacestode of Taenia endothoracicus in Arak, central Iran. J Helminthol. 2004;78(3):275–276. doi: 10.1079/joh2003229. [DOI] [PubMed] [Google Scholar]
- 19.Webster JP, Macdonald DW. Parasites of wild brown rats (Rattus norvegicus) on UK farms. Parasitology. 1995;111(3):247–255. doi: 10.1017/s0031182000081804. [DOI] [PubMed] [Google Scholar]
- 20.Kataranovski M, Mirkov I, Belij S, Popov A, Petrović Z, Gačić Z, Kataranovski D. Intestinal helminths infection of Rats (Ratus norvegicus) in the Belgrade Area (Serbia): The effect of sex, age and habitat. Parasite. 2011;18(2):189–196. doi: 10.1051/parasite/2011182189. [DOI] [PMC free article] [PubMed] [Google Scholar]