Abstract
Background
Despite Echinococcus granulosus, there are merely two old reports of E. multilocularis infection among Iranian canids of Moghan Plain, the only area known endemic for the species. We detected specific DNA markers in fecal samples by PCR (Copro-PCR) for differential diagnosis of Echinococcus species in living canids.
Methods
Totally 144 fecal samples from domestic dogs, red foxes and a golden jackal were examined for genus-specific Echinococcus coproantigens using ELISA. Forty two positive or ambiguous samples were further examined for Echinococcus species-specific DNA markers by two different set of nested-PCR.
Results
Twenty five out of 144 (17.4%) animals were contaminated with E. granulosus including 14 (23.7%) domestic dogs, 10 (11.9%) red foxes and one (100%) golden jackal. But none of them harboured E. multilocularis species-specific Copro-DNA. The overall prevalence of E. granulosus and E. multilocularis infections in canids of the area was estimated to be 17.4% and 0.0%, respectively. There was a significant relation between the results of Copro-PCR and CA-ELISA.
Conclusion
The lack of E. multilocularis infection, compared to previous reports may be due to the differences in used diagnostic methods and/or recently limited territories of wild canids and altered their food resources in this particular area.
Keywords: Echinococcus, Copro-DNA, Canids, Iran
Introduction
Echinococcosis is a near-cosmopolitan zoonosis caused by adult or larval stages of tapeworms belonging to the genus Echinococcus (family Taeniidae; class Cestoda). Larval infection known as hydatid disease or hydatidosis is characterized by long term growth of hydatid cysts in the intermediate hosts including humans, herbivores and rodents .In the parasite life cycle, carnivores especially canids, serve as definitive hosts and harbor the adult worms in their intestines. The two major species of medical and public health importance are Echinococcus granulosus and E. multilocularis, which cause cystic echinococcosis and alveolar echinococcosis, respectively (1, 2).
Echinococcus granulosus infection in definitive hosts is common throughout Iran (3) and its adult worms have been detected in various carnivores from rural and urban areas of many Iranian provinces including Kerman, Khuzestan, Fars, Tehran, Kurdestan, Khorasan, Isfahan and several western provinces (3–6).
But in the case of E. multilocularis infection in Iran, there are merely two old reports indicating that canids might serve as definitive hosts in Moghan plain, the only area in the country known to be endemic for this species. The first report on canine infection to adult worms of E. multilocularis, dates back to 1971, in which 10% red foxes (3 0ut of 30) were found infected (7, 8). The next study on 130 wild and domestic carnivores in Ardebil province, northwestern Iran in 1992 revealed that 22.9% of red foxes and 16% of jackals and 50% wild cats (one out of 2) were infected with adult stages of E. multilocularis (9, 10). The diagnosis in both aforementioned studies was based on morphological characteristics of adult worms at necropsy.
Because of high similarity between eggs of Echinococcus and Taenia species, the differential diagnosis of Echinococcus infection in fecal samples of canids is impossible (11). Additionally, the characteristic small Echinococcus proglottids may be absent in the feces or be easily overlooked (2). By the end of the 1980's the only reliable technique for diagnosis of intestinal Echinococcus infection in definitive hosts was Intestinal Scraping Technique (IST) at necropsy and examination of scraped materials under stereoscope. IST with maximum sensitivity of 78% in optimal conditions (1) is based on investigation of the dead animal's intestine and visual identification of the adult worms, based on morphological features (11). IST is considered as an expensive, bio-hazardous and laborious diagnostic method and is not recommended for examination of domestic live animals. Moreover, since dogs and red foxes are known to be susceptible to E. granulosus and E. multilocularis species, they might simultaneously be infected with both species (1).
In recent years, two new techniques, based on detection of the parasitic copro-DNA molecules by PCR (Copro-PCR) and Copro-Antigens by Enzyme-Linked ImmunoSorbent Assays (CA-ELISA) in animal fecal samples were introduced for the diagnosis of Echinococcus infections in living carnivores (2).
Briefly, Copro-PCR tests, involve purification of eggs from fecal materials, extraction of DNA, and identification of species-specific target sequences using DNA primers. The target sequences are often directed towards ribosomal and mitochondrial DNA, as there is generally a higher level of genetic heterogeneity and mutation within these sequences, increasing the presence of species-specific determinants (12). The first PCR-based method for the detection of E. multilocularis DNA in fecal samples of foxes was developed by Bretagne et al. (13) and later modified and improved (11, 14–24). The method is now recommended as an alternative method to the routine IST. Detection of E. multilocularis Copro-DNA by nested-PCR has showed a specificity of 100% and average sensitivity of 89%, ranging from 78% to 100%, influenced by worm burden (11). In summary, although the wide use of PCR for field studies will largely depend on the facilities and the costs, however Copro-DNA detection is already accepted and used as a confirmation test for positive samples in CA-ELISA (16) or in selected cases, especially in living dogs and cats (2).
To date, most of studies on prevalence of intestinal helminth infections of carnivores in Iran were merely based on the traditional method of IST. But recently, Siavashi et al. (25) used CA-ELISA for detection of canine echinococcosis in three provinces of Iran and reported the specificity and sensitivity of the method to be 74% and 72%, respectively. In our similar study using the same method in Moghan Plain, 21.6% of canids were found to be infected with Echinococcos Spp. (6), however, CA-ELISA could not identify the tapeworm species.
This study was aimed to determine the prevalences of E. granulosus and E. multilocularis infections among canine definitive hosts using nested-PCR in Moghan Plain, northwestern Iran, the only area in the country labeled as endemic for E. multilocularis infection.
Materials and Methods
Study area
This study was performed in the Moghan Plain (local name: Dasht-e-Moghan) in the province of Ardebil, northwestern Iran, bordered in north and east by Azerbaijan Republic. The area is located between 39°0' and 39°36' north latitude and 46°52' and 48°21' east longitudes. The region covers 5245 Km2 and includes three counties namely Pars Abad, Bileh Savar, and Germi. The population is approximately 310,000 of urban, rural and nomadic people. Most of the people are from Azeri ethnic group and mainly practice agriculture and Stockbreeding. The Moghan Plain consists of the plains with altitudes as low as 32 meters and highlands with heights as high as 1023 meters above sea level and has an average annual precipitation of 222.76 millimeters.
Samples
Totally 144 fecal samples including 59 from domestic dogs (Canis lupus f. familiaris) and 84 from red foxes (Vulpes vulpes), and one from a golden jackal (Canis aureus), were collected and examined for Echinococcus species by parasitological, serological and molecular methods.
The red foxes specimens were obtained either from rectums of necropsied foxes (n = 79) or from vicinity of red foxes dens (n = 5). Since fecal samples might contain infective eggs or proglottids of E. granulosus and E. multilocularis, all samples were frozen at least for one week in -70° C and then kept at -20° C until used.
The samples were first examined for genus-specific Echinococcus coproantigens using CA-ELISA as described before (6). Then 30 positive and 12 ambiguous samples (Table 1) were further investigated by two set of nested-PCR using specific primers.
Table 1.
Distribution of samples according to host species and the results of CA-ELISA
| Host species | Negative | Ambiguous | Positive | Total |
|---|---|---|---|---|
| Fox | 67 | 4 | 13 | 84 |
| Dog | 34 | 9 | 16 | 59 |
| Jackal | 0 | 0 | 1 | 1 |
| Total | 101 | 13 | 30 | 144 |
Copro-DNA nested PCR
a. DNA extraction from adult Echinococcus worms
DNA of an Iranian isolate of adult Echinococcus tapeworm was extracted and used as template to optimize the PCR, and as positive controls in PCR runs. For grinding of whole body of tapeworms in DNA extraction procedure, we evaluated five different methods including: a) using detergents; b) homogenizing by manual homogenizer; 3) sinking in liquid nitrogen followed by manual homogenizing; 4) squeezing adult worms between two glass slides; and 5) freezing in liquid nitrogen and thawing in hot water accompanied by manual homogenizing.
The best results were achieved in the last procedure. Then QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) was used to extract genomic DNA from adult worms. Extracted DNA was solved in 100µL distilled water.
b. DNA extraction from fecal samples
Because of low concentration of Copro-DNA in extracts, all fecal samples were concentrated by a modified sedimentation technique prior to DNA extraction. Briefly, fecal specimens were suspended in normal saline, filtered through strainer and gauze, poured in 50-mL Falcon® tubes and centrifuged one or more times in 1500 rpm for 10 minutes, until the supernatant came out clear.
DNA extraction from the sediments was performed using a commercial specific kit, QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to manufacturer protocols.
c. nested-PCR
According to recommendation of previous studies (2, 25), we merely used the PCR technique as a differential method and a confirmation test to CA-ELISA. Therefore, only positive or ambiguous (borderline) fecal samples in CA-ELISA (n = 42) were subjected to PCR to detect DNA molecules of Echinococcus species.
The extracted DNA samples were amplified with two nested-PCR protocols using the primers (as detailed in Table 2) and procedures described by Dinkel et al. (11) and Abbasi et al. (23) and optimized by some modifications in thermal cycles parameters and the concentrations of reagents as follows:
Table 2.
The details of primers used in nested-PCR protocols
| Species/target (Ref.) | Step | Name | Sequence | Product | Specificity |
|---|---|---|---|---|---|
| E. multilocularis /mitochondrial 12S rRNA gene (11) | 1st | P60.for. p375.rev. |
TTAAGATATATGTGGTACAGGATTAGATACCC AACCGAGGGTGACGGGCGGTGTGTACC |
373 bp | Genus |
| 2nd | Pnest.for. Pnest.rev |
CAATACCATATTACAACAATATTCCTATC ATATTTTGTAAGGTTGTTCTA |
255 bp | Species | |
| E. granulosus /EgG1 Hae III (24) | 1st | Eg21: Eg22 |
ACACCACGCATGAGGATTAC ACCGAGCATTTGAAATGTTGC |
269 bp | species |
| 2nd | Eg23 Eg24 |
GAATGCAAGCAGCAGATG GAGATGAGTGAGAAGGAGTG |
133 bp | species | |
c.1- Nested-PCR for E. multilocularis
The PCR with both pairs of primers was carried out in a volume of 25 µL PCR reaction that contained 10 mM Tris-HCl, pH 8.3, 50 mM KCl, 1.5 mM MgCl2, 200 M dNTP (each), 1U/reaction Taq DNA Polymerase, 5 l/reaction DNA Template and 20 pmol/reaction Primers (each).
The thermal profile optimized as 5 minutes at 95°C followed by 35 cycles, each of 45 seconds at 93°C, 35 seconds at 63.3°C, and one minute at 73°C and a final elongation phase for 5 minutes at 72°C.The
c.2- Nested-PCR for E. granulosus
In both two stages of PCR, reaction volumes of 25 µL contained 10 mM Tris-HCl, pH 8.3, 50 mM KCl, 1 mM MgCl2, 200 M dNTP (each), 1U/reaction Taq DNA Polymerase, 5 l/reaction DNA Template and 20 pmol/reaction Primers (each).
The thermal cycles for both steps was as 2 minutes at 95°C followed by 35 cycles, each of 30 seconds at 95°C, 45 seconds at 55°C, and 45 seconds at 72°C and a final elongation phase for 7 minutes at 72°C. All PCR runs were performed using Mastercyclers® thermocycler (Gradient 5331, version 2.03.31-09; Eppendorf AG, 22331 Hamburg). PCR products were subjected to electrophoresis on a 1% agarose gel in TAE buffer.
Results
Out of 42 samples subjected to nested-PCR, E. granolosus Copro-DNA was detected in 25 samples (Fig. 1). The 255-basepair amplicon of internal species-specific primers (Pnest. for. and Pnest.rew.) representative of E. multilocularis Copro-DNA, was not amplified in any sample. Although, the nonspecific products of E. multilocularis external primers (P60.for. and p375.rev.) were seen in four samples, but because of probable its amplification by Mesocestoides spp, E. granulosus, and or many other taenids (11) and of having been positive in early CA-ELISA, they could be evidence of E. granulosus infection.
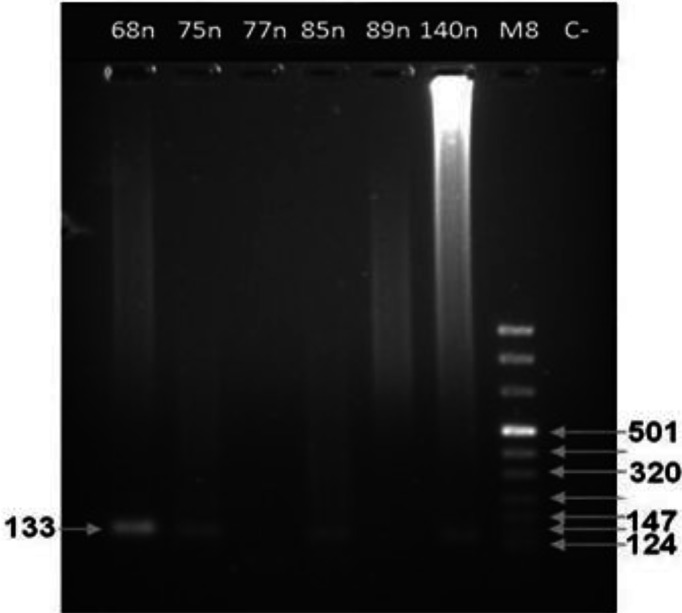
Fig. 1.
Representative results of second stage of nested-PCR with internal specific primers of Eg23 and Eg24 (see Table 2) on some fecal samples. Bands of a 133 base pair DNA product of E. granulosus were amplified in samples 68n, 75n, 85n and 140n, but not in samples 77n and 89n. Lane M8, DNA size marker; lane c-, negative control
Generally, according to the results of CA-ELISA (6) and Copro-PCR methods, we found that 25 out of 144 (17.4%) animals were contaminated with E. granulosus including 14 domestic dogs, 10 red foxes and one golden jackal.
There was a significant relation between the results of Copro-PCR and the net optical density (OD) values of samples run on a spectrophotometer at 450 nm wavelength in CA-ELISA (P = 0.041).
There was no significant relation between Copro-PCR results and the species of animal hosts, the geographical location of, and the seasons of sampling, as well as many other parasitic infections. But positive Copro-PCR results was statistically higher in animals with Toxocara canis (P< 0.05) and Mesocestoides spp. (P=0.075) infections.
Additionally, in preliminary parasitological examinations of fecal samples using wet mount, formol-ether and sucrose floatation methods, Taenia-like eggs were found in 10 (11.2%) dogs and 2 (3.4%) red foxes. The detailed parasitological results were presented in our other paper (26).
Discussion
CA-ELISA is based on polyclonal Echinococcus genus-specific antibodies and is not able to differentiate E. multilocularis from E. granulosus in fecal samples (2). Therefore we used Copro-PCR to detect species-specific Copro-DNA and to determine the prevalences of E. garnulosus and E. multilocularis infections in canids of the study area.
As expected, Copro-PCR had high consistency with CA-ELISA results. There was a significant relation between Copro-PCR results and the net optical density (OD) values of specimens in CA-ELISA. Furthermore, positive Copro-PCR cases were higher, albeit statistically insignificant (P-value = 0.136), in positive CA-ELISA specimens than negative ones (66.7% vs. 41.7%).
Regarding the relation between the results of Copro-PCR and other parasitic infections, it is worth mentioning that among 12 samples harboring Taenia eggs, Echinococcus Copro-DNA was detected only in one dog fecal sample, indicating the high specificity of Copro-PCR method.
The statistically significant relation between the results of Copro-PCR with Toxocara canis and Mesocestoides spp. infections was probably due to their high prevalence rates of 34.9% and 48.3%, respectively, obtained in our parasitological investigations (26), as well as it may be an indicative of a strong co-infection phenomenon.
If the Copro-PCR that has higher sensitivity and specificity than CA-ELISA (25), is considered as gold standard test, the sensitivity of CA-ELISA method would be 80%. In this study it was impossible to estimate the specificity of CA-ELISA, as only positive and ambiguous specimens in CA-ELISA were subjected to Copro-PCR.
Conclusion
The prevalence of E. granulosus in dogs and red foxes of the Moghan plain were 23.7%, 11.9%, respectively. The overall prevalences of E. granulosus and E. multilocularis infections in canids of the area is estimated to be 17.4% and 0.0%, respectively.
The lack of E. multilocularis infection in this study, compared to two previous works in 1971 and 1993 might be due to the differences in used diagnostic methods and/or extensive ecologic changes in recent years including the population growth and immigration, the establishment of new villages and towns around local rivers (Aras, Darehroud, Balharoud and aghbeiglar) and the building of new dams (Aras, Aslandouz and Khoda-afarin), new water reservoirs and irrigation networks. It seems that these factors could limited the territories of wild carnivores such as red foxes, jackals and wolves and also altered the their food resources as shifting from Microtus voles to other rodents e.g. Meriones spp, insects, birds and lizards in this particular area.
Acknowledgements
The study was financially supported in part by Pasteur Institute of Iran. We are very grateful to the staff of Health and Treatment Network of Pars Abad in Ardebil province for their cooperation. We wish to express our special thanks to Mr. Ojaroodi and Mr. Mehdizadeh for their sincere helps and efforts in the field activities and Ms. Naeimi and Ms. Ghazinezhad for kindly assistance in laboratory practice. The authors declare that there is no conflict of interest.
References
- 1.Zhang W, Li J, McManus DP. Concepts in immunology and diagnosis of hydatid disease. Clin Microbiol Rev. 2003;16(1):18–36. doi: 10.1128/CMR.16.1.18-36.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckert J, Gemmell MA, Meslin F-X, Pawłowski ZS. WHO/OIE Manual on Echinococcosis in Humans and Animals: a Public Health Problem of Global Concern; World Organisation for Animal Health, World Health Organization; 2001. [Google Scholar]
- 3.Rokni MB. The present status of human helminthic diseases in Iran. Ann Trop Med Parasit. Annals of Tropical Medicine and Parasitology. 2008;102(4):283–95. doi: 10.1179/136485908X300805. [DOI] [PubMed] [Google Scholar]
- 4.Eslami A, Hosseini SH. Echinococcus granulosus infection of farm dogs of Iran. Parasitol Res. 1998;84(3):205–7. doi: 10.1007/s004360050383. [DOI] [PubMed] [Google Scholar]
- 5.Sadjjadi SM. Present situation of ech-inococcosis in the Middle East and Arabic North Africa. Parasitol Int. 2006;55(Suppl):S197–202. doi: 10.1016/j.parint.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Zare-Bidaki M, Mobedi I, Naddaf SR, Kia EB, Mahmoudi M, Piazak N, et al. Prevalence of Echinococcus spp. Infection Using Coproantigen ELISA among Canids of Moghan Plain, Iran. Iran J Public Health. 2009;38(1):112–8. [Google Scholar]
- 7.Mobedi I, Bray RA, Arfaa F, Movafag K. A study on the cestodes of carnivores in the northwest of Iran. J Helminthol. 1973;97(3):277–81. doi: 10.1017/s0022149x00026559. [DOI] [PubMed] [Google Scholar]
- 8.Mobedi I, Sadighian A. Echinococcus multilocularis, Leukrart 1863, in red foxes, Vulpes vulpes, in Moghan, Azarbaijan province, north west Iran. J Parasitol. 1971;58:493. [PubMed] [Google Scholar]
- 9.Zarriffard MR. A study on helminthic parasites of wild carnivorous of east Azarbaijan with emphasis on Echinococcus multilocularis ; Tehran University of Medical Sciences; 1993. Tehran: PhD Thesis. [Google Scholar]
- 10.Zariffard MR, Massoud J. Study of Echinococcus granulosus and Echinococcus multilocularis infections in Canidiae in Ardabile province of Iran. Arch Razi Inst. 1998;48/49:47–52. [Google Scholar]
- 11.Dinkel A, von Nickisch-Rosenegk M, Bilger B, Merli M, Lucius R, Romig T. Detection of Echinococcus multilocularis in the definitive host: coprodiagnosis by PCR as an alternative to necropsy. J Clin Microbiol. 1998;36(7):1871–6. doi: 10.1128/jcm.36.7.1871-1876.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig P, Pawlowski Z. Cestode zoonoses: echinococcosis and cysticercosis: an emergent and global problem. Amsterdam, Netherlands: IOS Press; 2002. [Google Scholar]
- 13.Bretagne S, Guillou JP, Morand M, Houin R. Detection of Echinococcus multilocularis DNA in fox faeces using DNA amplification. Parasitology. 1993;106(Pt 2):193–9. doi: 10.1017/s0031182000074990. [DOI] [PubMed] [Google Scholar]
- 14.Mathis A, Deplazes P, Eckert J. An improved test system for PCR-based specific detection of Echinococcus multilocularis eggs. J Helminthol. 1996;70(3):219–22. doi: 10.1017/s0022149x00015443. [DOI] [PubMed] [Google Scholar]
- 15.Monnier P, Cliquet F, Aubert M, Bretagne S. Improvement of a polymerase chain reaction assay for the detection of Echinococcus multilocularis DNA in faecal samples of foxes. Vet Parasitol. 1996;67(3-4):185–95. doi: 10.1016/s0304-4017(96)01039-4. [DOI] [PubMed] [Google Scholar]
- 16.Smith GC, Gangadharan B, Taylor Z, Laurenson MK, Bradshaw H, Hide G, et al. Prevalence of zoonotic important parasites in the red fox (Vulpes vulpes) in Great Britain. Vet Parasitol. 2003;118(1-2):133–42. doi: 10.1016/j.vetpar.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 17.van der Giessen JW, Rombout YB, Franchimont JH, Limper LP, Homan WL. Detection of Echinococcus multilocularis in foxes in The Netherlands. Vet Parasitol. 1999;82(1):49–57. doi: 10.1016/s0304-4017(98)00263-5. [DOI] [PubMed] [Google Scholar]
- 18.Stefanic S, Shaikenov BS, Deplazes P, Dinkel A, Torgerson PR, Mathis A. Polymerase chain reaction for detection of patent infections of Echinococcus granulosus (“sheep strain”) in naturally infected dogs. Parasitol Res. 2004;92(4):347–51. doi: 10.1007/s00436-003-1043-y. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Bart JM, Giraudoux P, Craig P, Vuitton D, Wen H. Morphological and molecular characteristics of Echinococcus mult-ilocularis and Echinococcus granulosus mixed infection in a dog from Xinjiang, China. Vet Parasitol. 2006;139(1-3):244–8. doi: 10.1016/j.vetpar.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Trachsel D, Deplazes P, Mathis A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology. 2007;134(Pt 6):911–20. doi: 10.1017/S0031182007002235. [DOI] [PubMed] [Google Scholar]
- 21.Dyachenko V, Beck E, Pantchev N, Bauer C. Cost-effective method of DNA extraction from taeniid eggs. Parasitol Res. 2008;102(4):811–3. doi: 10.1007/s00436-007-0855-6. [DOI] [PubMed] [Google Scholar]
- 22.Knapp J, Guislain MH, Bart JM, Raoul F, Gottstein B, Giraudoux P, et al. Genetic diversity of Echinococcus multilocularis on a local scale. Infect Genet Evol. 2008;8(3):367–73. doi: 10.1016/j.meegid.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Abbasi I, Branzburg A, Campos-Ponce M, Abdel Hafez SK, Raoul F, Craig PS, et al. Copro-diagnosis of Echinococcus granulosus infection in dogs by amplification of a newly identified repeated DNA sequence. Am J Trop Med Hyg. 2003;69(3):324–30. [PubMed] [Google Scholar]
- 24.Siavashi MR, Motamedi GR. Evaluation of a coproantigen enzyme-linked immunosorbent assay for the diagnosis of canine echinococcosis in Iran. Helminthologia. 1996;43(1):17–9. [Google Scholar]
- 25.Eckert J. Predictive values and quality control of techniques for the diagnosis of Echinococcus multilocularis in definitive hosts. Acta Trop. 2003;85(2):157–63. doi: 10.1016/s0001-706x(02)00216-4. [DOI] [PubMed] [Google Scholar]
- 26.Zare-Bidaki M, Mobedi I, Ahari SS, Habibizadeh S, Naddaf SR, Siavashi MR. Prevalence of Zoonotic Intestinal Helminths of Canids in Moghan Plain, Northwestern Iran. Iran J Parasitol. 2010;5(2):42–51. [PMC free article] [PubMed] [Google Scholar]