Abstract
Objective
To compare the in-vitro efficiency of dual-switching monopolar (DSM) radiofrequency ablation (RFA) using a separable clustered electrode (Octopus® electrodes) with consecutive monopolar (CM) and switching monopolar (SM) RFA techniques to create an ablative zone in the explanted bovine liver.
Materials and Methods
For DSM-RFA, we used a prototype, three-channel, dual generator RFA Unit and Octopus® electrodes with three, 17 gauge internally cooled electrodes. The RFA Unit allowed simultaneous radiofrequency (RF) energy delivery to two electrodes of the Octopus® electrodes as well as automatic switching among the three electrode pairs according to the impedance changes. RF energy was sequentially applied to one of the three electrodes for 24 minutes (group A; CM mode, n = 10) or alternatively applied for 12 minutes (group B; SM mode, n = 10) or concurrently applied to a pair of electrodes for 12 minutes (group C; DSM mode, n = 10) in explanted bovine livers. Changes in the impedance and current during RFA as well as the dimensions of the thermal ablative zones were compared among the three groups.
Results
The mean, delivered RF energy amounts in groups A, B, and C were 63.15 ± 8.6 kJ, 72.13 ± 5.4 kJ, and 106.08 ± 13.4 kJ, respectively (p < 0.001). The DSM mode created a significantly larger ablation volume than did the other modes, i.e., 68.1 ± 10.2 cm3 (group A), 92.0 ± 19.9 cm3 (group B), and 115.1 ± 14.0 cm3 (group C) (p < 0.001). The circularity in groups A, B, and C were 0.84 ± 0.06, 0.87 ± 0.04 and 0.90 ± 0.03, respectively (p = 0.03).
Conclusion
DSM-RFA using Octopus® electrodes can help create large ablative zones within a relatively short time.
Keywords: Liver, interventional procedures; Radiofrequency ablation; Experimental study
INTRODUCTION
Radiofrequency ablation (RFA) is the most commonly used percutaneous ablation technique for the management of primary and metastatic liver tumors and its use continues to increase (1, 2). However, clinical studies have reported a high rate of inadequate treatment for index tumors with diameters greater than 3 cm as well as relatively high rates of local tumor progression (3). The single most important factor affecting the local tumor progression rate after RFA for hepatocellular carcinoma (HCC) or colorectal liver metastases (CRLM) could be the ablation of a tumor-free margin of hepatic parenchyma along the tumor margin as well as in the tumor itself (4-7). When the thickness of the ablative margin is evaluated by CT image fusion, a margin of 3-5 mm to 1 cm appears to be associated with a lower rate of local tumor progression after percutaneous RFA of HCC or CRLM (5, 6, 8, 9). Most clinically available electrodes, including internally cooled electrodes, multi-tined, expandable needle electrodes, and perfusion electrodes induce coagulation necrosis in the range of 3-4 cm in diameter after only a single ablation (10). Therefore, in order to treat liver tumors larger than 2 cm in diameter, multiple, overlapping ablations are often required in order to create a large ablative zone encompassing the tumor and the surrounding healthy tissue rim (7, 11). However, when ultrasound guidance is used, it is technically challenging to create a large ablative zone by the overlapping ablation technique, as RFA almost always creates numerous microbubbles in a heated tissue, which can obscure the unablated portions so that further electrode placement at the target site may thus be difficult or impossible (11). Recent technical development of the electrode design, improved ablation algorithms, and simplification of the exact electrode placement procedure using new, real-time fusion techniques of pre-interventional CT/MRI images or biplane fluoroscopic images with intra-interventional ultrasound have the potential to reduce the variable and sometimes the high rate of local tumor progression (12, 13). At the same time, there is a large clinical demand to improve the efficacy of RFA in order to create larger coagulation necrosis (14-17). The recent techniques that are used to improve the efficacy of the radiofrequency (RF) device in order to increase the coagulation with a single application include multiple electrode RFA in the switching monopolar (SM) (7, 18-20), bipolar or multipolar (20, 21) modes. Theoretically, the multiple electrode RFA approach, where three or four electrodes are placed in triangular or square arrays with equidistant inter-electrode spacing, may permit a creation of large, spherical-shaped ablative zones for the local treatment of human liver tumors (20-22). Using prior studies as references (18, 20-22), we assumed that the dual switching monopolar (DSM) RFA using a separable clustered electrode (Octopus® electrodes: STARmed, Goyang, Korea) having three, internally cooled electrodes and a dual generator system, where RF energy is applied to the two electrodes at the same time as well as the switching of RF energy between a pair of electrodes could create a large ablative zone with a spherical shape and thus, with better efficiency for delivering RF energy during a given treatment time.
Therefore, we attempted to compare the in-vitro efficiency of DSM RFA using a separable clustered electrode with consecutive monopolar (CM) and SM RFA techniques in order to create an ablative zone in the explanted bovine liver.
MATERIALS AND METHODS
Development of a Three-Channel, Dual-Generator RFA Unit
In order to improve the efficiency of the multiple-electrode RFA using a separable clustered electrode (Octopus®; STARmed, Goyang, Korea) by allowing the delivery of a large amount of RF energy within a given amount of time, a prototype of a three-channel dual-generator RFA unit (VIVA Multi®; STARmed, Goyang, Korea) was developed. In order for it to be possible to control the RF power of each RF amplifier independently based on independent impedance changes, and to deliver RF energy to the electrodes simultaneously, we developed the multichannel RF system instead of using a single generator RF system. The three-channel, dual-generator RFA Unit features simultaneous RF energy delivery to two electrodes using two independent 200 W generators. It is also able to automatically switch the RF energy between the two pairs of three electrodes according to the impedance changes similar to those of a multi-channel generator (7, 18, 19) (Fig. 1). This system is based on the use of a simultaneous mode and a SM mode which are used to heat the tissue; RF power is then simultaneously applied to a pair of electrodes; however, when impedance changes occur, RF energy is then delivered in an alternating fashion to three possible pairs of active tips of the separable clustered electrode in order to obtain improved efficiency for delivering RF energy at a given time. Although in theory, simultaneous RF energy delivery with two generators to two electrodes could create interference in multiple RF electrodes (Faraday Cage Effect), which could result in an unheated portion between the electrodes, we theorized that if the degree of tissue heating around each electrode is strong enough, the thermal conduction between the areas of active heating may create a large, single ablation area.
Fig. 1.
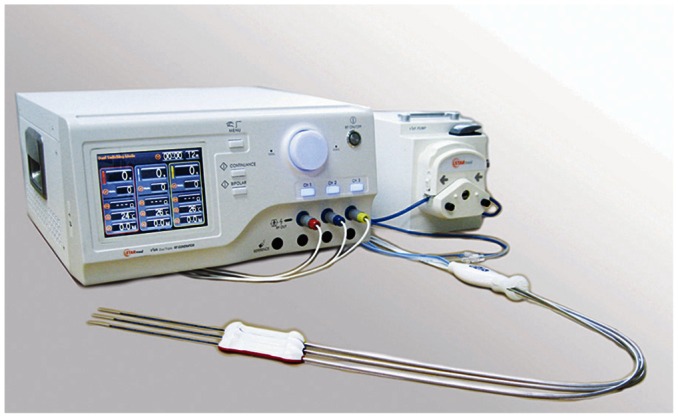
Photographs of single Octopus® electrode and prototype three-channel dual generator radiofrequency ablation unit.
The RFA unit uses two generators with a maximum power of 200 W at a frequency of 480 kHz, thus allowing simultaneous RF energy delivery; we used the unit at maximum power (200 W) in the impedance control mode. The circuitry incorporated into the RF system allows continuous monitoring of the impedance between the active portion of the electrode and the grounding pads.
In Vitro Experimental Setting
We performed RFA in 20 freshly excised bovine livers weighing an average of 7 kg each. The liver was cut into one or two 12 × 12 × 7 cm3 blocks, which were immersed in a 50 × 20 × 25 cm3 saline-filled bath. The RFA system used in our experiments was comprised of a separable clustered electrode (Octopus® electrode; STARmed) with three internally cooled electrodes with a 3 cm long active tip each and a prototype of a three-channel dual generator RFA unit (VIVA Multi®; STARmed) used at maximum wattage (- 200 W). The Octopus® electrode is a separable clustered electrode with a specialized handle portion that can be incorporated into a larger handle in a single unit (18). As each electrode has a flexible, 50-cm-long cable, an Octopus® electrode can be placed in the liver with a variable inter-electrode distance determined by the size of the tumor. Recently, Lee et al. (18) demonstrated that RFA using 17 gauge Octopus® electrodes with three separate, individual electrodes at a 10 mm inter-electrode distance was more efficient for creating a larger coagulation area than a conventional, monopolar RFA using clustered electrodes. Based on some of the previous study results of SM RFA (19), we decided to place the three electrodes of the Octopus® electrode in a triangular array with equidistant, inter-electrode spacing at 3 cm through an acrylic plate containing multiple holes at 5 mm intervals. The electrode tips were advanced at least 4 cm deep into the target tissue. A peristaltic pump (Watson-Marlow, Medford, MA, USA) was used to infuse the normal saline solution at 0℃ into the electrode lumens at a rate sufficient to maintain a tip temperature of 20-25℃. The applied current, power output, and impedance were continuously monitored by the generator during RFA and were recorded automatically using a computer program (VIVA Monitor Software V 1.0; STARmed, Goyang, Korea). The technical aspects of RFA, including impedance and wattage changes, as well as the dimensions of the RF-coagulated area, were compared with each technique.
Ablation Protocol
Based on the results of the previous study (19), the electrodes were placed in a triangular array with equidistant 3 cm inter-electrode spacing. A total of 30 ablation areas were created in the CM mode (group A, total 24 minutes [8 minutes × 3], n = 10), in the SM mode (group B, 12 minutes, n = 10), and in the DSM mode (group C, 12 minutes, n = 10) (Fig. 2). In the CM mode, RF energy (- maximum 200 W) was consecutively applied to each electrode for eight minutes by changing the current flow to the second electrode just after ablation with the first electrode. To prevent tissue cooling between ablations, we attempted to minimize the time between sequential RF ablations, and succeeded in reducing it to less than 5 seconds, which was needed in all cases to change the connection in one of the three electrodes as well as in the generator (21). RF energy delivery was performed at maximum wattage (200 W) using an impedance-controlled algorithm to optimize the energy administration to the tissue. The circuitry incorporated into the generator allowed continuous monitoring of impedance between the active portion of the electrode and the grounding pads, and the generator automatically controlled the power output with the pulsing algorithm.
Fig. 2.
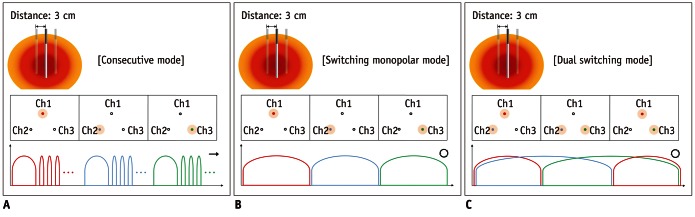
Diagram showing typical patterns of radiofrequency energy delivery during radiofrequency ablation in three groups.
A. Consecutive mode: radiofrequency (RF) energy was consecutively applied to each electrode for eight minutes by changing current flow to second electrode just after ablation with first electrode (in total 24 minutes). B. Switching monopolar mode: RF energy (- maximum 200 W) was applied to one of three electrodes and was switched between three electrode tips of Octopus® electrode depending on tissue impedance changes for total of 12 minutes. C. Dual switching mode: synchronous parallel RF energy (- maximum 400 W; 200 + 200 W) was applied to pair of three electrodes; similar to switching monopolar mode, RF energy was delivered in alternating fashion to three possible pairs of electrodes of Octopus® electrode.
In the SM mode, the RF energy (- maximum 200 W) was applied to one of the three electrodes and was switched between the three electrode tips of the separable clustered electrode depending on the tissue impedance changes for a total of 12 minutes (Fig. 2) (19, 23). In the SM mode, the maximum power switched to the other electrode at approximately 30 seconds if there was no impedance rise for 50 ohm above the baseline value (18). However, if the impedance of one of the electrodes rose 50 ohm above the baseline value, the current was automatically switched to the other electrode; if the impedance immediately rose 300 ohm above the baseline value, no power was applied to that particular electrode for 15 seconds (18, 19).
In the DSM mode, synchronous parallel RF energy (- maximum 400 W; 200 + 200 W) was applied to a pair of the three electrodes; similar to the SM mode, the RF energy was delivered in an alternating fashion to the three possible pairs of electrodes of the separable clustered electrode (Fig. 2). The switching of RF energy delivery between the pair of electrodes was automatically controlled by the microprocessor of the prototype triple channel dual RF generator system according to the continuously measured impedance values. The maximum power (2 × 200 W) switches at approximately 30 seconds if there is no impedance rise of 170% of the baseline value (initial impedance) for example, the first pair of electrodes is energized for 30 seconds, after which the second pair of electrodes is energized for 30 seconds, etc. However, if the impedance of one of the electrodes rises higher than 170% of the baseline impedance value the baseline value, the current is automatically switched to the other pair of electrodes. Consequently, the switching time can vary depending on the tissue impedance changes during the RF energy delivery.
Ablative Zone Size Measurement and Shape Analysis
The liver blocks containing ablative zones were dissected along the axis of the electrode insertion, and the blocks were sliced again in the transverse plane perpendicular to the electrode tracks. As the white, central area of the RF-induced ablative zone has been shown to correspond to the zone of coagulation necrosis (24), two observers (technicians with at least three years of clinical experience participating in RF experiments) measured the long-axis diameter (Dmx), the vertical diameter (Dv), and the short-axis diameter (Dmi) of the central, white region of the RF-induced ablative zones at the slice showed the maximum area in consensus. Further, in order to avoid any bias in the measurements of the ablative zones, the slices were then placed on an optical platform and photographed along with a ruler using a digital camera (Canon EOS 600D; Canon Inc., Tokyo, Japan); the pictures were then saved to a computer equipped with Image J software (http://rsb/info/nig/gov) (19). The ablative zone size measurements were reconfirmed by one reviewer among the authors (J.M.L.) who had clinical experience, performing more than 2000 RFA procedures; he measured the diameters of the white, central areas of the ablative zone using the Image J program and was blinded to all information regarding the RFA techniques used (18, 19).
The volume of the ablative zone was evaluated by approximating the lesion to a sphere using the following formula: π (Dv × Dmx × Dmi) / 6. If the ablative zone was clover leaf shaped, we drew an ellipse, which passed by three tangent points, and measured the maximum (Dmx-eff) and minimum (Dmi-eff) diameters of the ellipse. The volumes of the ablative zones created were evaluated by approximating the coagulation zone to a sphere using the following formula: Volume = π / 6 × Dmx × Dmi × Dv and Volumeeff = π / 6 × Dmi3 (21).
The shape of the RF-induced ablative zone was characterized by the ratio between the Dmx and Dmi (18, 19). The area analysis was performed on a computer equipped with Image J software (http://rsb/info/nig/gov). The shape of the ablative zone was evaluated using a rough estimate of the ablative zone "roundness" in two dimensions by computing the isoperimetric ratio for each ablative zone in the most representative slice. This value was computed using the following formula: R = 4πA / l2, where R is the isoperimetric ration and A is the area of the measured zone; l was obtained using the computer program National Institutes of Health image J.
Statistical Analysis
For all of the experiments, the results were reported as mean ± standard deviation (SD) values. The normal distribution of the values of the dimensions of the thermal ablative zone and the technical parameters of the three groups (consecutive vs. SM vs. DSM modes) were tested with the Kolmogorov-Smirnov test. Next, the dimensions of the thermal ablative zone and the technical parameters of the three groups were compared using the analysis of variance (ANOVA) test. In order to test the variability of the dimensions of the ablative zone in the three groups, we calculated the coefficient of variation (CV) of the ablation volume. CV was defined as the ratio of the SD to the mean size of the ablative zone in each group (18). For all statistical analyses, a p value less than 0.05 was considered statistically significant. Statistical analysis was performed using the summary statistics and the ANOVA test with MedCalc statistical software, version 12.2.1 (MedCalc Software, Mariakerke, Belgium).
RESULTS
Technical Parameters
During RFA procedure, tissue impedance was automatically well-controlled by the impedance control mode, and the mean impedance for the three groups was in 62-66 ohm. In the SM and DSM modes, there was no dead time not to deliver RF energy during the procedure time as RF energy was continually delivered to at least one electrode. Therefore, the mean RF energy delivered was significantly higher in the DSM mode than in either the SM mode or the CM mode (p < 0.001), and the SM mode also delivered a higher amount of RF energy than the CM mode; the average of the delivered wattage during the procedure time was 74.15 ± 12.1 Watts in the CM mode (group A), 115.94 ± 5.95 Watts in the SM mode (group B), and 237.92 ± 14.5 Watts in DSM mode (group C) (p < 0.001). The mean amounts of the cumulative, delivered RF energy in groups A, B, and C were 63.15 ± 8.6 kJ, 72.13 ± 5.4 kJ, and 106.08 ± 13.4 kJ, respectively (p < 0.001). Furthermore, the DSM mode provided the highest efficiency for delivering RF energy per minute among the three modes, i.e., 1.51 kJ/min in the CM mode; 6.01 kJ/min in the SM mode; and 8.84 kJ/min in the DSM mode (p < 0.001).
Ablative Zone Size Measurement and Shape Analysis
There was no case which showed a separated ablative zone; moreover, all three modes generated unified ablative zones along the three electrodes (Fig. 3). The mean values of Dmx of the RF-induced central white zones measured in the gross specimens of the three groups were 5.51 ± 0.42 cm in Group A, 5.97 ± 0.45 cm in Group B, and 6.49 ± 0.30 cm in Group C (p < 0.01) (Table 1). The Dmi of the ablation areas in groups A, B, and C were 5.06 ± 0.31 cm, 5.53 ± 0.54, and 6.18 ± 0.35 cm, respectively (p < 0.01).
Fig. 3.
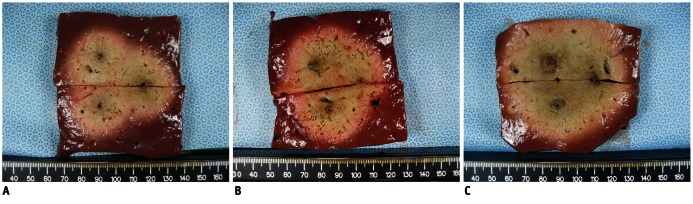
Comparison of radiofrequency ablation (RFA)-induced coagulation created by applying radiofrequency in consecutive, switching, and dual switching modes and with 3 cm, inter-electrode distance.
A. Transverse cut section of specimen created in consecutive monopolar RFA showed 4.54 × 5.03 sized ablation area. B. Transverse cut section of specimen created in switching monopolar RFA. Long-axis and short-axis diameters of ablative zone were 5.77 cm and 5.29 cm, respectively. C. Transverse cut section of specimen created in dual switching RFA. Long-axis and short-axis diameters of ablative zone were 6.78 cm and 6.55 cm, respectively.
Table 1.
Mean Values of Parameters in Each Group
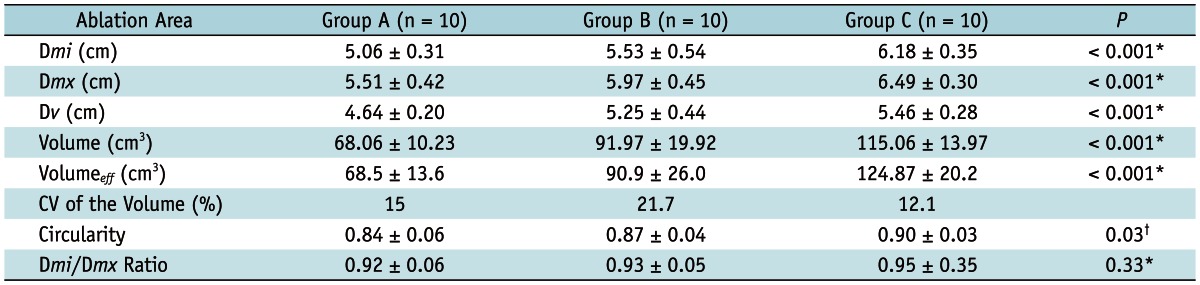
Note.- Values are mean ± standard deviation. *Stands for statistical difference between group A, group B, and group C, *Represents differences in dimension of ablative zone between group A and group B + C. Dmi = minimum diameter of the ablative zone, Dmx = maximum diameter of the ablative zone, Dv = vertical diameter of the ablative zone, Volumeeff = effectively ablated volume, CV = coefficient of variation
With regard to the ablation volume, the DSM RFA (group C) created a significantly larger volume of ablative zones than did the other modes (groups A and B): 68.06 ± 10.23 cm3 (group A); 91.97 ± 19.92 cm3 (group B); and 115.06 ± 13.97 cm3 (group C) (p < 0.001, thus indicating that the difference between each group was significant) (Table 1). The CV values of the ablation volume were 15% in group A, 21.7% in group B, and 12.1% in group C, respectively.
Regarding the shape of the ablative zones, the ratios between the Dmx and the Dmi of the ablative zones in groups A, B, and C were 0.92 ± 0.06, 0.93 ± 0.05, and 0.95 ± 0.35, respectively (p = 0.33), thus indicating that there was no significant difference among the three groups. However, the circularity of the ablative zones measured using the Image J program by one of the authors was 0.84 ± 0.06 in group A, 0.87 ± 0.04 in group B, and 0.90 ± 0.03 in group C, respectively; there were significant differences in the three groups (p = 0.03). Compared with the CM (group A), the SM mode (group B) and the DSM mode (group C) thus produced round ablative zones with a less prominent waist formation between the electrodes (p < 0.05).
DISCUSSION
In our study, DSM RFA using a single Octopus® electrode and a prototype three-channel dual generator RFA unit created a significantly larger ablation volume (115.1 cm3) than the consecutive mode (68.1 cm3) (p < 0.001) (Table 1). The mean short-axis diameter (6.18 cm) of the ablative zone in group C was also significantly larger than those of groups A (5.06 cm) and B (5.53 cm). Furthermore, DSM RFA (group C) created larger ablative zone in only 50% of the ablation time compared with the CM mode (group A), primarily due to its improved efficiency for delivering RF energy at a given time. In addition, compared with the CM mode (group A), which created rather elongated, oval-shaped ablative zones, the SM and DSM modes tended to produce round-shaped ablative zones with less prominent waist formation between electrodes (p = 0.03). As it is quite difficult to reposition the electrode during overlapping ablations, as numerous micro-bubbles form in the heated tissue during RFA, based on our study results, DSM RFA may improve the therapeutic efficacy of RFA for the treatment of both HCC and CRLM by reducing local recurrence along with reduced treatment time; it may also increase the RFA indications to include larger liver malignancies. Although placing three electrodes into the index tumor can make the RFA procedure slightly more difficult, it is only minimally more complicated compared to the insertion of a single electrode. Furthermore, inserting the electrodes before RF energy instillation may avoid the problem of moving the electrode from one site to another (18).
The greater efficiency of DSM RFA using the prototype three-channel dual-generator RFA unit mode, and a single Octopus® electrode compared with CM or SM RFA in our study could be attributed to several factors. First, it could be caused by the greater RF power delivery during the procedure time through the simultaneous delivery of two 200 W RF generators. The means of the cumulative delivered RF energy in groups A, B, and C were 63.15 ± 8.6 kJ, 72.13 ± 5.4 kJ, and 106.08 ± 13.4 kJ, respectively (p < 0.001). Second, it could be related to the greater efficiency of the DSM mode for delivering RF energy to the target tissue compared with the consecutive overlapping RFA (18). The average of the delivered wattage during the procedure time of SM RFA (237.92 ± 14.5 Watt) was significantly greater than that of the CM mode (74.15 ± 12.1 Watt) or the SM mode (115.94 ± 5.95 Watt) (p < 0.001). Third, the greater efficiency of DSM RFA for creating a large ablative zone could be related to heat trapping between the pair of electrodes (23). In the CM mode, heat is diverted from the ablation site in all directions. In the DSM mode, as heat is trapped between the electrodes and higher temperatures are thus achieved, there is less cooling in the direction of the collateral electrode compared with the CM mode (23).
Currently, RFA procedures are widely performed at many hospitals using a single electrode, a cooled-tip electrode, a clustered electrode, or an umbrella-type electrode (18, 25). In a large number of cases in clinical practice, overlapping ablation is required in order to create a sufficient number of safety zones around the index tumor in order to increase the dimensions of the RF-induced ablative zones. However, the single electrode approach is limited for creating a large ablative zone despite of its improved performance or for creating a spherical-shaped ablative zone in a controlled fashion (26). As demonstrated in several previous studies regarding the multiple electrode approach as a strategy to increase the dimension of the spherical-shaped ablative zone (7, 18-20), we believe that the separable clustered electrode (Octopus®) could provide the same advantages as those of the multiple-electrode RF approaches.
Previous investigations (20, 27) demonstrated that bipolar or switching bipolar RFA show greater energy efficiency than monopolar RFA. In several previous studies, in order to use multiple electrodes (two or more than three) for RFA, several modes can be used to deliver RF energy to the electrodes, i.e., CM, simultaneous monopolar, SM, and switching bipolar and multipolar modes (7, 18-23, 27-31). The conventional, simultaneous monopolar mode using a single generator and a cluster electrode allows synchronous energy application to all electrodes, and therefore, the maximal energy of any electrode is a reduced equivalent to the number of electrodes used (Rule of Ohm) (21). Furthermore, the simultaneous mode has the intrinsic problem of the Faraday Cage Effect, which represents the interference in multiple RF electrodes and which may result in an unheated portion between the electrodes (29). Although DSM RFA may exhibit the same problem with the conventional simultaneous mode RFA, we assume that if the degree of tissue heating around each electrode is strong enough, the thermal conduction between the areas of active heating can create a large, single ablation area. Based on our study results with DSM-RFA using a prototype three-channel dual-generator RFA unit, which created large unified ablative zones along the three electrodes, we believe that if the degree of tissue heating around each electrode was strong enough, the thermal conduction might be more critical for creating an ablative zone than an electronic conduction in the liver tissue.
Although DSM RFA using a single Octopus® electrode and a prototype three-channel dual-generator RFA unit created a significantly larger volume of ablation (115.1 cm3) than did the consecutive mode (68.1 cm3), it has certain drawbacks. First, the multiple electrodes RFA technique is more costly than the single, consecutive RF application. Second, in DSM RFA, it is optimal that the electrodes be placed equidistant from each other in order to create a single, large ablation sphere. In the clinical environment, the insertion of the three electrodes at equidistance might be difficult for operators with less clinical experience.
Our experimental study has certain limitations. First, the application of our study results to the human liver tumor is limited because our study was performed under ex-vivo conditions using normal liver parenchyma. However, despite these concerns, we believe that our study results provide a reliable basis for future comparative studies regarding the efficiency of various RF modes in in vivo large animals, particularly the DSM mode, which provided a relatively larger amount of energy to the tissue. Second, we tested the efficacy of the SM and DSM RF modes for creating large ablative zones using a 24-minute CM RFA. However, in theory, with increasing RFA time longer than 24 minutes, the CM RFA might be able to create a larger ablative zone than that seen in our study results. However, we believe that a 24-minute RFA time is not less than the usual RFA time seen in clinical practice. Third, we did not perform a comparative study between the DSM mode and the multipolar mode. Fourth, even though the DSM mode RFA using an Octopus® electrode showed better performance in creating large ablative zones when compared to the monopolar RFA, the placement of the three electrodes through intercostal spaces may present technical difficulties in the clinical setting. Lastly, although the creation of large ablative zones with a single RF application is beneficial for achieving complete necrosis of the index tumor, it also has an increased risk of unexpected thermal injury to the adjacent vital structures, such as the bile duct. In addition, in DSM-RFA mode, synchronous parallel RF energy (- maximum 400 W; 200 + 200 W) can be applied to a pair of the three electrodes in an ideal environment, which might increase the risk of the RFA procedure. Therefore, so as to resolve the possible safety issues, an experimental study in a large animal tumor model of clinically relevant size will be necessary before the clinical application of DSM RFA using the dual generator system and a separable clustered electrode for the treatment of human liver malignancies.
In conclusion, as DSM RFA can achieve large ablative zones with improved time efficiency, this technology may provide a useful tool for the treatment of large malignant tumors of the liver.
Acknowledgments
We thank Bonnie Hami, M.A. (USA), for her editorial assistance and Dong Un Kim, BA, for his contribution for technical assistance for radiofrequency ablation experiments.
References
- 1.Wu YZ, Li B, Wang T, Wang SJ, Zhou YM. Radiofrequency ablation vs hepatic resection for solitary colorectal liver metastasis: a meta-analysis. World J Gastroenterol. 2011;17:4143–4148. doi: 10.3748/wjg.v17.i36.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salhab M, Canelo R. An overview of evidence-based management of hepatocellular carcinoma: a meta-analysis. J Cancer Res Ther. 2011;7:463–475. doi: 10.4103/0973-1482.92023. [DOI] [PubMed] [Google Scholar]
- 3.Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities--part II. J Vasc Interv Radiol. 2001;12:1135–1148. doi: 10.1016/s1051-0443(07)61670-4. [DOI] [PubMed] [Google Scholar]
- 4.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761–768. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- 5.Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010;195:758–765. doi: 10.2214/AJR.09.2954. [DOI] [PubMed] [Google Scholar]
- 6.Kim KW, Lee JM, Klotz E, Kim SJ, Kim SH, Kim JY, et al. Safety margin assessment after radiofrequency ablation of the liver using registration of preprocedure and postprocedure CT images. AJR Am J Roentgenol. 2011;196:W565–W572. doi: 10.2214/AJR.10.5122. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Lee JM, Yoon JH, Lee JY, Kim SH, Lee JE, et al. Percutaneous radiofrequency ablation with multiple electrodes for medium-sized hepatocellular carcinomas. Korean J Radiol. 2012;13:34–43. doi: 10.3348/kjr.2012.13.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Sofocleous CT, Erinjeri JP, Petre EN, Gonen M, Do KG, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36:166–175. doi: 10.1007/s00270-012-0377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Baere T. [Ablation of liver metastases by radiofrequency] Cancer Radiother. 2012;16:339–343. doi: 10.1016/j.canrad.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Dodd GD, 3rd, Frank MS, Aribandi M, Chopra S, Chintapalli KN. Radiofrequency thermal ablation: computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol. 2001;177:777–782. doi: 10.2214/ajr.177.4.1770777. [DOI] [PubMed] [Google Scholar]
- 12.Bangard C. [Radiofrequency of the liver - an update] Rofo. 2011;183:704–713. doi: 10.1055/s-0029-1246082. [DOI] [PubMed] [Google Scholar]
- 13.Min JH, Lee MW, Rhim H, Choi D, Kim YS, Kim YJ, et al. Radiofrequency ablation for viable hepatocellular carcinoma around retained iodized oil after transcatheter arterial chemoembolization: usefulness of biplane fluoroscopy plus ultrasound guidance. Korean J Radiol. 2012;13:784–794. doi: 10.3348/kjr.2012.13.6.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound. 2001;13:129–147. doi: 10.1016/s0929-8266(01)00126-4. [DOI] [PubMed] [Google Scholar]
- 15.Widmann G, Bodner G, Bale R. Tumour ablation: technical aspects. Cancer Imaging. 2009;9 Spec No A:S63–S67. doi: 10.1102/1470-7330.2009.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McWilliams JP, Yamamoto S, Raman SS, Loh CT, Lee EW, Liu DM, et al. Percutaneous ablation of hepatocellular carcinoma: current status. J Vasc Interv Radiol. 2010;21(8 Suppl):S204–S213. doi: 10.1016/j.jvir.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 17.Seong NJ, Yoon CJ, Kang SG, Chung JW, Kim HC, Park JH. Effects of arsenic trioxide on radiofrequency ablation of VX2 liver tumor: intraarterial versus intravenous administration. Korean J Radiol. 2012;13:195–201. doi: 10.3348/kjr.2012.13.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee ES, Lee JM, Kim WS, Choi SH, Joo I, Kim M, et al. Multiple-electrode radiofrequency ablations using Octopus® electrodes in an in vivo porcine liver model. Br J Radiol. 2012;85:e609–e615. doi: 10.1259/bjr/61619687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JM, Han JK, Kim HC, Choi YH, Kim SH, Choi JY, et al. Switching monopolar radiofrequency ablation technique using multiple, internally cooled electrodes and a multichannel generator: ex vivo and in vivo pilot study. Invest Radiol. 2007;42:163–171. doi: 10.1097/01.rli.0000252495.44818.b3. [DOI] [PubMed] [Google Scholar]
- 20.Stoffner R, Kremser C, Schullian P, Haidu M, Widmann G, Bale RJ. Multipolar radiofrequency ablation using 4-6 applicators simultaneously: a study in the ex vivo bovine liver. Eur J Radiol. 2012;81:2568–2575. doi: 10.1016/j.ejrad.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 21.Lee JM, Han JK, Kim HC, Kim SH, Kim KW, Joo SM, et al. Multiple-electrode radiofrequency ablation of in vivo porcine liver: comparative studies of consecutive monopolar, switching monopolar versus multipolar modes. Invest Radiol. 2007;42:676–683. doi: 10.1097/RLI.0b013e3180661aad. [DOI] [PubMed] [Google Scholar]
- 22.Clasen S, Schmidt D, Boss A, Dietz K, Kröber SM, Claussen CD, et al. Multipolar radiofrequency ablation with internally cooled electrodes: experimental study in ex vivo bovine liver with mathematic modeling. Radiology. 2006;238:881–890. doi: 10.1148/radiol.2382050571. [DOI] [PubMed] [Google Scholar]
- 23.Lee JM, Han JK, Lee JY, Kim SH, Choi JY, Lee MW, et al. Hepatic radiofrequency ablation using multiple probes: ex vivo and in vivo comparative studies of monopolar versus multipolar modes. Korean J Radiol. 2006;7:106–117. doi: 10.3348/kjr.2006.7.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JD, Lee JM, Kim SW, Kim CS, Mun WS. MR imaging-histopathologic correlation of radiofrequency thermal ablation lesion in a rabbit liver model: observation during acute and chronic stages. Korean J Radiol. 2001;2:151–158. doi: 10.3348/kjr.2001.2.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrean S, Hering J, Saied A, Helton WS, Espat NJ. Radiofrequency ablation of primary and metastatic liver tumors: a critical review of the literature. Am J Surg. 2008;195:508–520. doi: 10.1016/j.amjsurg.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Lin SM. Recent advances in radiofrequency ablation in the treatment of hepatocellular carcinoma and metastatic liver cancers. Chang Gung Med J. 2009;32:22–32. [PubMed] [Google Scholar]
- 27.Ritz JP, Lehmann KS, Reissfelder C, Albrecht T, Frericks B, Zurbuchen U, et al. Bipolar radiofrequency ablation of liver metastases during laparotomy. First clinical experiences with a new multipolar ablation concept. Int J Colorectal Dis. 2006;21:25–32. doi: 10.1007/s00384-005-0781-y. [DOI] [PubMed] [Google Scholar]
- 28.Clasen S, Rempp H, Schmidt D, Schraml C, Hoffmann R, Claussen CD, et al. Multipolar radiofrequency ablation using internally cooled electrodes in ex vivo bovine liver: correlation between volume of coagulation and amount of applied energy. Eur J Radiol. 2012;81:111–113. doi: 10.1016/j.ejrad.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 29.Lee FT, Jr, Haemmerich D, Wright AS, Mahvi DM, Sampson LA, Webster JG. Multiple probe radiofrequency ablation: pilot study in an animal model. J Vasc Interv Radiol. 2003;14:1437–1442. doi: 10.1097/01.rvi.0000096771.74047.c8. [DOI] [PubMed] [Google Scholar]
- 30.Lee IJ, Kim YI, Kim KW, Kim DH, Ryoo I, Lee MW, et al. Radiofrequency ablation combined with transcatheter arterial embolisation in rabbit liver: investigation of the ablation zone according to the time interval between the two therapies. Br J Radiol. 2012;85:e987–e994. doi: 10.1259/bjr/90024696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JM, Han JK, Kim SH, Choi SH, An SK, Han CJ, et al. Bipolar radiofrequency ablation using wet-cooled electrodes: an in vitro experimental study in bovine liver. AJR Am J Roentgenol. 2005;184:391–397. doi: 10.2214/ajr.184.2.01840391. [DOI] [PubMed] [Google Scholar]


